Abstract
Background
A behavioral phenotype that characterizes nicotine dependence, the time to first cigarette after waking, is hypothesized to increase the risk of lung cancer.
Methods
A case-control study of histologically-confirmed lung cancer was conducted. The current analysis included 4775 lung cancer cases and 2835 controls who were regular cigarette smokers.
Results
Compared to subjects who smoked more than 60 minutes after waking, the pack-years -adjusted odds ratio was 1.31 (95% confidence intervals [CI] 1.11–1.54) for subjects who smoked 31–60 minutes after waking and 1.79 (95% CI 1.56–2.07) for subjects who smoked within 30 minutes. The risk estimates were similar when smoking was modeled as total years, smoking status (current vs. former), cigarettes per day, years since quitting, and excess odds ratio. The findings were consistent for all histologic types of lung cancer.
Conclusion
These findings indicate that a specific nicotine dependence phenotype that is associated with the amount of smoke uptake per cigarette is independently associated with lung cancer risk. The findings may help identify high risk individuals who would benefit from targeted interventions.
Keywords: Nicotine, addiction, dependence, lung cancer, smoking, cotinine, case-control
INTRODUCTION
Since the first studies of cigarette smoking and lung cancer in the 1950’s, cigarette smoking has been considered a ‘lifestyle’ factor that has been proven to increase the risk of many cancers and cardiovascular disease in a dose-dependent relationship (1–6). The conceptual approach of quantifying smoking in terms of age of initiation, cigarette frequency and duration over the past several decades served well to underscore both the magnitude of the risks and the benefits of smoking cessation.
The recognition of nicotine dependence as a psychological or physiological problem by the American Psychiatric Institute did not occur until 1980. Although this occurred after the health risks of smoking were well-established, DSM-IV nicotine dependence criteria, which are based primarily on symptomology and used as the basis for pharmacologic treatment decisions, have not been examined in relation to cancer risk. The diagnostic criteria for nicotine dependence may have limited utility in risk studies since many young and adult daily moderate and heavy smokers do not meet the clinical criteria for nicotine dependence (7–10). There is only a modest concordance between the criteria and the daily frequency of smoking. Behavioral scales of nicotine dependence, such as the Fagerstrom Test for Nicotine Dependence (FTND), have also not been examined in relation to lung cancer risk although cigarette frequency is the major contributor to the FTND index. Most other FTND items are subjective feelings regarding smoking or refraining from smoke.
Nicotine uptake can be measured biochemically by cotinine in saliva, blood and urine. There is wide variation in cotinine levels among smokers which is only partially explained by smoking frequency. This indicates that other manifestations of nicotine dependence affect nicotine and smoke uptake.
One specific behavior that explains a substantial amount of the variation in cotinine levels in active smokers is the time to first cigarette after waking (TTFC). Like cigarette frequency, TTFC is the other item of the FTND that is quantifiable (11). An increasing TTFC interval is significantly associated with decreased cotinine levels (12). Two nicotine dependence phenotypes are characterized by the TTFC time interval. The "low" dependent phenotype are smokers who smoke >30 minutes after waking and smoke ≤20 cigarettes per day. The "high" dependent phenotype are smokers who smoke ≤30 minutes after waking, but in contrast to the low dependent phenotype, have a wide range of daily cigarette consumption (e.g. 6–70).
The time to first cigarette is also associated with behavioral traits of nicotine addiction including smoking amount (13), inability to quit (14–16), smoking relapse (17), and tolerance (18). The current study therefore examined whether the time to first cigarette is a nicotine dependent phenotype that predicts lung cancer risk independent of cigarette smoking frequency and duration.
MATERIALS AND METHODS
The methods for the study were previously described in detail (1). The study was conducted primarily in large academic medical centers in the New York Metropolitan area to study the effects of tobacco exposure and cancer risk. In brief, all newly diagnosed patients with histologically-confirmed lung cancer were requested to participate in the study. Subjects were asked to provide informed consent and were interviewed by a trained interviewer using a structured questionnaire. Controls were consented patients admitted to the same hospital for conditions unrelated to tobacco smoke exposure. Controls were frequency matched to cases by sex, age (within five years), race and month of diagnosis. Controls included subjects with a wide range of conditions such as acute conditions, fractures and injuries, nonmalignant diagnosis such as benign prostatic hypertrophy and cancers not caused by tobacco including breast and prostate. The study dates were 1977–1999 and the response rates were over 90% for both cases and controls. Reasons for nonresponse included not feeling well or lack of interest. All subjects were required to speak English and be free of any mental impairment. In the current analysis, we restricted the database to subjects who had a smoking history of at least one cigarette per day for one or more years. The current analysis included 7,610 subjects, including 4775 cases and 2835 controls. There were 6812 whites, 759 blacks and 39 subjects belonging to other racial groups.
The data were analyzed using R (R Foundation for Statistical Computing, Austria) and SAS (Cary, NC) statistical software packages. All tests were two-sided. Unconditional logistic regression procedures were used to derive odds ratios (OR) and 95% confidence intervals (CI).
The question “Approximately how many minutes after you wake (woke) up do(did) you have your first cigarette?” was asked of all subjects. Subjects were given the following categories of responses: 1–30 minutes; 31–60 minutes; > 1 hour (reference category) and do not know. None of the subjects responded as not knowing. In the initial models using pack-years as a measure of tobacco smoke exposure, a significant lack-of-fit was found. Subsequent models using a squared term for pack-years and an interaction term between pack-years and a categorical term for cigarettes per day (≤20, >20) showed no evidence for lack of fit. We also fitted models that used other measures of cigarette smoking history besides pack-years. These included models with terms for intensity (e.g. cigarettes per day), smoking status (current vs. former), years since quitting (0 years [current smoker], 1–5 years, 6–10 years and >10 years) and the excess OR (EOR) where pack-years is linear and the logarithm of cigarettes per day and its square is exponential (6). As the risk for lung cancer varies by smoking intensity, the risk associated with time to first cigarette adjusted for EOR was further stratified by smoking intensity. A few subjects reported quitting less than one year prior to the interview, and were classified as current smokers. The following covariates were included in the final models: age (≤50, 51–60, 61–70 and >70), sex (male vs. female), race (blacks vs. whites), education (≤12 years, 12 years, 13–15 years and ≥16 years), and body mass index (Weight[lbs]*703/(height[in.])2). Histologic-specific models were developed by comparing cases with lung adenocarcinoma or squamous cell cancer to the entire control series. For all the analyses, statistical significance was set at P < 0.05, and all tests were 2-sided. There were no missing data for the variables analyzed in this report. A goodness of fit test for every model was performed using the Hosmer and Lemeshow chi-square statistic (19).
RESULTS
The basic characteristics of the study subjects are shown in Table 1. Since the current analysis excluded never smokers, there were a larger number of cases than controls (4775 vs. 2835). Lung cancer was more common among men, and about 90% of all subjects were white. Sixty percent of cases and 36% of controls were current smokers. In logistic regression models, the association between the dose of smoking and lung cancer risk showed the expected dose-response pattern. Since the risk is calculated among ever smokers, the ORs are substantially lower than what is typically observed in studies that include never smokers. The OR for current vs. former smoking was 2.32 (95% CI 2.8–2.59). Relative to smoking 1–9 cigarettes per day, the OR was 1.4 (95% CI 1.09–1.8) for 10–19 cigarettes per day, 2.20 (95% CI 1.86–2.59) for 20–29 cigarettes per day, 2.6 (95% CI 2.1–3.2) for 30–39 cigarettes per day, 3.5 (95% CI 2.9–4.3) for 40–49 cigarettes per day and 4.0 (95% CI 1.1–1.5) for 50+ cigarettes per day.
Table 1.
Characteristics of lung cancer cases and controls
| Characteristic | Cases N=4775 (%) |
Controls N=2835 (%) |
|---|---|---|
| Mean Age | 60 | 60 |
| Sex | ||
| Men | 2973 (62.3) | 1896 (66.9) |
| Women | 1802 (37.7) | 939 (33.1) |
| Race | ||
| White | 4251 (89.0) | 2561 (90.0) |
| Black | 493 (10.3) | 266 (9.4) |
| Other | 31 (0.7) | 8 (0.3) |
| Smoking Status | ||
| Current | 2843 (59.5) | 1024 (36.1) |
| Former | 1932 (40.5) | 1811 (63.9) |
| Time to First Cigarette | ||
| >60 minutes | 575 (12.0) | 833 (29.4) |
| 31–60 minutes | 722 (15.1) | 588 (20.7) |
| 1–30 minutes | 3478 (72.8) | 1414 (49.9) |
Relative to smoking the fist cigarette more than one hour after waking up, the unadjusted odds ratio for lung cancer was 1.78 (95% CI 1.53–2.07 ) for 31–60 minutes and 3.56 (95% CI 3.15–4.03) for 1–30 minutes. There was a significant association between TTFC and sex, age, years of education, smoking status, and pack-years (P<0.01). No significant differences for TTFC by race were found. In multivariate models controlling for sociodemographic characteristics, the pack-year adjusted odds ratios were 1.31 (95% CI: 1.11–1.54) and 1.79 (95% CI: 1.56–2.07) (Table 2).
Table 2.
Odds ratios and 95% confidence intervals for lung cancer and time to first cigarette in ever smokers, adjusting for different measures of smoking history.
| Time to first cigarette* | OR adjusted for pack- years of smoking | 95% CI | OR adjusted for total years of smoking | 95% CI | OR adjusted for cigarettes per day | 95% CI | OR adjusted for smoking status | 95% CI | OR adjusted for years since quitting | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|
| >60 min. | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| 31–60 min. | 1.31 | 1.11–1.54 | 1.47 | 1.25–1.72 | 1.58 | 1.35–1.84 | 1.68 | 1.44–1.97 | 1.66 | 1.41–1.94 |
| 1–30 min. | 1.79 | 1.56–2.07 | 2.34 | 2.10–2.68 | 2.64 | 2.31–3.02 | 3.0 | 2.64–3.42 | 2.82 | 2.49–3.22 |
| Trend X2 | P<0.01 | P<0.01 | P<0.01 | P<0.01 | P<0.01 | |||||
| Whites | ||||||||||
| >60 min. | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| 31–60 min. | 1.39 | 1.17–1.65 | 1.53 | 1.29–1.81 | 1.65 | 1.40–1.95 | 1.75 | 1.48–2.06 | 1.65 | 1.4–1.95 |
| 1–30 min. | 1.93 | 1.66–2.24 | 2.44 | 2.12–2.82 | 2.82 | 2.45–3.25 | 3.15 | 2.75–3.61 | 2.82 | 2.45–3.25 |
| Blacks | ||||||||||
| >60 min. | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| 31–60 min. | 0.89 | 0.51–1.54 | 1.06 | 0.62–1.83 | 1.04 | 0.68–1.79 | 1.21 | 0.71–2.05 | 1.04 | 0.61–1.79 |
| 1–30 min. | 1.12 | 0.71–1.77 | 1.63 | 1.28–2.06 | 1.66 | 1.07–2.56 | 1.79 | 1.17–2.75 | 1.66 | 1.07–2.56 |
Odds ratios adjusted for age, sex, race, education, and body mass index. The odds ratios adjusted for years since quitting included current smokers.
The results for models using alternative measures of smoking history including total years of smoking, cigarettes per day, smoking status (current vs. former) and years since quitting showed similar results (Table 2). The total-smoking years adjusted odds ratio was 1.47 (95% CI: 1.25–1.72) for 31-60 minutes after waking, and 2.34 (95% CI: 2.10–2.68) for within 30 minutes after waking.
The cigarettes per day adjusted odds ratio was 1.58 (95% CI: 1.35–1.84) for 31–60 minutes after waking, and 2.64 (95% CI: 2.31–3.02) for within 30 minutes after waking. The smoking status adjusted odds ratio was 1.68 (95% CI 1.44–1.97) for 31–60 minutes after waking, and 3.0 (95% CI: 2.64–3.42) for within 30 minutes after waking. When smoking was modeled by years since quitting, the risk was 1.66 (95% CI: 1.41–1.94) for 31–60 minutes after waking, and 2.82 (95% CI 2.49–3.42) for within 30 minutes after waking (Table 1). In another model that adjusted for total years of smoking and cigarettes per day, the respective odds ratios were 1.40 (95% CI: 1.20–1.66) and 2.12 (95% CI 1.85–2.43).
In whites only, TTFC was statistically significant regardless of the method for smoking adjustment. In blacks, smoking within 30 minutes after waking but not 31–60 minutes after waking was consistently associated with a significant increased risk of lung cancer.
The adjusted ORs associated with each additional year of smoking stratified by the number of cigarettes per day were all statistically significant (P<0.01). The OR was 1.04 for 1–10 cigarettes per day, 1.04 for 11–20 cigarettes per day, 1.06 for 21–30 cigarettes per day, 1.06 for 31–40 cigarettes per day and 1.07 for more than 40 cigarettes per day. The risk of lung cancer associated with TTFC adjusted for the EORs was 1.47 (95% CI: 1.26–1.72) for 31–60 minutes and 2.23 (95% CI: 1.95–2.56). Figure 1 shows the association between the TTFC and lung cancer risk adjusted for the EOR, stratified by seven categories of smoking intensity. The results were consistent with the other models. Compared to a TTFC of 60 or more minutes, the odds ratios were increased for a TTFC of 31–60 minutes and even higher OR were found for a TTFC of 1–30 minutes. The only intensity subcategory where the risk for TTFC was not elevated was for 10–15 cigarettes per day, and 30+ cigarettes per day for just those subjects with a TTFC of 31–60 minutes.
Figure 1.
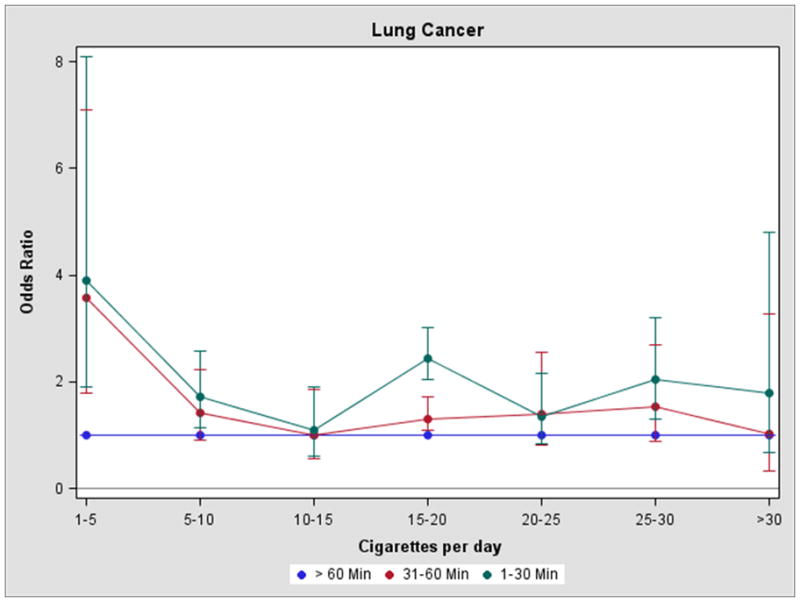
Legend: Time to first cigarette and lung cancer risk in ever smokers adjusting for the excess odds ratio per pack-year, by cigarette intensity (cigarettes per day)
The association between TTFC and lung cancer risk was observed for all major histologic types of cancer (Table 3). The odds ratios were slightly higher for the lung histologies that are most strongly related to cigarette smoking including small cell carcinoma and squamous cell carcinoma. Findings for other or mixed histologies are not shown.
Table 3.
Odds ratios and 95% confidence intervals for lung cancer and time to first cigarette in ever smokers by histologic type, adjusting for pack-years of smoking
| Time to first cigarette* | OR for lung adeno- carcinoma | 95% CI | OR for squamous cell carcinoma | 95% CI | OR for small cell carcinoma | 95% CI | OR for large cell carcinoma | 95% CI |
|---|---|---|---|---|---|---|---|---|
| >60 min. | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 31–60 min. | 1.39 | 1.12– 1.55 | 1.48 | 1.08– 2.03 | 1.24 | 0.76– 2.02 | 1.15 | 0.68– 1.93 |
| 1–30 min. | 1.47 | 1.13– 1.71 | 2.13 | 1.62– 2.81 | 2.00 | 1.33– 3.01 | 1.62 | 1.05– 2.51 |
| Trend X2 | P<0.01 | P<0.01 | P<0.01 | P<0.05 |
Odds ratios adjusted for age, sex, race, education, and body mass index.
DISCUSSION
These results show that a specific nicotine dependence phenotype, TTFC, is an independent predictor of lung cancer after adjustment for smoking history. The risk of lung cancer has been historically modeled based on measures of cigarette frequency, which is linearly related to cotinine concentrations up to about 20 cigarettes per day. The correlation between cigarette frequency and cotinine is moderate in light smokers and low in heavy smokers (12). There is clearly inter-individual variability in the way smokers regulate their nicotine intake per cigarette with increasing frequency in a natural setting and in attempting to quit (20). It is not feasible to measure how smokers regulate their nicotine uptake in studies of disease risk, but the time to first cigarette is a behavior that is strongly associated with cotinine per cigarette smoked. Nicotine and cotinine are not carcinogenic, but the correlation between the urinary level of cotinine and the tobacco-carcinogen 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) from mainstream cigarette smoke is fairly high (r=0.69) (21). The TTFC is a distinct nicotine dependence phenotype, that is also shown to be an independent risk factor for lung cancer in this study.
There have been few studies of nicotine dependence and cancer risk. One report of 55 lung cancer cases and 49 controls found a significant trend in risk associated with the FTND score, although cigarette frequency is the biggest contributor to the FTND index and TTFC was not examined separately (22). The current study found that the risk of lung cancer is almost doubled in smokers who took their first cigarette within 30 minutes after waking compared to smokers with an interval of an hour or more. It is possible that had this response category been defined by 15 minute intervals, an even higher risk might have been observed for smoking within the first 15 minutes after waking.
To account for potential confounding by smoking duration or intensity or both, the lung cancer risk was modeled using a number of traditional methods to characterize smoking including total years of smoking, smoking status, cigarettes per day and years since quitting. The odds ratios for TTFC were all similar after adjustment for these measures. Similarly, since the effects of total smoke exposure (pack-years) may vary for a fixed intensity, the risk associated with TTFC was adjusted for the excess odds ratio at different smoking intensities. The association between decreasing TTFC and the EOR-adjusted lung cancer risk was consistent with the other methods of smoking adjustment. Compared to a TTFC of 60 or more minutes, the odds ratio was increased for TTFC across almost all intensity levels, with higher risks observed for a TTFC of 1–30 minutes compared to a TTFC of 30–60 minutes. The only intensity subcategory where the risk for TTFC was not elevated was for 10–15 cigarettes per day,and 30+ cigarettes per day for just those subjects with a TTFC of 31–60 minutes. The reasons for this are unclear, however the differences in odds ratios between strata were within the margin of error.
Recall bias is always a concern in case-control studies. Smoking habits are usually reported with a high degree of reliability. This was tested previously in a subset of subjects from this study. In repeat interviews scheduled 6-weeks apart, the OR for lung cancer associated with smoking intensity changed very little (e.g. 1–19 cigarettes per day: OR = 2.4 vs. 2.4; 20–29 cigarettes per day (OR = 2.8 vs. 3.0) and 30+ cigarettes per day (OR = 3.8 vs. 3.6). Any misclassification regarding TTFC due to poor recall would be expected to be more common at a younger age when distant habits are less easily recalled. The similar findings in the age-stratified analysis (≤50, >50) would seem to suggest that recall bias concerning TTFC isn’t large.
The study included a large number of individuals and had a high response rate. We believe the findings are generalizeable to whites. It is less certain if the findings can be generalized to African Americans. Blacks, like whites had increased risks for a TTFC of 1–30 minutes, but unlike whites there was no increased risk for a TTFC of 31–60 minutes. This may reflect the relatively small number of blacks in this category. In addition, the participating institutions were located primarily in New York County, and not Brooklyn County or other boroughs that have a large African American population. No inferences can be made regarding Asian smokers.
It is uncertain what explains the relationship between shorter interval between waking and the first cigarette and increased cotinine levels. The reasons might reflect genetic variation in nicotine dependence, non-genetic behavioral and social factors, and cigarette brand characteristics such as taste or combinations of genetic and social factors. Early morning smokers might have a greater craving for nicotine after overnight abstinence. In a British study, the time to first cigarette was not associated with more intense puffing although cotinine was not measured (23). Still, the findings emphasize the importance of nicotine dependence in cancer risk. While the smoking prevalence has declined in the United States, remaining younger smokers are more highly addicted to nicotine than older smokers (24).
Recent findings from genome-wide scans have identified variants in the 15q25 cholinergic nicotine receptor genes that are associated with both a phenotype of nicotine dependence (e.g. cigarette frequency) and as a consequence increased lung cancer risk (25). Similarly, identifying specific smoking behavior phenotypes and the role of genetic variability is emerging as a new field in the pharmacogenetics of smoking cessation (26). These studies collectively indicate that variation in nicotine dependence caused either by genetic susceptibility or behavioral factors or both affect lung cancer risk. They also indicate that quantifying the health risks from tobacco smoke should conceptualize cigarette smoking as a physiological dependence and not a lifestyle factor. This will help identify those smokers who are at especially high risk of cancer and help develop targeted smoking interventions to reduce their risk.
Acknowledgments
Support: This study was supported by research grants (PO1 CA68384 and K07 CA104231) from the National Cancer Institute and (PA-DOH 4100038714) from the Pennsylvania Department of Health.
References
- 1.Stellman SD, Muscat JE, Thompson S, Hoffmann D, Wynder EL. Risk of squamous cell carcinoma and adenocarcinoma of the lung in relation to lifetime filter cigarette smoking. Cancer. 1997;80:382–388. doi: 10.1002/(sici)1097-0142(19970801)80:3<382::aid-cncr5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Khuder SA, Mutgi AB. Effect of smoking cessation on major histologic types of lung cancer. Chest. 2001;120:1577–1583. doi: 10.1378/chest.120.5.1577. [DOI] [PubMed] [Google Scholar]
- 3.Bach PB, Kattan MW, Thornquist MD, Kris MG, Tate RC, Barnett MJ, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst. 2003;95:470–478. doi: 10.1093/jnci/95.6.470. [DOI] [PubMed] [Google Scholar]
- 4.Leffondre K, Abrahamowicz M, Siemiatycki J, Rachet B. Modeling smoking history: a comparison of different approaches. Am J Epidemiol. 2002;156:813–23. doi: 10.1093/aje/kwf122. [DOI] [PubMed] [Google Scholar]
- 5.Flanders WD, Lally CA, Zhu BP, Henley SJ, Thun MJ. Lung cancer mortality in relation to age, duration of smoking, and daily cigarette consumption: results from Cancer Prevention Study II. Cancer Res. 2003;63:6556–6562. [PubMed] [Google Scholar]
- 6.Lubin JH, Caporaso NE. Cigarette smoking and lung cancer: modeling total exposure and intensity. Cancer Epidemiol Biomarkers Prev. 2006;15:517–523. doi: 10.1158/1055-9965.EPI-05-0863. [DOI] [PubMed] [Google Scholar]
- 7.Breslau N, Johnson EO, Hiripi E, Kessler R. Nicotine dependence in the United States: prevalence, trends, and smoking persistence. Arch Gen Psychiatry. 2001;58:810–816. doi: 10.1001/archpsyc.58.9.810. [DOI] [PubMed] [Google Scholar]
- 8.Kandel DB, Chen K. Extent of smoking and nicotine dependence in the United States: 1991–1993. Nicotine Tob Res. 2000;2:263–274. doi: 10.1080/14622200050147538. [DOI] [PubMed] [Google Scholar]
- 9.Donny EC, Dierker LC. The absence of DSM-IV nicotine dependence in moderate-to-heavy daily smokers. Drug Alcohol Depend. 2007;89:93–96. doi: 10.1016/j.drugalcdep.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dierker LC, Donny E, Tiffany S, Colby SM, Perrine N, Clayton RR. The association between cigarette smoking and DSM-IV nicotine dependence among first year college students. Drug Alcohol Depend. 2007;86:106–114. doi: 10.1016/j.drugalcdep.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addict. 86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 12.Muscat JE, Stellman SD, Caraballo RS, Richie JP., Jr Time to first cigarette after waking predicts cotinine levels. Cancer Epidemiol Biomarkers Prev. 2009;18:3415–3420. doi: 10.1158/1055-9965.EPI-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heatherton TF, Kozlowski LT, Frecker RC, Rickert W., JR Measuring the Heaviness of Smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addictc. 1989;84:791–800. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- 14.Kozlowski LT, Porter CQ, Orleans CT, Pope MA, Heatherton T. Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug Alcohol Depend. 1994;34:211–216. doi: 10.1016/0376-8716(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 15.Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim S-Y, et al. Time to first cigarette in the morning as an Index of ability to quit smoking: Implications for nicotine dependence. Nicotine Tob Res. 2007;9:S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabat GC, Wynder EL. Determinants of quitting smoking. Am J Public Health. 1987;77:1301–1305. doi: 10.2105/ajph.77.10.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toll BA, Schepis TS, O'Malley SS, McKee SA, Krishnan-Sarin S. Subjective reactivity to the first cigarette of the day as a predictor of smoking relapse: A preliminary study. Drug Alcohol Depend. 2007;89:302–305. doi: 10.1016/j.drugalcdep.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pillitteri JL, Kozlowski LT, Sweeney CT, Heatherton TF. Individual Differences in the Subjective Effects of the First Cigarette of the Day: A Self-Report Method for Studying Tolerance. Exp Clin Psychopharmacol. 1997;5:83–90. doi: 10.1037//1064-1297.5.1.83. [DOI] [PubMed] [Google Scholar]
- 19.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: Wiley; 1989. [Google Scholar]
- 20.Benowitz NL, Jacob P, 3rd, Kozlowski LT, Yu L. Influence of smoking fewer cigarettes on exposure to tar, nicotine, and carbon monoxide. N Engl J Med. 1986;315:1310–1313. doi: 10.1056/NEJM198611203152102. [DOI] [PubMed] [Google Scholar]
- 21.Joseph AM, Hecht SS, Murphy SE, Carmella SG, Le CT, Zhang Y, et al. Relationships between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiol Biomarkers Prev. 2005;14:2963–2968. doi: 10.1158/1055-9965.EPI-04-0768. [DOI] [PubMed] [Google Scholar]
- 22.Kubíka An, Petr Z, Peter B, Chris R, Sara G, Tomášekc L, et al. A case-control study of lung cancer among Czech women. Lung cancer. 2001;31:111–112. doi: 10.1016/s0169-5002(00)00178-1. [DOI] [PubMed] [Google Scholar]
- 23.Grainge MJ, Shahab L, Hammond D, O'Connor RJ, McNeill A. First cigarette on waking and time of day as predictors of puffing behaviour in UK adult smokers. Drug Alcohol Depend. 2009;101:191–195. doi: 10.1016/j.drugalcdep.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Goodwin RD, Keyes KM, Hasin DS. Changes in cigarette use and nicotine dependence in the United States: Evidence from the 2001-2002 wave of the national epidemiologic survey of alcoholism and related conditions. Am J Public Health. 2009;99:1471–1417. doi: 10.2105/AJPH.2007.127886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lips EH, Gaborieau V, McKay JD, Chabrier A, Hung RJ, Boffetta P, et al. Association between a 15q25 gene variant, smoking quantity and tobacco-related cancers among 17 000 individuals. Int J Epidemiol. 2010;39:563–577. doi: 10.1093/ije/dyp288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray R, Tyndale RF, Lerman C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J Neurogenet. 2009;23:252–261. doi: 10.1080/01677060802572887. [DOI] [PMC free article] [PubMed] [Google Scholar]


