Abstract
Introduction
Statin medications have anti-inflammatory effects. We sought to determine whether statin use in persons with inflammatory bowel disease (IBD) was associated with reduced rates of steroid use or other markers of disease activity.
Methods
We performed a retrospective cohort study using administrative data. Statin users with IBD were compared to statin-unexposed IBD subjects. The primary outcome was an oral steroid prescription; secondary outcomes included anti-TNF initiation, hospitalization, or abdominal surgery. Cox proportional hazard models were used to estimate hazard ratios (HR) adjusted for potential confounders.
Results
The study cohort included 1,986 statin-exposed and 9,871 unexposed subjects. Statin use was associated with an 18% reduction in the rate of steroid initiation [HR 0.82 (95% CI 0.71, 0.94)]. A statistically significant result was seen with atorvastatin only [HR 0.76 (95% CI 0.60, 0.96)]. Statins were associated with a reduced rate of steroids in ulcerative colitis [HRs 0.75 (95% CI 0.62, 0.91)], but not in Crohn’s disease [HR 0.91 (95% CI 0.74, 1.12)]. Statin use was associated with reduced hazard of anti-TNF use [HR 0.72 (95% CI 0.46, 1.11)], abdominal surgery [HR 0.80 (95% CI 0.63, 1.02)], and hospitalization [HR 0.88 (95% CI 0.74, 1.05)], but these results did not reach statistical significance.
Conclusion
In this large retrospective cohort study, statin use amongst persons with IBD was associated with reduced use of oral steroids, particularly for UC. Prospective clinical trials are needed to confirm whether adjuvant treatment of IBD with statin drugs may spare immunosuppressant therapy or ameliorate flares.
Keywords: Hydroxymethylglutaryl-CoA Reductase Inhibitors, Inflammatory Bowel Diseases, Ulcerative Colitis, Crohn’s Disease, Glucocorticoids
Introduction
Inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), affect 1-1.5 million Americans (1, 2). Both UC and Crohn’s are associated with substantial morbidity, including frequent hospitalization and surgery (3, 4), reductions in quality of life (5), an increased risk of colorectal cancer (6), and increased mortality (7). The current medical armamentarium for IBD includes drugs that are either incompletely effective, have serious potential adverse effects, are difficult to administer, or are very expensive. The recent development and use of biologic therapies in IBD has brought increased effectiveness (8, 9), but also concerns about safety (10, 11), and the associated increase in the cost of treating IBD (12, 13). Pharmacoepidemiology studies that explore novel uses of existing medications could lead to the identification of safe and inexpensive treatment options for patients with IBD.
Statin drugs are widely used worldwide for treatment of hyperlipidemia (14, 15). In addition to cholesterol-lowering, statins reduce many of the mediators involved in IBD-specific inflammation including C-reactive protein, interferon gamma, interleukins 6 and 8, and NF-kappa B (16-18). Furthermore, studies in animal models of IBD suggest that statins can attenuate colitis (19, 20). In humans, a few small studies suggest a possible beneficial effect of statins. A randomized controlled trial of atorvastatin vs. placebo in UC patients reported a reduction in disease activity and a higher rate of remission in patients treated with atorvastatin compared to placebo (21). A small uncontrolled trial of atorvastatin treatment in CD patients demonstrated a measurable reduction in pro-inflammatory markers, and a non-statistically significant decrease in disease activity on treatment (22). However, a second open-label trial of pravastatin and rosuvastatin in CD did not show a benefit of statin therapy (23).
Importantly, statins are associated with minimal toxicity, and are substantially less expensive than most existing IBD therapies (<$1/day for generic versions). It is unknown whether statins may reduce the rate of flares of disease or other important patient-related outcomes. This study was designed to address the question of whether use of statin drugs could result in steroid sparing and reduce IBD exacerbations in a large cohort of patients.
Materials and Methods
Study design and data source
This was a retrospective cohort study using an administrative claims database. The MarketScan database (Thompson Reuters©) is a proprietary database that contains claims data on over 35 million individuals covered by employer-sponsored commercial health insurance from over 100 health plans, large employers, and government and public organizations. Data elements include inpatient and outpatient diagnoses ((International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM)), procedures (Current Procedural Terminology, 4th Edition (CPT-4)), prescription records, demographic information, and enrollment details. Data from January 2005 to December 2007 were used in this study. This database has been used in other epidemiologic studies of IBD (24, 25) and statins (26), and is representative of the commercially-insured population of the US (27, 28).
Cohort identification and assessment of exposure
We selected IBD patients initiating a statin medication and a comparator group of statin-unexposed IBD patients using the following process. First, the entire source population (n=35,568,227) was limited to those aged 35-64 years who met the IBD case definition: 1) at least 1 healthcare contact associated with an ICD-9-CM diagnosis code for CD (555.xx) or UC (556.xx) and 2) at least 1 pharmacy claim for any of the following medications: mesalamine, olsalazine, balsalazide, sulfasalazine, 6-mercaptopurine, azathioprine, or enteral budesonide. To qualify for the study, exposed subjects had a new prescription for one of the six statin drugs on the US market at the time of this study (atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, or simvastatin). This “new user design” was used to minimize bias resulting from analysis of prevalent medication use such as the healthy user effect (29). Statin use was continuous, though gaps in prescribing dates of ≤60 days beyond days supplied were allowed (30). Subjects were excluded if they did not meet the IBD diagnostic criteria or did not have continuous health plan enrollment and pharmacy benefits during the 6 month pre-exposure period and the first month of the post-exposure period.
For each new statin user, we randomly sampled statin-unexposed persons (who met the same criteria for IBD and enrollment in the 6 month pre-exposure period) in a 5:1 ratio using an incidence density design, matched on age (in 5 year increments) and sex. In addition, we required unexposed persons to have received at least 1 outpatient pharmacy claim for a non-statin prescription during the month they were matched in order to ensure a comparator population with similar healthcare access and utilization.
After these procedures, 2,022 statin-exposed subjects and 10,110 matched unexposed subjects were identified. Subsequently, 275 subjects were eliminated because they had claims for either a colectomy or enterostomy (n=184) or a diagnosis of colon cancer (n=91) in the pre-exposure period. The final study population included 1,986 exposed and 9,871 unexposed subjects (Figure 1).
Figure 1.
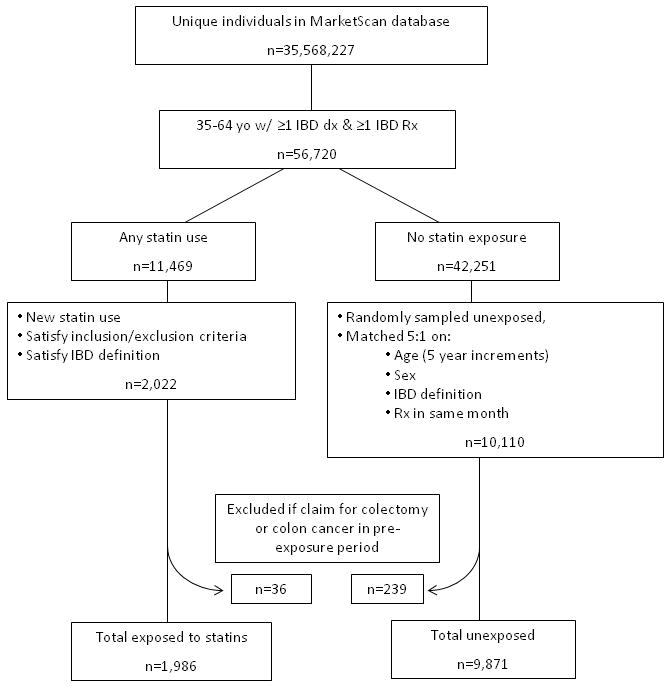
Study flow diagram for a historical cohort study of statin use and inflammatory bowel disease using administrative claims
Assessment of covariates of interest
In addition to demographic information on age, sex, and geographic region (Northeast, North Central, South, and West based on US Census regions), data on potential confounders were collected during the 6 month pre-exposure period. These covariates included type of IBD (UC or CD, based on majority of diagnosis claims), use of other IBD medications [aminosalicylate drugs (mesalamine, olsalazine, balsalazide, sulfasalazine), 6-mercaptopurine (6-MP) or azathioprine, anti-TNF drugs (infliximab or adalimumab), cholestyramine, rectal steroids (enema, suppository, or foam), methotrexate, or cyclosporine], markers of disease severity [weight loss (ICD-9 783.2x), malnutrition (ICD-9 262.xx - 263.xx) and anemia (ICD-9 280.xx)], non-colectomy abdominal surgery (CPT 44500-44979, 49000-49999, 45000-45190, 45395-45999, 46020-46211, 46270-46288, 46700-46762, 46937-46942, 46999), comorbidities [Deyo modification of the Charlson comorbidity index (31, 32), claims for coronary artery disease (ICD-9 410.xx – 414.xx), stroke (ICD-9 433.xx- 435.xx), or diabetes (ICD-9 250.xx)], and markers of healthcare utilization [number of inpatient and outpatient contacts, and endoscopic procedures (upper or lower endoscopy, CPT codes: 43200-43259, 44360-44386, 44388-44397, or 45300-45392)].
Outcomes and censoring events
The primary outcome for this study was defined as a 1st prescription for an oral steroid (days supplied ≥ 14), a marker of flare of disease (33). Secondary outcomes included a 1) 1st use of an anti-TNF agent (infliximab or adalimumab, if not used in 6 months prior to cohort entry); 2) colectomy or other abdominal surgery (excluding outpatient anal procedures such as hemorrhoidectomy); and 3) first hospitalization. A composite outcome was also analyzed (i.e. any primary or secondary outcome).
Participants were censored if they experienced a lapse in plan enrollment/pharmacy benefit > 1 month, if they stopped using a statin (for statin group only- defined as a gap of >60 days beyond days supplied, according to a previously published definition (30)), if they started using a statin (for the unexposed group only), or if they reached the end of study period (December 31, 2007).
Statistical Analysis
Bivariate analysis was performed between covariates and the exposure (using chi-squared tests for categorical variables, or Student’s t-tests for continuous variables), and the primary outcome (using Kaplan Meier plots and log rank tests). Covariates were evaluated for confounding and effect modification if they were significantly associated with the exposure and outcome at an alpha of ≤ 0.2, or if they were believed to be important confounders based on previously published data. Prior to inclusion in modeling, each variable was evaluated with log-log plots and Schoenfeld residuals to ensure the proportional hazards assumption was not violated.
Multivariate analysis
Cox proportional hazard modeling was performed to estimate hazard ratios (HR) for the association between statin use and the primary and secondary outcomes. To determine which covariates should be included in the final multivariable models, a full model with all potential confounders was constructed. Covariates were then removed from the model using backwards elimination with a threshold of <10% change in beta coefficients and a likelihood ratio test p value of >0.05. The Charlson index was retained in each model in order to ensure adjustment for comorbid illness. Because this process was repeated iteratively for each outcome, the adjustment set differed slightly for models for the primary and secondary outcomes. All analyses were performed with STATA version 10.1 (College Station, TX).
Dosage, duration, and adherence analyses
For each statin preparation, a dichotomous variable for dose was created for low and high dose, based on the mean dose for each drug. These variables were then combined into a dichotomous high/low dose variable for all statin-exposed subjects to test the effects of increasing dose. Duration of statin treatment was dichotomized as ≤90 days vs. >90 days. In addition, the medication possession ratio [MPR: calculated as: (sum of days supplied) ÷ (study days)](34) was calculated for each exposed subject and was categorized using cutoffs of 0.80 and 0.90 to determine whether different levels of adherence modified the effect of statin use (35).
Sensitivity analyses
We conducted a number of sensitivity analyses to further evaluate our findings, including restricting the population to: 1) subjects meeting a more stringent diagnosis criteria prior to study entry: ≥ 4 IBD diagnosis claims + ≥ 1 IBD prescription; 2) subjects between the ages of 35-50; 3) subjects without pre-exposure diagnoses of coronary artery disease, diabetes, or stroke; 4) subjects taking statins for >30 days; and 5) subjects who did not receive steroids in the pre-exposure period.
Ethical Considerations
The study protocol was granted an exemption by the Institutional Review Board at University of North Carolina because it involved the use of de-identified data.
Results
Study population
The study cohort included 1,986 statin-exposed subjects and 9,871 matched unexposed subjects (Figure 1, Table 1). Age and sex distributions were expectedly similar given the matching design. Study groups differed based on comorbidities (a higher proportion of statin-exposed subjects had diagnoses of coronary artery disease, diabetes, and stroke). Statin-exposed subjects had slightly more outpatient contacts and hospitalizations. Medication use and endoscopy utilization was similar between groups.
TABLE 1.
Characteristics of the study population
| Characteristic | Statin exposed (n=1,986) n (%) | Unexposed (n=9,871) n (%) | p* |
|---|---|---|---|
| Sex | 0.9 | ||
| Female | 1,013 (51.0) | 5,025 (50.9) | |
| Male | 973 (49.0) | 4,846 (49.1) | |
| Age (years) | 1.0 | ||
| 35-39 | 108 (5.4) | 531 (5.4) | |
| 40-44 | 177 (8.9) | 881 (8.9) | |
| 45-49 | 303 (15.3) | 1,501 (15.2) | |
| 50-54 | 440 (22.2) | 2,188 (22.2) | |
| 55-59 | 558 (28.1) | 2,777 (28.1) | |
| 60-64 | 400 (20.1) | 1,993 (20.2) | |
| Region | <0.0001 | ||
| Northeast | 215 (10.8) | 1,190 (12.1) | |
| North Central | 534 (26.9) | 2,854 (28.9) | |
| South | 905 (45.6) | 3,962 (40.1) | |
| West | 321 (16.2) | 1,800 (18.2) | |
| Unknown/other | 11 (0.6) | 65 (0.7) | |
| Disease manifestation | 0.8 | ||
| Crohn’s disease | 824 (41.5) | 4,176 (42.3) | |
| Ulcerative colitis | 1,132 (57.0) | 5,544 (56.2) | |
| Indeterminate IBD | 30 (1.5) | 151 (1.5) | |
| Comorbidities† (pre-exposure) | |||
| Coronary artery disease | 246 (12.4) | 456 (4.6) | <0.0001 |
| Diabetes mellitus | 429 (21.6) | 918 (9.3) | <0.0001 |
| Stroke | 67 (3.4) | 112 (1.1) | <0.0001 |
| Charlson index (mean ± SD) | 0.65 ± 1.03 | 0.41 ± 0.93 | <0.0001 |
| Disease severity (pre-exposure) | |||
| Weight loss | 12 (0.6) | 112 (1.1) | 0.03 |
| Malnutrition | 5 (0.3) | 33 (0.3) | 0.6 |
| Anemia | 71 (3.6) | 409 (4.1) | 0.2 |
| Abdominal surgery‡ | 83 (4.2) | 453 (4.6) | 0.4 |
| IBD drugs (pre-exposure) | |||
| Oral steroid (≥ 14 days) | 341 (17.2) | 1,768 (17.9) | 0.4 |
| Anti-TNF | 131 (6.6) | 513 (5.2) | 0.01 |
| 5-ASA | 1,718 (86.5) | 8,633 (87.5) | 0.2 |
| Azathioprine/6-MP | 476 (24.0) | 2,209 (22.4) | 0.1 |
| Rectal steroid | 112 (5.6) | 635 (6.4) | 0.2 |
| Cholestyramine | 22 (1.1) | 241 (2.4) | <0.0001 |
| Methotrexate | 23 (1.1) | 123 (1.3) | 0.7 |
| Cyclosporine | 19 (1.0) | 90 (0.9) | 0.8 |
| Healthcare utilization (pre-exposure) | |||
| # outpatient visits (mean ± SD) | 11.3 ± 9.3 | 9.4 ± 8.4 | <0.0001 |
| # hospitalizations (mean ± SD) | 0.2 ± 0.5 | 0.1 ± 0.5 | <0.0001 |
| Any hospitalization | 282 (14.2) | 1,031 (10.4) | <0.0001 |
| IBD hospitalization | 121 (6.1) | 566 (5.7) | 0.5 |
| Endoscopy (pre-exposure) | |||
| EGD/enteroscopy | 168 (8.5) | 863 (8.7) | 0.7 |
| Colonoscopy/sigmoidoscopy | 759 (38.2) | 4,024 (40.8) | 0.04 |
| Any endoscopy§ | 794 (40.0) | 4,224 (42.8) | 0.02 |
p values obtained via Chi-squared tests for categorical variables and Student’s t-tests for continuous variables
Identified by any (≥1) associated ICD-9 coded diagnosis
Excluding colectomy/enterostomy and minor/outpatient procedures (e.g. hemorrhoidectomy)
Includes EGD, enteroscopy, colonoscopy, or flexible sigmoidoscopy and associated interventions
EGD: Esophagogastroduodenoscopy; IBD: inflammatory bowel disease; Anti-TNF: anti-tumor necrosis factor therapies (i.e. infliximab or adalimumab); 5-ASA: aminosalicylate drugs (e.g. sulfasalazine); 6-MP: 6-mercaptopurine; SD: standard deviation
Primary outcome
The absolute frequencies of primary and secondary outcomes are included in Supplementary Table A. The crude incidence of steroid use in the post exposure period was 20.9 per 100 patient years for all study subjects. Overall, statin exposure was associated with an 18% reduction in the rate of receiving steroids [HR 0.82 (95% CI 0.71, 0.94)], after adjusting for the Charlson comorbidity index, pre-exposure use of anti-TNF, 5-ASA, 6-MP/azathioprine, or rectal steroids, the number of outpatient contacts and hospitalization prior to exposure (Table 2, Figure 2). When stratified by disease type, statins were associated with a reduced rate of steroid use in UC [HRs 0.75 (95% CI 0.62, 0.91), but not CD. The hazard ratios of other covariates in the model are presented in Supplementary Table B.
TABLE 2.
Hazard Ratios (95% CI) for primary outcome of 1st oral steroid prescription associated with statin exposure in patients with inflammatory bowel disease overall, and for ulcerative colitis and Crohn’s disease
| Exposure | IBD* |
UC
|
CD
|
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Statin use | |||
| Unadjusted | 0.90 (0.78, 1.04) | 0.81 (0.67, 0.99) | 1.01 (0.82, 1.24) |
| Adjusted | 0.82 (0.71, 0.94) | 0.75 (0.62, 0.91) | 0.91 (0.74, 1.12) |
HRs were determined via Cox proportional hazard models, adjusting for the Charlson comorbidity index, pre-exposure use of anti-TNF, 5-ASA, 6-MP/azathioprine, or rectal steroids, the number of outpatient contacts and hospitalization prior to exposure.
IBD includes cases of Crohn’s disease, ulcerative colitis, and indeterminate IBD.
IBD: inflammatory bowel disease; UC: ulcerative colitis; CD: Crohn’s disease; HR: Hazard ratio
Figure 2.
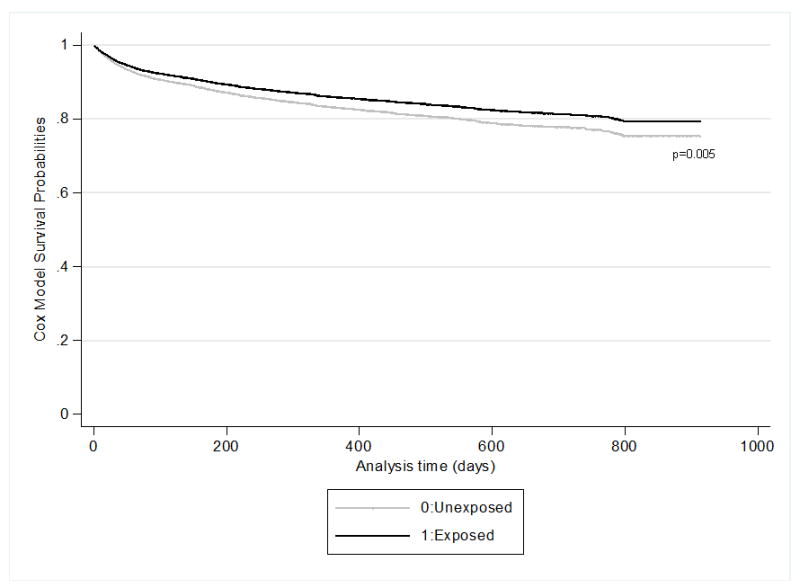
Probability of remaining steroid free for subjects with inflammatory bowel disease who were exposed (black line) and unexposed (gray line) to statin drugs. Curves represent adjusted Cox Model survival probabilities for the primary outcome (steroid prescription ≥ 14 days).
Secondary outcomes
Statin exposure was associated with a trend towards decreased rate of anti-TNF agent use, abdominal surgery, and hospitalization for overall IBD and after stratifying by disease type (CD versus UC), though only the association between statin use and abdominal surgery in CD was statistically significant (Table 3). Statin exposure was significantly associated with a reduction of the composite outcome for overall IBD.
TABLE 3.
Hazard Ratios (95% CI) for secondary outcomes of anti-TNF use, abdominal surgery, or hospitalization associated with statin exposure for all IBD patients, and stratified by ulcerative colitis and Crohn’s disease.
| Anti-TNF† |
Abdominal Surgery‡ |
Hospitalization
|
Combined§ |
|
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| All IBD* | 0.72 (0.46, 1.11) | 0.80 (0.63, 1.02) | 0.88 (0.74, 1.05) | 0.88 (0.78, 0.98) |
| UC | 0.48 (0.19, 1.20) | 0.97 (0.69, 1.37) | 0.87 (0.67, 1.13) | 0.86 (0.74, 1.01) |
| CD | 0.84 (0.50, 1.40) | 0.65 (0.45, 0.93) | 0.89 (0.70, 1.13) | 0.90 (0.76, 1.07) |
HRs were determined via Cox proportional hazard models, adjusting for potential confounders including geographic region, Charlson comorbidity index, a diagnosis of coronary artery disease, weight loss or anemia, pre-exposure use of aminosalicylates, 6MP or azathioprine, rectal steroids, outpatient contacts and hospitalizations prior to exposure. The adjustment set differed slightly for each outcome.
IBD includes cases of Crohn’s disease, ulcerative colitis, and indeterminate IBD.
HRs estimated after excluding subjects who received anti-TNFs in the 180 day pre-exposure period.
Any abdominal surgery (excluding minor outpatient procedures; see Methods section for details of ICD-9 codes used, etc.)
First occurrence of any primary or secondary outcome
IBD: inflammatory bowel disease; UC: ulcerative colitis; CD: Crohn’s disease; HR: Hazard ratio
Dose, duration, and adherence analysis
Dose, duration, and adherence to statin medication were examined to determine whether there was evidence of a dose response effect (Table 4). Compared to unexposed persons, those taking high dose statins had the lowest rate of new steroid prescriptions. With regard to duration of statin therapy, we found that longer duration was associated with the lowest rates of the primary outcome compared to unexposed persons. Increasing duration reduced the rate of the secondary outcomes as well, though these estimates were not statistically significant. Longer duration was associated with the lowest rate of the composite outcome. When adherence to statin therapy was analyzed, better adherence (MPR >0.90) was associated with the lowest rate of steroid prescriptions, anti-TNF initiation, abdominal surgery, hospitalization, and the composite outcome. When stratified by disease type, increasing dose and duration of statin therapy was associated with the lowest rates of steroid use for UC, but not CD (data not shown).
TABLE 4.
Dose, duration, and adherence analysis for primary and secondary outcomes for IBD overall
| Statin usage | n(%) | Steroid use
|
Anti-TNF
|
Abdominal surgery
|
Hospitalization
|
Composite outcome
|
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Dose* | ||||||
| Unexposed | 9,871 (83.3) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Low dose | 1,334 (11.3) | 0.86 (0.73, 1.01) | 0.55 (0.31, 0.99) | 0.80 (0.60, 1.06) | 0.86 (0.70, 1.06) | 0.87 (0.76, 0.99) |
| High dose | 652 (5.5) | 0.72 (0.56, 0.93) | 1.08 (0.58, 1.98) | 0.80 (0.54, 1.20) | 0.92 (0.69, 1.21) | 0.90 (0.74, 1.09) |
| Duration | ||||||
| Unexposed | 9,871 (83.3) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| ≤90 days | 644 (5.4) | 0.84 (0.62, 1.14) | 1.16 (0.42, 3.20) | 0.84 (0.41, 1.70) | 0.93 (0.59, 1.47) | 0.92 (0.70, 1.20) |
| >90 days | 1,342 (11.3) | 0.81 (0.70, 0.95) | 0.67 (0.41, 1.07) | 0.80 (0.62, 1.02) | 0.87 (0.73, 1.05) | 0.87 (0.77, 0.98) |
| Adherence | ||||||
| Unexposed | 9,871 (83.3) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| MPR ≥0.80 | 1,472 (12.4) | 0.88 (0.75, 1.02) | 0.71 (0.43, 1.15) | 0.80 (0.61, 1.04) | 0.84 (0.69, 1.02) | 0.89 (0.79, 1.01) |
| MPR ≥0.90 | 1,220 (10.3) | 0.82 (0.69, 0.98) | 0.68 (0.39, 1.17) | 0.73 (0.54, 0.99) | 0.71 (0.57, 0.89) | 0.80 (0.69, 0.93) |
HRs were determined via Cox proportional hazard models, adjusting for potential confounders including including geographic region, Charlson comorbidity index, a diagnosis of coronary artery disease, weight loss or anemia, pre-exposure use of aminosalicylates, 6MP or azathioprine, rectal steroids, outpatient contacts and hospitalizations prior to exposure. The adjustment set differed slightly for each outcome.
High and low dose based on mean and median dose for each statin type.
HR: Hazard ratio; MPR: medication possession ratio
Statin type
Of the 6 different statin medications used in this study, simvastatin and atorvastatin were the most commonly prescribed, representing 40% and 32% of exposures respectively. The HRs of the primary and secondary outcomes associated with each different statin preparation are shown in Table 5. Though small size of some strata limited the precision of these estimates, atorvastatin use was associated with the lowest statistically significant estimate. Simvastatin was not associated with a reduced rate of steroid usage (HR 0.91 (95% CI 0.74, 1.11)
TABLE 5.
Effect of individual statins on primary and secondary outcomes in subjects with IBD.
| Statin type | n(%) | Steroid use
|
Anti-TNF
|
Abdominal surgery
|
Hospitalization
|
Composite outcome
|
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Atorvastatin | 639 (32.2) | 0.76 (0.60, 0.96) | 0.72 (0.35, 1.46) | 0.71 (0.47, 1.07) | 0.92 (0.70, 1.21) | 0.81 (0.67, 0.99) |
| Fluvastatin | 14 (0.7) | 0.67 (0.09, 4.74) | NE | NE | 1.18 (0.17, 8.40) | 0.50 (0.07, 3.53) |
| Lovastatin | 146 (7.4) | 0.92 (0.58, 1.47) | NE | 0.55 (0.21, 1.48) | 0.82 (0.45, 1.49) | 0.90 (0.61, 1.31) |
| Pravastatin | 132 (6.7) | 0.68 (0.40, 1.18) | 0.92 (0.23, 3.71) | 1.93 (1.09, 3.43) | 0.91 (0.50, 1.66) | 0.86 (0.57, 1.30) |
| Rosuvastatin | 271 (13.7) | 0.75 (0.52, 1.08) | 1.01 (0.38, 2.73) | 0.74 (0.40, 1.39) | 0.73 (0.45, 1.16) | 0.85 (0.63, 1.14) |
| Simvastatin | 784 (39.5) | 0.91 (0.74, 1.11) | 0.66 (0.32, 1.34) | 0.77 (0.53, 1.13) | 0.90 (0.70, 1.17) | 0.95 (0.80, 1.12) |
HRs were determined via Cox proportional hazard models, adjusting for potential confounders including including geographic region, Charlson comorbidity index, a diagnosis of coronary artery disease, weight loss or anemia, pre-exposure use of aminosalicylates, 6MP or azathioprine, rectal steroids, outpatient contacts and hospitalizations prior to exposure. The adjustment set differed slightly for each outcome. Unexposed persons served as the referent group.
HR: Hazard ratio; NE: could not estimate (e.g. due to small cell size)
Sensitivity analyses
Additional sensitivity analyses were performed to further evaluate these findings (Table 6). To reduce the possibility of contamination of the study population with misdiagnosed or miscoded IBD, we restricted the cohort to subjects meeting more stringent IBD diagnosis criteria prior to study which did not change the HR estimates substantially. To evaluate the effect of statins in younger patients given that the peak incidence of IBD is in the 2nd and 3rd decades of life, we performed an analysis restricted to patients aged 35-50. In this age-restricted subset, statins remained associated with a reduced rate of the primary outcome in all patients. To address the possibility of channeling bias (because statins are frequently prescribed in patients with metabolic syndrome), we restricted the population to subjects without any diagnosis of coronary artery disease, diabetes, or stroke. In general, these restrictions resulted in loss of precision, but the strength of the association remained unchanged. Because some statin-exposed subjects had a single prescription for statins which could have occurred in absence of actual exposure or could be associated with only trivial exposure to statins, we restricted the exposed population to those with >30 days supplied of statin medication and found the association unchanged. When patients with pre-exposure steroids were excluded the HR for the primary outcome increased but still remained <1. In order to evaluate the effect of statins in persons with more quiescent IBD, we excluded those who were hospitalized in the 180 days prior to starting statins, and those with higher Charlson comorbidity index (≥2) and found the associations unchanged.
TABLE 6.
Sensitivity analyses for primary outcome
| Restricted analysis | IBD
|
UC
|
CD
|
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| More stringent IBD definition* | 0.81 (0.69, 0.94) | 0.74 (0.60, 0.91) | 0.88 (0.70, 1.10) |
| Age 35-50 only | 0.77 (0.61, 0.99) | 0.71 (0.50, 1.00) | 0.88 (0.62, 1.24) |
| Exclude CAD | 0.82 (0.70, 0.95) | 0.77 (0.62, 0.95) | 0.91 (0.72, 1.14) |
| Exclude DM | 0.87 (0.74, 1.02) | 0.86 (0.70, 1.07) | 0.89 (0.70, 1.13) |
| Exclude Stroke | 0.82 (0.71, 0.95) | 0.74 (0.61, 0.91) | 0.93 (0.76, 1.15) |
| Exclude CAD, DM, or stroke† | 0.87 (0.73, 1.04) | 0.86 (0.68, 1.09) | 0.92 (0.71, 1.19) |
| Exclude statin ≤30 days | 0.81 (0.70, 0.93) | 0.75 (0.61, 0.91) | 0.88 (0.71, 1.09) |
| Exclude pre-exposure steroids | 0.95 (0.77, 1.18) | 0.92 (0.69, 1.24) | 1.03 (0.74, 1.43) |
| Exclude pre-exposure hospitalization | 0.80 (0.68, 0.94) | 0.76 (0.61, 0.95) | 0.87 (0.69, 1.10) |
| Charlson index <2 | 0.82 (0.70, 0.96) | 0.77 (0.63, 0.96) | 0.87 (0.69, 1.10) |
Definition included at least 4 diagnosis claims for IBD in addition to at least 1 IBD-related prescription
Any subject with a diagnosis claim for coronary artery disease, diabetes, or stroke in the 180 day pre-exposure period was excluded
HRs were determined via Cox proportional hazard models, adjusting for potential confounders including including Charlson comorbidity index, pre-exposure use of aminosalicylates, 6MP or azathioprine, rectal steroids, outpatient contacts and hospitalizations prior to exposure.
HR: Hazard ratio; IBD: Inflammatory bowel disease; CAD: coronary artery disease; DM: diabetes mellitus; UC: Ulcerative colitis; CD: Crohn’s disease
Discussion
In this retrospective cohort study using administrative claims data, we have found that statin use was associated with a small but measurably decreased rate of steroid prescriptions, a marker of IBD flares. This association appeared to be strongest in UC patients, but there was also some evidence of protection in CD patients as well. Statin use tended to decrease the rates of anti-TNF initiation, abdominal surgery, and hospitalization, but these estimates generally did not reach statistical significance. Increasing dose and duration of statin therapy was associated with the lowest rates of steroid initiation, indicating a possible dose-response phenomenon. The association between statins and reduced steroid use persisted in a number of sensitivity analyses, indicating that these findings were likely not attributable to bias.
To our knowledge, this is the first large scale epidemiologic study to address the question of whether statin use is beneficial in IBD patients. If statins are indeed associated with a steroid-sparing effect and reduced flares in IBD, this could potentially result in reduced morbidity, reduced toxicity of steroid and immunosuppressant therapy, and reduced costs of care for IBD sufferers. However, this is an observational study, and our results must be considered hypothesis-generating until prospective studies are available to confirm or refute a protective effect of adjunctive statin therapy in IBD.
We found that statin use was associated with a more pronounced effect for ulcerative colitis vs. Crohn’s disease. This could possibly be explained by several factors. Firstly, since UC and CD differ in their pathophysiology and treatment, statins may have different effects in these two diseases. For instance, if the observed effect of statins is due to their anti-inflammatory actions, statins could be more effective in reducing the mucosal inflammation seen in UC vs. more transmural inflammation (or fibrotic or fistulizing disease) seen in CD. Furthermore, steroid use may be a better marker of flares in ulcerative colitis patients than in Crohn’s patients. Lastly, since we had fewer Crohn’s disease subjects (and fewer statin-exposed CD subjects) the power for effect estimation was lower for CD than UC, which resulted in less precision of this HR estimate.
Based on the stratified analysis of statin-type, while all statin preparations generally resulted in reduced hazard of the primary outcome, due to limited precision, it’s unclear whether this represents a class effect for all statins. We found some evidence that atorvastatin may be associated with the “strongest” protective effect. These data are consistent with other studies in which atorvastatin has been shown to have anti-inflammatory effects in IBD (17, 22). It is worth noting that simvastatin was not associated with a reduced rate of steroid usage despite being the most commonly used statin type.
There are several important strengths to this study. We used a large population representative of the commercially-insured population of the US in order to maximize precision and external validity of our results. Also, we used clinically-relevant outcomes that are likely to be accurately measured with claims data. A “new user” design was used to avoid the biases associated with prevalent use of exposure medications such as the healthy user bias. Additionally, we performed an extensive evaluation for potential confounders of the relationship between statins and IBD flares including demographics, IBD medication use, comorbidities, and markers of disease severity and healthcare utilization.
There are limitations associated with using administrative claims data, which we attempted to address with our study design. As with any study using claims data, there exists the possibility of misclassification bias. In order to minimize misclassification of the study population, we used a previously reported administrative claims definition for IBD that is similar or more rigorous that others that have been reported or validated elsewhere (2, 36), and using a more stringent IBD definition did not change our results. The exposure and primary outcome were measured by prescriptions filled vs. patient report, which would be expected to minimize misclassification, but it is possible that some people who were prescribed statins actually did not take them. To address this possibility, we excluded persons with a ≤30 day supply, but the HR for the primary outcome was essentially unchanged. Also, given the limitations of administrative data and the lack of clinical detail, we were unable to examine the potentially important effects of smoking history, disease phenotype, or use of non-prescription drugs such as aspirin. Additionally, we recognize that steroid initiation may be an imperfect marker of flares of IBD, but even in absence of a 100% correlation with flare of disease, steroid use is an important clinical outcome in itself given the associated toxicities of glucocorticoid therapy.
Because this is an observational study, there are potential systematic differences between statin users and non-users apart from statin exposure. To address this, our exposed and unexposed subjects were matched on age, sex, and having a prescription in the same month, in order to ensure comparability of subjects with respect to demographic characteristics and access to healthcare. Comorbid illness was included in all models using the Charlson comorbidity index, a validated measure typically used for this purpose. The positive effect of statins persisted even after excluding specific populations in which statins are commonly prescribed (i.e. coronary artery disease, diabetes, or stroke). Nevertheless, it is possible that residual confounding of the relationship between statins and IBD activity exists that we were either unable to measure or control for. However, it is worth noting that adjustment for the confounders we could measure actually strengthened our effect estimates, so it is possible that if we were able to measure and adjust for more confounders the relationship would be even stronger.
In conclusion, in this large retrospective cohort study, statin use amongst persons with IBD was associated with a possible protective, steroid-sparing effect. Prospective controlled clinical trials are needed to confirm whether adjuvant treatment of IBD patients with statin drugs may ameliorate flares or spare immunosuppressant therapy.
Supplementary Material
Acknowledgments
The authors would like to thank Frances Olchart who also contributed to compiling the dataset for analysis, as well as the UNC GI Epidemiology Group for their thoughtful input during the planning stages of this project.
Financial support: This study was supported by a grant from the North Carolina TraCS institute (Award number UL1RR025747 from the National Center for Research Resources), as well as by funding from the National Institutes of Health: T32 DK 07634 (SDC), and KL2 RR025746 (MDK).
Footnotes
Conflicts of Interest: None to declare for all authors
References
- 1.Herrinton LJ, Liu L, Lewis JD, et al. Incidence and prevalence of inflammatory bowel disease in a Northern California managed care organization, 1996-2002. Am J Gastroenterol. 2008;103:1998–2006. doi: 10.1111/j.1572-0241.2008.01960.x. [DOI] [PubMed] [Google Scholar]
- 2.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Solberg IC, Vatn MH, Hoie O, et al. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5:1430–1438. doi: 10.1016/j.cgh.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Ritchie JK, Powell-Tuck J, Lennard-Jones JE. Clinical outcome of the first ten years of ulcerative colitis and proctitis. Lancet. 1978;1:1140–1143. doi: 10.1016/s0140-6736(78)90312-4. [DOI] [PubMed] [Google Scholar]
- 5.Reinisch W, Sandborn WJ, Bala M, et al. Response and remission are associated with improved quality of life, employment and disability status, hours worked, and productivity of patients with ulcerative colitis. Inflamm Bowel Dis. 2007;13:1135–1140. doi: 10.1002/ibd.20165. [DOI] [PubMed] [Google Scholar]
- 6.Terzic J, Grivennikov S, Karin E, et al. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114 e2105. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 7.Card T, Hubbard R, Logan RF. Mortality in inflammatory bowel disease: a population-based cohort study. Gastroenterology. 2003;125:1583–1590. doi: 10.1053/j.gastro.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 8.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 9.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 10.Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353:362–368. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- 11.Stallmach A, Hagel S, Bruns T. Adverse effects of biologics used for treating IBD. Best Pract Res Clin Gastroenterol. 24:167–182. doi: 10.1016/j.bpg.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Cohen RD. The pharmacoeconomics of biologic therapy for IBD. Nat Rev Gastroenterol Hepatol. 7:103–109. doi: 10.1038/nrgastro.2009.232. [DOI] [PubMed] [Google Scholar]
- 13.Kappelman MD, Rifas-Shiman SL, Porter CQ, et al. Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology. 2008;135:1907–1913. doi: 10.1053/j.gastro.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li M, Ong KL, Tse HF, et al. Utilization of lipid lowering medications among adults in the United States 1999-2006. Atherosclerosis. 2009 doi: 10.1016/j.atherosclerosis.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Primatesta P, Poulter NR. Lipid concentrations and the use of lipid lowering drugs: evidence from a national cross sectional survey. BMJ. 2000;321:1322–1325. doi: 10.1136/bmj.321.7272.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JY, Kim JS, Kim JM, et al. Simvastatin inhibits NF-kappaB signaling in intestinal epithelial cells and ameliorates acute murine colitis. Int Immunopharmacol. 2007;7:241–248. doi: 10.1016/j.intimp.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Grip O, Janciauskiene S. Atorvastatin reduces plasma levels of chemokine (CXCL10) in patients with Crohn’s disease. PLoS One. 2009;4:e5263. doi: 10.1371/journal.pone.0005263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grip O, Janciauskiene S, Lindgren S. Circulating monocytes and plasma inflammatory biomarkers in active Crohn’s disease: elevated oxidized low-density lipoprotein and the anti-inflammatory effect of atorvastatin. Inflamm Bowel Dis. 2004;10:193–200. doi: 10.1097/00054725-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki S, Tajima T, Sassa S, et al. Preventive effect of fluvastatin on ulcerative colitis-associated carcinogenesis in mice. Anticancer Res. 2006;26:4223–4228. [PubMed] [Google Scholar]
- 20.Sasaki M, Bharwani S, Jordan P, et al. The 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor pravastatin reduces disease activity and inflammation in dextran-sulfate induced colitis. J Pharmacol Exp Ther. 2003;305:78–85. doi: 10.1124/jpet.102.044099. [DOI] [PubMed] [Google Scholar]
- 21.Higgins PD, Khan T, Mapili J, et al. Atorvastatin decreases Seo Index in patients with short duration of disease in ulcerative colitis: A randomized placebo-controlled clinical trial. Gastroenterology. 2006;130:A120–A120. [Google Scholar]
- 22.Grip O, Janciauskiene S, Bredberg A. Use of atorvastatin as an anti-inflammatory treatment in Crohn’s disease. Br J Pharmacol. 2008;155:1085–1092. doi: 10.1038/bjp.2008.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behm B, Pizarro T, Cominelli F. Statin Therapy in Active Crohn’s Disease. American Journal of Gastroenterology. 2009;104:S482. [Google Scholar]
- 24.Ha C, Magowan S, Accortt NA, et al. Risk of arterial thrombotic events in inflammatory bowel disease. Am J Gastroenterol. 2009;104:1445–1451. doi: 10.1038/ajg.2009.81. [DOI] [PubMed] [Google Scholar]
- 25.Loftus EV, Jr, Delgado DJ, Friedman HS, et al. Colectomy and the incidence of postsurgical complications among ulcerative colitis patients with private health insurance in the United States. Am J Gastroenterol. 2008;103:1737–1745. doi: 10.1111/j.1572-0241.2008.01867.x. [DOI] [PubMed] [Google Scholar]
- 26.Motsko SP, Russmann S, Ming EE, et al. Effectiveness of rosuvastatin compared to other statins for the prevention of cardiovascular events-a cohort study in 395 039 patients from clinical practice. Pharmacoepidemiol Drug Saf. 2009;18:1214–1222. doi: 10.1002/pds.1843. [DOI] [PubMed] [Google Scholar]
- 27.Zhou F, Harpaz R, Jumaan AO, et al. Impact of varicella vaccination on health care utilization. JAMA. 2005;294:797–802. doi: 10.1001/jama.294.7.797. [DOI] [PubMed] [Google Scholar]
- 28.Cohen FJ, Neslusan CA, Conklin JE, et al. Recent antihyperglycemic prescribing trends for US privately insured patients with type 2 diabetes. Diabetes Care. 2003;26:1847–1851. doi: 10.2337/diacare.26.6.1847. [DOI] [PubMed] [Google Scholar]
- 29.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 30.Yeaw J, Benner JS, Walt JG, et al. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009;15:728–740. doi: 10.18553/jmcp.2009.15.9.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 32.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 33.Lewis JD, Aberra FN, Lichtenstein GR, et al. Seasonal variation in flares of inflammatory bowel disease. Gastroenterology. 2004;126:665–673. doi: 10.1053/j.gastro.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Robertson TA, Cooke CE, Wang J, et al. Effect of medication burden on persistent use of lipid-lowering drugs among patients with hypertension. Am J Manag Care. 2008;14:710–716. [PubMed] [Google Scholar]
- 35.LaFleur J, Thompson CJ, Joish VN, et al. Adherence and persistence with single-dosage form extended-release niacin/lovastatin compared with statins alone or in combination with extended-release niacin. Ann Pharmacother. 2006;40:1274–1279. doi: 10.1345/aph.1G646. [DOI] [PubMed] [Google Scholar]
- 36.Herrinton LJ, Liu L, Lafata JE, et al. Estimation of the period prevalence of inflammatory bowel disease among nine health plans using computerized diagnoses and outpatient pharmacy dispensings. Inflamm Bowel Dis. 2007;13:451–461. doi: 10.1002/ibd.20021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


