Abstract
Ghrelin is a pleiotropic hormone that was originally described as promoting feeding and stimulating growth hormone release in adults. A growing body of evidence suggests that ghrelin may also exert developmental and organizational effects during perinatal life. The perinatal actions of ghrelin include the regulation of early developmental events such as blastocyst development and perinatal growth. Moreover, alterations in perinatal ghrelin levels result in structural differences in various peripheral organs, such as the pancreas and gastrointestinal tract. Recent data have also suggested that ghrelin acts on appetite-related brain centers in early life. Together, these observations indicate that exposure to factors that alter how ghrelin impacts development may induce lasting effects on physiological regulation.
Keywords: hormone, GHSR, brain, development, hypothalamus, proliferation, pancreas
Introduction
Ghrelin is a 28-amino-acid peptide that is primarily secreted by gastric mucosal cells and plays a key role in appetite regulation (see [1] for review). It is now widely recognized that ghrelin acts primarily on the brain to mediate its effects on feeding and energy balance. Consistent with this idea, direct intracerebroventricular administration of ghrelin produces a marked increase in food intake and body weight [31]. Among brain regions involved in ghrelin’s orexigenic effects, a primary importance has been given to the hypothalamus. The hypothalamus contains the highest density of ghrelin receptors (GHSRs) of any brain region [16, 38], and administration of ghrelin induces cFos immunoreactivity (a marker of neuronal activation) in various hypothalamic nuclei involved in feeding regulation, including the arcuate (ARH), ventromedial (VMH), and paraventricular (PVH) nuclei of the hypothalamus [11, 19]. More recent work has also suggested that extra-hypothalamic sites, such as the ventral tegmental area (in the midbrain), may also be crucial for relaying ghrelin’s orexigenic actions [27]. In addition to regulating feeding, ghrelin is involved in other physiological regulation, such as cardiovascular function [17], gastrointestinal motility [15], and growth hormone release [35]. Ghrelin has also been shown to exert more complex behavioral effects and is involved in learning and memory [7], reward/addiction [13, 20], and depression/anxiety [9]. Recent studies have demonstrated the pleiotropic nature of ghrelin by showing that it can also influence a variety of developmental processes during perinatal development.
This review summarizes the developmental and structural changes that have been observed in various organs in response to alterations in ghrelin levels during critical periods of development. In particular, this review will describe the developmental effects of ghrelin on the neural and endocrine systems involved in metabolic regulation.
Very Early Developmental Effects of Ghrelin
Following its discovery, it soon became evident that ghrelin may play a role during development. The earliest developmental event that appears to be influenced by ghrelin is blastocyst development. Ghrelin and Ghsr mRNAs are detected in embryos as early as the morula stage [14]. In addition to being expressed by the embryo, ghrelin is expressed in the uterus and secreted in uterine fluid [14]. In vitro experiments have indicated that ghrelin acts on GHSRs to inhibit the development of embryos from the morula to the blastocyst stage and from the blastocyst to the expanded blastocyst stage [14]. Consistent with these observations, ghrelin inhibits the proliferation of both the inner cell mass and the trophectoderm, which are the two cell lineages found in the blastocyst [14]. Notably, uterine ghrelin levels are influenced by the maternal diet (e.g., maternal fasting increases both the plasma and uterine ghrelin levels [14]), raising the possibility that nutritional manipulation of ghrelin secretion may affect the development of pre-implantation embryos.
Developmental Regulation of Ghrelin and GHSR Expression
As mentioned above, ghrelin is expressed in the embryo as early as the morula stage and continues to be expressed in the developing embryo and fetus. In rodents, high levels of ghrelin mRNA are detected in fetuses at embryonic day (E) 12, and E17 fetuses contain significant levels of acylated and deacylated ghrelin in their blood [18, 30]. A single ghrelin injection into the mother is capable of increasing the circulating ghrelin levels in the fetus within 5 minutes, indicating that maternal ghrelin can easily transfer to the fetal circulatory system and increase fetal ghrelin levels [18]. It is also worth noting that the total ghrelin levels found in fetuses are three times higher than those found in their dams [4], suggesting that the ghrelin found in a fetus may be produced by the fetus itself.
In the fetus, the pancreas appears to be a major source of ghrelin expression during perinatal life. Ghrelin mRNA and protein are found at high levels in the fetal pancreas, whereas low levels of ghrelin are detected in the fetal stomach [4, 33] (Fig. 1 and Table 1). These observations suggest that, in contrast to the adult, the source of circulating fetal ghrelin may be the pancreas, not the stomach. Stomach ghrelin expression increases gradually after birth to reach adult-like levels by 3–5 weeks of life [10, 30]. Meanwhile, pancreatic ghrelin expression declines progressively from birth to weaning and becomes barely detectable in the adult pancreas [33] (Fig. 1). In addition to being transiently expressed in the developing pancreas, ghrelin mRNA is also expressed in the pituitary gland, lung, gonads, and gut during perinatal development [4, 24, 29, 30] (Table 1).
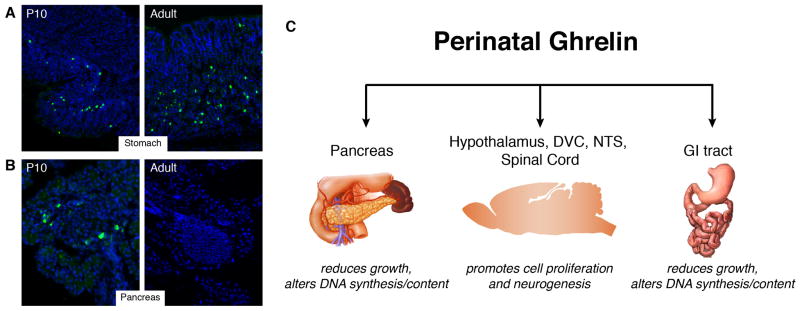
Figure 1. Primary sites of expression and developmental effects of perinatal ghrelin.
Ghrelin immunoreactivity (green fluorescence) is detected in both the pancreas and stomach of P10 mouse pups (A, B). Postnatal stomach ghrelin expression increases gradually to reach adult-like levels by 3–5 weeks of life (A). Meanwhile, pancreatic ghrelin expression declines progressively and is barely detectable in the adult pancreas (B). See Supplemental Online Material for detailed methods. Instead of regulating food intake, perinatal ghrelin appears to be an important signal for the growth and development of various tissues, including the pancreas, gastrointestinal tract, and central nervous system (C).
Table 1.
Expression of ghrelin and Ghsr mRNA in peripheral and central tissues during perinatal development.
| Organ | Ages | References |
|---|---|---|
| Ghrelin mRNA | ||
| pancreas | E20 | [4] |
| stomach | E20 | [4] |
| gut | P2, P5, P10, P12 | [30] |
| pituitary | P2, P5, P10, P12 | [30] |
| lung | E12, E15, E17, E19, E21 | [24] |
| gonads | P15 | [29] |
| Ghsr mRNA | ||
| skin | E14, E15, E17, E19 | [12, 18] |
| bone | E14, E15, E17, E19 | [12, 18] |
| eye | E14, E15, E19 | [18] |
| heart | E14, E15, E17, E19 | [12, 18] |
| lung | E14, E15, E19 | [18] |
| liver | E14, E15, E17, E19 | [12, 18] |
| stomach | E14, E15, E17, E19, P2 | [12, 18] |
| intestine | E14, E15, E17, E19, P2 | [12, 18] |
| kidney | E19 | [18] |
| spleen | E19 | [18] |
| testis | E19, P15 | [18, 29] |
| ovary | E19 | [18] |
| pituitary | E19 | [12, 18] |
| spinal cord | E14, E15, E17, E19, P2 | [12, 18] |
| brain | E14, E15, E17, E19, P2 | [12, 18] |
Ghrelin acts on growth hormone secretagogue receptors (GHSRs) to mediate its effects [28]. In adult animals and neonates, GHSRs are expressed in both the central nervous system and the periphery (Table 1). In peripheral fetal organs, Ghsr mRNA is detected in the skin, bone, eye, heart, lung, liver, stomach, intestine, kidney, spleen, testis, ovary, and pituitary gland [18]. Although these qualitative studies provide valuable information regarding expression sites of ghrelin and GHSR during development, further studies will be needed to quantitatively compare the expression of ghrelin and its receptor in various tissues during development. RT-PCR analysis has also indicated that Ghsr mRNA is highly expressed in the central nervous system during both pre- and post-natal life. Ghsr mRNA is found in the brain and spinal cord as early as E12 and continues to be expressed in these tissues during postnatal life [12, 18]. Unfortunately, a detailed distribution of GHSR expression in the neonatal brain has not yet been described. Similarly, whether central (and peripheral) GHSR expression is subjected to developmental regulations remains unknown. Nevertheless, we know that Ghsr mRNA is primarily expressed in the adult hypothalamus, specifically in the nuclei area involved in food intake and body weight regulation, such as the arcuate, ventromedial, dorsomedial, and paraventricular nuclei of the hypothalamus [16, 38]. Additionally, GHSRs are expressed in other appetite-related brain structures, such as the ventral tegmental area and the nucleus of the tractus solitarius [16, 38].
Ghrelin and Perinatal Growth
The widespread expression of ghrelin and its receptor during development suggests that ghrelin may be involved in perinatal growth. The capacity of ghrelin to influence perinatal growth has been summarized in reviews by Chanoine et al. [2, 3]. One key observation is that chronic administration of ghrelin into pregnant dams from day 14 of gestation until delivery results in higher birth weights [18]. In contrast, passive immunization against ghrelin during pregnancy results in low birth weights in the offspring [18]. Ghrelin injection into either postnatal day (P) 7 or P10 rat pups stimulates growth hormone secretion, supporting a role for ghrelin in somatic growth [10, 22]. However, a lifelong ghrelin deficiency, such as that observed in either ghrelin-knockout mice or GHSR null mice, does not impair early growth and development, suggesting that there may be compensatory pathways that can adjust for the loss of ghrelin and promote growth [26, 28, 34]. Remarkably, although adult GHSR null mice develop obesity when fed a high-fat diet, the same mutant mice appear resistant to diet-induced obesity when exposure to the high-fat diet occurs shortly after weaning [26, 28, 34]. These observations indicate that ghrelin and GHSR affect the maturation of metabolic systems involved in energy balance.
Effects of Ghrelin on Pancreatic and Gastrointestinal Development
During perinatal development, ghrelin is transiently expressed in the α islet cells in the pancreas, where it colocalizes with glucagon [5]. Recently, it has been shown that ghrelin is also produced by a distinct population of pancreatic islet cells, the ε cells [23]. Interestingly, insulin- and ghrelin-secreting cells appear to be derived from a common progenitor cell. In this common progenitor, both the Nkxx2.2 and Pax4 transcription factors are involved in regulating the cell fate decision in which the progenitors differentiate into either β (i.e., insulin-producing) or ε (i.e., ghrelin-producing) cells [23]. In fact, in mice that lack either Nkxx2.2 or Pax4, all pancreatic endocrine cells are replaced by ε cells that produce ghrelin. Following the discovery that ghrelin is expressed in the endocrine pancreas during perinatal life, Dembinski et al. reported a role for ghrelin in pancreatic development. They found that newborn rats exposed to ghrelin for 7 or 14 days had reduced pancreatic weights, attenuated pancreatic DNA synthesis and reduced DNA content [6]. Remarkably, similar ghrelin injections in older rats lead to the opposite effects (i.e., increased pancreatic weights, DNA synthesis and DNA content) [6]. These data highlight the ability of ghrelin to induce biphasic effects on gastric growth depending on the age of exposure. Similar effects have been observed in the stomach, in which chronic neonatal ghrelin injections reduce gastric growth, as evidenced by a decrease in gastric weight, DNA synthesis and DNA content. Conversely, ghrelin injections in adults increase gastric weight, DNA synthesis and DNA content [32].
Ghrelin’s Effects on the Developing Hypothalamus
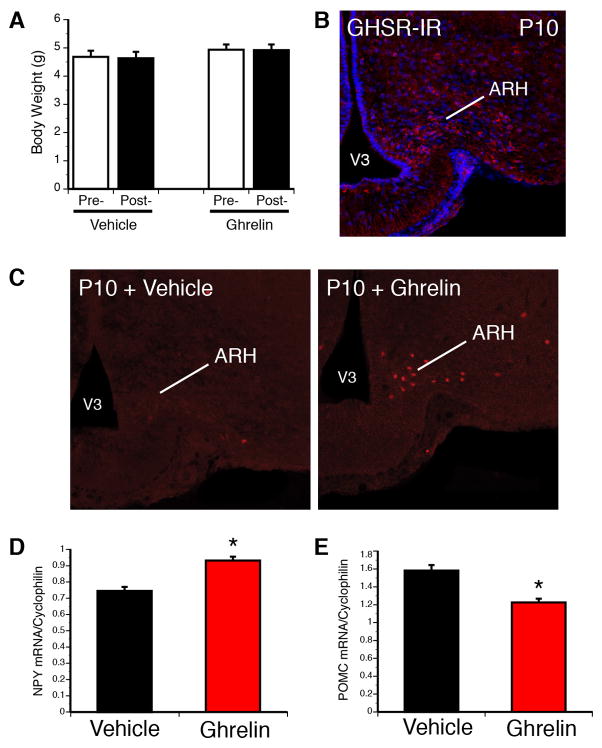
The central function of ghrelin in adults is to promote feeding. However, accumulating evidence suggests that there are physiological differences in the regulation of energy balance between adults and neonates. In sharp contrast to the effects of ghrelin on adults, exogenous ghrelin does not significantly promote food intake in the first 2–3 postnatal weeks. More specifically, Piao and colleagues have shown that acute ghrelin injections do not induce weight gain in rats until P20 [21]. We also found that a systemic ghrelin injection does not promote weight gain in a P10 mouse neonate (Fig. 2). One possible explanation for the inability of ghrelin to promote feeding in early life is that the brain, which normally conveys the feeding effects of ghrelin, may be relatively insensitive to ghrelin during neonatal life. However, GHSRs are found in the nuclei known to regulate feeding, including in the ARH, (Fig. 2) and recent findings suggest that hypothalamic neurons can respond to exogenous ghrelin during postnatal life. For example, acute peripheral ghrelin treatment activates ARH neurons during early postnatal life, as evidenced by the presence of cFos-immunoreactive (-IR) cells in the ARH 120 minutes following ghrelin administration in P10 mice (Fig. 2). Additionally, the mRNA levels of Proopiomelanocortin (Pomc) and Neuropeptide Y (Npy) mRNA, two metabolically relevant neuropeptides in the ARH, are decreased and increased, respectively, in the ARH following ghrelin adminstration to mouse pups at P10 (Fig. 2). Similarly, ghrelin administration to ARH organotypic explants derived from P7-P9 rats directly increases Npy and Agouti-related peptide (Agrp) mRNA expression [8]. Collectively, these results provide convincing evidence that ghrelin receptors are present and functional in the hypothalamus in the postnatal period. These data further suggest that the “ghrelin insensitivity” observed in this developmental period may be due to a failure of hypothalamic neurons to relay ghrelin signals to other parts of the brain to initiate feeding.
Figure 2. Ghrelin acts on appetite-related brain regions during neonatal life.
In contrast to adults, acute ghrelin injection in P10 mouse pups does not result in changes in body weight (A). However, neurons located in the arcuate nucleus of the hypothalamus (ARH) contain GHSR immunoreactivity (red fluorescence) at P10 (B). Ghrelin increases the number of cFos-immunopositive cells (a marker of neuronal activation) in P10 ARH neurons 120 min after ghrelin injection (2 mg/kg, i.p.) (C). Acute ghrelin injection (2 mg/kg, i.p.) also increases Npy mRNA expression (D), while it decreases Pomc mRNA expression (E) in the hypothalamus of P10 mice. See Supplemental Online Material for detailed methods.
Effects of Ghrelin on Brain Growth and Neurogenesis
The brain undergoes tremendous growth beginning early in gestation and continuing throughout the postnatal period. During this developmental period, a variety of processes shape the brain nuclei. The cellular mechanisms proposed to explain the formation of brain circuits fall into five main categories: neurogenesis, neuronal migration, cell death, axonal growth, and synapse formation. Much of what we know about the role of ghrelin in brain development has been inferred from classic studies using experimental tools such as the thymidine analog bromodeoxyuridine, which monitors cell proliferation and neurogenesis. These studies have clearly demonstrated that the exposure of neural cells derived from a variety of brain regions to ghrelin promotes cell proliferation. For example, when cells from the traditional ghrelin target areas of the adult brain (i.e., the hypothalamus, dorsal motor nucleus of the vagus, and nucleus of the solitary tract) are incubated in vitro with ghrelin, cell proliferation increases in a dose-dependant manner [12, 36, 37]. Importantly, many of the resultant newborn cells acquire a neuronal and/or glial phenotype. Also, the neurogenic effects of ghrelin appear to be widespread, because ghrelin can also induce cell proliferation in the developing spinal cord in vitro [25]. These same in vitro studies further showed that ghrelin exerts its maximal neurogenic effect on fetal neural tissues. Exposure of either cultured spinal cord or hypothalamic cells to ghrelin markedly increases cell proliferation, but these effects were greater when the cells were taken from E17 embryos instead of P2 pups [12]. Intriguingly, both acyl and des-acyl ghrelin promote cell proliferation and neurogenesis, with the acylated form of ghrelin exerting greater proliferative effects as opposed to the acylated form of ghrelin [18, 25]. These observations suggest that the proliferative effects of ghrelin do not require the octanoic acid modification of ghrelin, and that ghrelin acts through both a GHSR-dependent and GHSR-independent mechanisms to mediate its neurodevelopmental effects.
Conclusions
In conclusion, this relatively new area of research has provided convincing evidence that the effects of ghrelin extend well beyond those originally described in adults. It is now clear that, in addition to its regulatory role in mature animals, ghrelin acts as a signal that can influence developmental processes in a variety of organs including the pancreas, gastrointestinal tract, and brain (Fig. 1). However, more research is needed to gain a better understanding of the cellular and molecular mechanisms mediating the effects of ghrelin on perinatal growth and development, and the periods of maximal sensitivity of various organs to changes in ghrelin levels. Nevertheless, by affecting the development and function of a variety of organs, ghrelin may be a major signal mediating perinatally acquired predispositions to adult diseases
Supplementary Material
Highlights.
Ghrelin and GHSRs are expressed in the developing embryo and fetus.
Ghrelin influences perinatal growth.
Alterations in perinatal ghrelin levels cause structural differences in the pancreas, gastrointestinal tract, and brain.
Acknowledgments
The work in Dr. Bouret’s lab was supported by the National Institutes of Health (grant DK84142), the “Fondation pour la Recherche Medicale”, the Danone Institute, the European Union Seventh Framework Programme (FP7/2007-2013) EU FP7 integrated project (grant agreement n° 266408, “Full4Health”), and the “Agence Nationale de la Recherche” (grant ANR-08-JCJC-0055-01).
Abbreviation list
- AgRP
agouti-related peptide
- ARH
arcuate nucleus of the hypothalamus
- E
embryonic day
- GHSR
growth hormone secretagogue receptor
- IR
immunoreactive
- NPY
neuropeptide Y
- P
postnatal day
- POMC
proopiomelanocortin
- PVH
paraventricular nucleus of the hypothalamus
- VMH
ventromedial nucleus of the hypothalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Castaneda TR, Tong J, Datta R, Culler M, Tschoep MH. Ghrelin in the regulation of body weight and metabolism. Frontiers in Neuroendocrinology. 2010;31:44–60. doi: 10.1016/j.yfrne.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Chanoine J-P. Ghrelin in growth and development. Horm Res. 2005;63:129–38. doi: 10.1159/000084688. [DOI] [PubMed] [Google Scholar]
- 3.Chanoine J-P, De Waele K, Walia P. Ghrelin and the growth hormone secretagogue receptor in growth and development. International Journal of Obesity. 2009;33:S48–S52. doi: 10.1038/ijo.2009.17. [DOI] [PubMed] [Google Scholar]
- 4.Chanoine J-P, Wong ACK. Ghrelin gene expression is markedly higher in fetal pancreas compared with fetal stomach: effect of maternal fasting. Endocrinology. 2004;145:3813–20. doi: 10.1210/en.2004-0053. [DOI] [PubMed] [Google Scholar]
- 5.Date Y, Nakazato M, Hashiguchi S, Dezaki K, Mondal MS, Hosoda H, et al. Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes. 2002;51:124–9. doi: 10.2337/diabetes.51.1.124. [DOI] [PubMed] [Google Scholar]
- 6.Dembinski A, Warzecha Z, Ceranowicz P, Bielanski W, Cieszkowski J, Dembinski M, et al. Variable effect of ghrelin administration on pancreatic development in young rats. Role of insulin-growth faftor-1. Journal of Physiology and Pharmacology. 2005;56:555–70. [PubMed] [Google Scholar]
- 7.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nature Neuroscience. 2006;9:381–8. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 8.Goto M, Arima H, Watanabe M, Hayashi M, Banno R, Sato I, et al. Ghrelin increases neuropeptide Y and agouti-related peptide gene expression in the arcuate nucleus in rat hypothalamic organotypic cultures. Endocrinology. 2006;147:5102–9. doi: 10.1210/en.2006-0104. [DOI] [PubMed] [Google Scholar]
- 9.Hansson C, Haage D, Taube M, Egecioglu E, Salomé N, Dickson SL. Central administration of ghrelin alters emotional responses in rats: behavioural, electrophysiological and molecular evidence. Neuroscience. 2011;180:201–211. doi: 10.1016/j.neuroscience.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Hayashida T, Nakahara K, Mondal MS, Date Y, Nakazato M, Kojima M, et al. Ghrelin in neonatal rats: distribution in stomach and its possible role. J Endocrinol. 2002;173:239–45. doi: 10.1677/joe.0.1730239. [DOI] [PubMed] [Google Scholar]
- 11.Hewson AK, Dickson SL. Systemic administration of ghrelin induces Fos and egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. Journal of Neuroendocrinology. 2000;12:1047–9. doi: 10.1046/j.1365-2826.2000.00584.x. [DOI] [PubMed] [Google Scholar]
- 12.Inoue Y, Nakahara K, Kangawa K, Murakami N. Transitional change in rat fetal cell proliferation in response to ghrelin and des-acyl ghrelin during the last stage of pregnancy. Biochemical and Biophysical Research Communications. 2010;393:455–60. doi: 10.1016/j.bbrc.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, et al. Requirement of central ghrelin signaling for alcohol reward. Proceedings of the National Academy of Sciences. 2009;106:11318–23. doi: 10.1073/pnas.0812809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawamura K, Sato N, Fukuda J, Kodama H, Kumagai J, Tanikawa H, et al. Ghrelin Inhibits the Development of Mouse Preimplantation Embryos in Vitro. Endocrinology. 2003;144:2623–33. doi: 10.1210/en.2003-0033. [DOI] [PubMed] [Google Scholar]
- 15.Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochemical and Biophysical Research Communications. 2000;276:905–8. doi: 10.1006/bbrc.2000.3568. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell V, Bouret SBJ, Schilling A, Perret M, Kordon C, Epelbaum J. Comparative distribution of mRNA encoding the growth hormone secretagogue-receptor (GHS-R) in Microcebus murinus (Primate, lemurian) and rat forebrain and pituitary. J Comp Neurol. 2001;429:469–89. doi: 10.1002/1096-9861(20010115)429:3<469::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Nagaya N, Uematsu M, Kojima M, Ikeda Y, Yoshihara F, Shimizu W, et al. Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation. 2001;104:1430–5. doi: 10.1161/hc3601.095575. [DOI] [PubMed] [Google Scholar]
- 18.Nakahara K, Nakagawa M, Baba Y, Sato M, Toshinai K, Date Y, et al. Maternal ghrelin plays an important role in rat fetal development during pregnancy. Endocrinology. 2006;147:1333–42. doi: 10.1210/en.2005-0708. [DOI] [PubMed] [Google Scholar]
- 19.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 20.Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, et al. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biological psychiatry. 2010;67:880–6. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piao H, Hosoda H, Kangawa K, Murata T, Narita K, Higuchi T. Ghrelin stimulates milk intake by affecting adult type feeding behaviour in postnatal rats. Journal of Neuroendocrinology. 2008;20:330–4. doi: 10.1111/j.1365-2826.2007.01644.x. [DOI] [PubMed] [Google Scholar]
- 22.Pinilla L, Barreiro ML, Tena-Sempere M, Aguilar E. Role of ghrelin in the control of growth hormone secretion in prepubertal rats: interactions with excitatory amino acids. Neuroendocrinology. 2003;77:83–90. doi: 10.1159/000068652. [DOI] [PubMed] [Google Scholar]
- 23.Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2924–9. doi: 10.1073/pnas.0308604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos M, Bastos P, Gonzaga S, Roriz Jâ-MÅ, Baptista MJ, Nogueira-Silva C, et al. Ghrelin expression in human and rat fetal lungs and the effect of ghrelin administration in nitrofen-Induced congenital diaphragmatic hernia. Pediatric Research. 2006;59:531–7. doi: 10.1203/01.pdr.0000202748.66359.a9. [DOI] [PubMed] [Google Scholar]
- 25.Sato M, Nakahara K, Goto S, Kaiya H, Miyazato M, Date Y, et al. Effects of ghrelin and des-acyl ghrelin on neurogenesis of the rat fetal spinal cord. Biochemical and Biophysical Research Communications. 2006;350:598–603. doi: 10.1016/j.bbrc.2006.09.088. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Ahmed S, Smith RG. Deletion of Ghrelin Impairs neither Growth nor Appetite. Mol Cell Biol. 2003;23:7973–81. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–37. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4679–84. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tena-Sempere M, Barreiro ML, Gonzalez LC, Gaytan F, Zhang FP, Caminos JE, et al. Novel expression and functional role of ghrelin in rat testis. Endocrinology. 2002;143:717–25. doi: 10.1210/endo.143.2.8646. [DOI] [PubMed] [Google Scholar]
- 30.Torsello A, Scibona B, Leo G, Bresciani E, Avallone R, Bulgarelli I, et al. Ontogeny and tissue-specific regulation of ghrelin mRNA expression suggest that ghrelin is primarily involved in the control of extraendocrine functions in the rat. Neuroendocrinology. 2003;77:91–9. doi: 10.1159/000068653. [DOI] [PubMed] [Google Scholar]
- 31.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodent. Nature. 2000:908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 32.Warzecha Z, Dembinski A, Ceranowicz P, Dembinski M, Cieszkowski J, Bielanski W, et al. Dual age-dependent effect of ghrelin administration on serum level of insulin-like growth factor-1 and gastric growth in young rats. European Journal of Pharmacology. 2006;529:145–50. doi: 10.1016/j.ejphar.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 33.Wierup N, Svensson H, Mulder H, Sundler F. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regulatory Peptides. 2002;107:63–9. doi: 10.1016/s0167-0115(02)00067-8. [DOI] [PubMed] [Google Scholar]
- 34.Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, et al. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8227–32. doi: 10.1073/pnas.0402763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–8. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, Hu Y, Lin TR, Fan Y, Mulholland MW. Stimulation of neurogenesis in rat nucleus of the solitary tract by ghrelin. Peptides. 2005;26:2280–8. doi: 10.1016/j.peptides.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W, Lin TR, Hu Y, Fan Y, Zhao L, Stuenkel EL, et al. Ghrelin stimulates neurogenesis in the dorsal motor nucleus of the vagus. The Journal of Physiology. 2004;559:729–37. doi: 10.1113/jphysiol.2004.064121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zigman JM, ones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. The Journal of Comparative Neurology. 2006;494:528–48. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.