Abstract
Indian mustard (Brassica juncea L.) accumulates high tissue Se concentrations and volatilizes Se in relatively nontoxic forms, such as dimethylselenide. This study showed that the presence of bacteria in the rhizosphere of Indian mustard was necessary to achieve the best rates of plant Se accumulation and volatilization of selenate. Experiments with the antibiotic ampicillin showed that bacteria facilitated 35% of plant Se volatilization and 70% of plant tissue accumulation. These results were confirmed by inoculating axenic plants with rhizosphere bacteria. Compared with axenic controls, plants inoculated with rhizosphere bacteria had 5-fold higher Se concentrations in roots (the site of volatilization) and 4-fold higher rates of Se volatilization. Plants with bacteria contained a heat-labile compound in their root exudate; when this compound was added to the rhizosphere of axenic plants, Se accumulation in plant tissues increased. Plants with bacteria had an increased root surface area compared with axenic plants; the increased area was unlikely to have caused their increased tissue Se accumulation because they did not accumulate more Se when supplied with selenite or selenomethionine. Rhizosphere bacteria also possibly increased plant Se volatilization because they enabled plants to overcome a rate-limiting step in the Se volatilization pathway, i.e. Se accumulation in plant tissues.
Se pollution is a major concern for agriculture in California and other parts of the western United States because irrigation of seleniferous soils derived from shale rocks has led to leaching of Se and other naturally occurring trace elements such as B and As into the drainage water (Aubert and Pinta, 1977; Presser and Ohlendorf, 1987). The predominant form of Se in agricultural drainage water is selenate (McNeal and Balisteri, 1989), which is known to bioaccumulate with significant ecotoxicological effects (Presser and Ohlendorf, 1987). At the Kesterson, California, reservoir, Se-laden agricultural runoff accumulated in the food chain, resulting in the deformity and death of birds and fish (Ohlendorf et al., 1986; Saiki and Lowe, 1987).
Se is also a significant contaminant in industrial wastewater (Manceau and Gallup, 1997; Hansen et al., 1998). Constructed wetlands have been shown to efficiently remove Se from contaminated wastewater: 89% of the Se entering a constructed wetland as selenite-contaminated oil refinery effluent was removed (Hansen et al., 1998). A large proportion of the Se removed by the wetland was accumulated in plant tissues and sediments, and it was estimated that 10% to 30% of the removed Se was volatilized. Se volatilization is the process by which inorganic Se is converted to volatile forms (Lewis et al., 1966; Evans et al., 1968; Lewis, 1971; Frankenberger and Karlson, 1994; Terry and Zayed, 1994). Dimethylselenide, the predominant form of volatile Se, is 500 to 700 times less toxic than inorganic forms of Se (McConnell and Portman, 1952; Ganther et al., 1966; Wilber, 1980).
Phytoremediation, the use of plants to remove, stabilize, or detoxify pollutants, is an environmentally responsible and efficient way to clean up Se-contaminated soil and water (Terry and Zayed, 1998). Many plant species have been shown to very efficiently accumulate and volatilize Se (Terry et al., 1992; Pilon-Smits et al., 1998), and these processes make them excellent candidates for the phytoremediation of Se-contaminated sites (Terry and Zayed, 1994, 1998; Zayed and Terry, 1994; Banuelos et al., 1995). Phytoextraction is an important remediation process by which plants remove trace elements (such as Se) from contaminated environments and absorb them into their tissue (Kumar et al., 1995). The plant tissue can be harvested, thereby removing the Se from the site. Phytovolatilization is also an important remediation process because it prevents Se from entering the food chain. Atmospheric studies on the fate of dimethylselenide show that the volatile Se is deposited away from the contaminated site in soils that might be deficient in Se (Atkinson et al., 1990).
A laboratory screening study conducted with 20 crop species identified Indian mustard (Brassica juncea L.) as one of the best plant species for Se phytoremediation because it grows rapidly, produces a large biomass, and efficiently volatilizes and accumulates Se (Terry et al., 1992; Terry and Zayed, 1998). Compared with the other species tested, Indian mustard was the most efficient in extracting inorganic Se from hydroponic solution. Some of the extracted Se was bioconcentrated in plant tissues and some was volatilized. These laboratory data are consistent with results from field trials that showed that Indian mustard efficiently removed Se from selenate-contaminated soil (Banuelos and Meek, 1990; Banuelos et al., 1993), with up to 50% of the soil Se removed from a depth of 0 to 75 cm during a 3-year period (Banuelos et al., 1995). Se-volatilization rates from this plant species have not been measured in the field.
In addition to Se volatilization by plants, the production of volatile Se has been measured from algae, fungi, and bacteria, and from samples collected from upland and wetland environments (this literature was recently reviewed by Terry and Zayed [1998] and Frankenberger and Karlson [1994]). Experiments with bactericide and fungicide amendments to wetland sediment-enrichment cultures showed that bacteria were more important than fungi in Se volatilization in wetlands (Azaizeh et al., 1997). Enrichment cultures of wetland sediment rhizosphere microbes volatilized Se at higher rates than bulk sediment microbes. This is because there are greater numbers of microbes and higher microbial activity in the rhizosphere than in bulk soils and sediments (Anderson et al., 1993; Sorensen, 1997). Because microbes in the rhizosphere interact with plants by affecting plant growth, enhancing mineral and water uptake, producing antibiotics to inhibit soil pathogens, and producing plant-growth regulators (Kapulnik, 1996), it is possible that they influence Se assimilation by plants and, therefore, affect Se phytoremediation.
Preliminary data have shown that bacteria may increase the ability of plants to volatilize Se. The rate of Se volatilization from selenate was higher from detopped broccoli plants (plants with shoots that had been removed) than from intact plants (Zayed and Terry, 1994). When bactericides were added to the nutrient solution of these detopped plants, the rate of Se volatilization was inhibited, presumably because the bacteria in the rhizosphere of the detopped plants were inhibited. Cycloheximide, a fungicide, did not have any effect on the rate of Se volatilization, suggesting that fungi were not involved in Se assimilation and volatilization by these plants. Therefore, the objective of this study, using Indian mustard as a model species, was to determine the role of rhizosphere bacteria in Se accumulation and volatilization by plants.
MATERIALS AND METHODS
Antibiotic Experiments
Indian mustard (Brassica juncea L.) seeds (accession no. 173874) were obtained from the North Central Regional Plant Introduction Station (Ames, IA). Seeds were germinated on moistened filter paper and transferred after 2 d into 4-inch pots containing coarse sand. The pots were maintained in a greenhouse with controlled temperature (25°C–30°C) and a short-day (9 h) photoperiod. The plants were watered twice a day, once with tap water and once with half-strength Hoagland solution (Hoagland and Arnon, 1938). After 1 month the plants were carefully removed from the sand to avoid damage to the roots, washed in deionized water, and placed in plastic containers containing half-strength Hoagland solution. The hydroponic solutions were aerated. After 1 week in hydroponic solution the plants were transferred into solutions containing 0.1 mg mL−1 ampicillin (to inhibit bacteria) and 20 μm Se supplied as sodium selenate. Ampicillin inhibits both gram-positive and gram-negative bacteria; it was the antibiotic of choice because it interferes with bacterial cell wall synthesis and is therefore expected to have the least effect on plant physiology. For use as controls, another set of plants was maintained in Se-containing hydroponic solution without ampicillin.
After 1 week on Se, the hydroponic solution was replaced with fresh half-strength Hoagland solution with or without ampicillin (depending on the treatment) and 20 μm selenate. Se volatilization was measured for a 24-h period as described below. Microbial numbers were estimated in 50-mL samples of nutrient solution from ampicillin-treated and untreated plants by acridine orange direct counts (Hobbie et al., 1977). The plants were then washed thoroughly in running water to remove any Se that may have adhered to the roots, dried at 70°C for 3 d, and then weighed. Roots and shoots were ground separately using a Wiley mill, and Se analysis in the tissues was carried out by acid digestion (Martin, 1975) followed by vapor generation-atomic absorption spectroscopy (Logan et al., 1987). The detection limit of Se in this analytical method was 1 μg L−1. A wheat flour standard (Se at 1.1 mg kg−1) and a blank were used with all digestions. Se dioxide reference solution (Fisher) was diluted in 6 m HCl and used as a standard. All samples were diluted in 6 m HCl to give absorbances in the linear portion of the standard curve.
Three replicate plants were used for each treatment in all experiments. Statistical analyses were performed using the JMP IN statistical package (SAS Institute, Cary, NC) using analysis of variance procedures.
Se-Volatilization Measurements
Se volatilization was measured by placing the plants in Magenta boxes (Sigma) containing half-strength Hoagland solution and 20 μm selenate. The Magenta boxes were placed in gas tight acrylic volatilization chambers (3-L volume) through which a continuous air flow (1.5 L min−1) was passed by applying suction at the outlet and by bubbling incoming air into the hydroponic solution. Volatile Se was quantitatively trapped in alkaline peroxide liquid traps as described previously (Zayed and Terry, 1992). For the axenic plant experiments, the chambers were disinfected with 20% bleach, sterile hydroponic solutions were used, and sterile air (0.22-μm-filter sterilized) and sterile tubes were used to aerate the hydroponic solution. The Se-volatilization chambers were placed in a plant growth chamber with a 24-h photoperiod and maintained at 25°C with an irradiance of 400 μmol m−2 s−1 photosynthetic photon flux (mainly as fluorescent light with some incandescent light). Aliquots of trap solution were kept at 4°C until analysis. The trap-solution samples were heated at 95°C to remove the peroxide. The selenate-Se was reduced to selenite by adding an equal volume of concentrated HCl and heating at 95°C for 30 min. The Se concentration was measured by vapor-generation atomic absorption spectroscopy as described above.
Isolation of Bacteria
Indian mustard plants were grown in soil and then transferred to hydroponic medium containing 20 μm selenate as described above. The plant shoots were detopped and individual roots were rinsed in sterile water. Excess water was removed by blotting the roots on sterile filter paper. The plant roots were then inserted (using sterile forceps) into tubes containing a basal salts medium (de Souza and Yoch, 1995) of 0.3% agar; the medium was similar to that described earlier except that it contained 20 μm selenate and 5 mm acetate instead of acrylate as the C source. Se was used in the medium to obtain Se-tolerant bacterial isolates. The tubes were incubated in the dark at room temperature for 3 d, after which bacterial colonies were visible in the medium next to the roots. Phenotypically different bacterial colonies were picked out of the medium using sterile inoculation needles and streaked out on plates containing the same Se-containing basal salts medium solidified with 1.5% agar. These bacteria, which also grew on tryptic soy agar plates, can be characterized morphologically and phylogenetically.
Axenic Plants and Bacterial Inoculations
Indian mustard seeds were surface-sterilized by treatment with 70% ethanol for 30 s, 20% hypochlorite for 30 min, and five washes with sterile water. During these treatments the seeds were kept in tightly closed, sterile, plastic tubes and kept on a rocking platform to ensure equal access of the bleach and alcohol to all of the seeds. After the seeds were blotted on filter paper in a laminar flow hood, they were transferred into sterile double Magenta boxes containing 200 mL of 0.22-μm-filter sterilized half-strength Hoagland solution. The Magenta boxes contained sterile wire mesh grids that were made to perfectly fit the sides of the box and just touch the surface of the hydroponic solution. A small square was cut in one corner of the grid and a Nalgene (Milwaukee, WI) tube was inserted through it so that the nutrient solution could be aerated during the Se-volatilization measurements. Fifty seeds were placed on each grid and allowed to germinate. The seedlings were kept in a growth chamber maintained at the same conditions as the Se-volatilization chambers (see above) for 1 month.
For inoculation of axenic plants with bacteria to determine the optimum plant-microbe combinations for Se accumulation and Se volatilization, pure cultures of all bacterial strains used in this study were grown in tryptic soy broth for 18 h on a shaker at 200 rpm. The only exception was strain BJ2, which was grown for 36 h because of its slow growth rate. The cultures were centrifuged at 8000g for 10 min. The pellet was resuspended in 50 mm phosphate buffer, pH 7.0, recentrifuged, and finally resuspended in 5 mL of phosphate buffer. Equal numbers of bacteria (approximately 104 colony-forming units mL−1) were added to the rhizospheres of axenic plants maintained in hydroponic solution containing 20 μm selenate. The number of viable bacterial cells was measured by serial dilution in sterile 0.85% NaCl, followed by viable counts on tryptic soy agar plates. After a 7-d treatment with Se and bacteria, the plants were rinsed in sterile water and placed in fresh, sterile hydroponic solution containing 20 μm selenate to minimize the contribution of bacteria to volatile Se production. After 7 d the bacteria were assumed to colonize the root, and therefore, no further addition of bacteria was made when the hydroponic solution was replaced. Procedures described above for the antibiotic experiments were used to measure tissue Se concentration and the rate of Se volatilization from axenic plants and from axenic plants to which bacteria had been added.
Controls for the Se-volatilization experiment from axenic plants inoculated with strain BJ2 or BJ15 were set up as follows. Strains BJ2 and BJ15 were grown in tryptic soy broth and washed as described above. Different volumes (0.1–1 mL) of the washed cell suspensions were added to 200 mL of half-strength hydroponic solution with 20 μm selenate to generate samples with different bacterial cell numbers, and Se-volatilization rates were measured for a 24-h period using the same incubation conditions as for plants. Se-volatilization rates were linearly dependent on the bacterial cell numbers (data not shown). After measuring the rate of Se volatilization from plants that had been inoculated with strain BJ2 or BJ15, bacterial cell numbers were estimated in the nutrient solution, and the rate of Se volatilization for that number of bacteria was estimated from the curve of Se-volatilization rate versus bacterial cell numbers. The amount of Se volatilized during a 24-h period by strains BJ2 and BJ15 alone was subtracted from that volatilized by the plant-bacterial combination during the same period.
Root Exudate Experiments
The hydroponic solution that was used to culture the plants was considered to contain diluted root exudate. The root exudate was filtered through a 0.22-μm membrane to remove any bacteria present in the solution. The amino acid content of bacteria-free root exudate was determined by phenol:HCl hydrolysis followed by ion-exchange chromatography with a sodium citrate buffer on an amino acid analyzer (model 6300, Beckman) at the University of California, Davis, Protein Structure Laboratory. The protein content of root exudate was estimated using the Bradford procedure (Bio-Rad) and BSA as a standard.
To determine if bacteria cause the production of a heat-labile bioactive compound in root exudate, and to determine if this compound was involved in enhancing Se accumulation in plants, 75 mL of filter-sterilized root exudate from axenic and inoculated plants was boiled for 5 min or left untreated, and then added to new batches of axenic plants growing in sterile Magenta boxes containing 75 mL of sterile, half-strength Hoagland solution. Selenate was added at 20 μm, and the Se content of tissues was measured after 1 week, as described above.
Effect of Bacteria and Se on Plant Growth
Indian mustard seeds were surface-sterilized as described above. Sterilized seeds were blotted dry on sterile filter paper in a laminar flow hood. The seeds were then allowed to soak for 20 min in a sterile 0.5% methylcellulose solution that did or did not contain a pure culture of bacterial cells at a density of 108 colony-forming units mL−1. The seeds were removed from the methylcellulose solution containing bacteria and dried on a sterile paper towel placed in a laminar flow hood. Fifty seeds were transferred aseptically into sterile Magenta boxes containing 50 mL of autoclaved half-strength Murashige and Skoog medium (without Suc) that had been amended with 0, 20, 50, 100, or 250 μm Se as sodium selenate. The selenate was filter sterilized through a 0.22-μm filter and added to the medium before it solidified. The Magenta boxes were incubated in a growth chamber maintained at 25°C, 40% humidity, and constant light. After 1 week plants were removed from the agar medium and the length of the longest root and the fresh weights of the seedlings were measured. Some seedlings were processed for microscopy as described below. The remaining seedlings were carefully washed to remove any agar, digested, and analyzed for their Se content, as described above.
To determine the effect of bacteria on root morphology, both axenic and bacteria-inoculated seedlings were fixed in 0.05% Tween 20 for 10 min, washed with water, stained with a 10−5 dilution of acridine orange for 10 min, and washed for 10 min in water. The acridine orange-stained samples were optically sectioned using the 10× objective (0.45 numerical aperture) of a confocal laser scanning microscope (Sarastro 1000, Molecular Dynamics, Sunnyvale, CA) with an Ar ion laser (excitation, 488 nm; emission, 525 nm). Image files were acquired and three-dimensional projections were rendered on an SGI Indy R 4400 using ImageSpace 3.2 (Molecular Dynamics). The resulting images were saved as TIFF files for use in Adobe Photoshop for Macintosh (Apple). The same samples were viewed using a fluorescence microscope (Zeiss Axiophot) with a HBO 100-W mercury light source and a 5× objective. Samples were excited using a 450- to 490-nm excitation filter and viewed with a 520-nm emission filter. Images were captured using a color video camera (Optronics 450, Optronics International, Chelmsford, MA) and a Scion C67 frame-grabber board on a Macintosh computer using Scion Image (color NIH Image v. 1.62). All images were saved as TIFF files for Adobe Photoshop for Macintosh.
RESULTS
Use of Antibiotic Experiments to Determine the Role of Bacteria in Se Accumulation and Volatilization
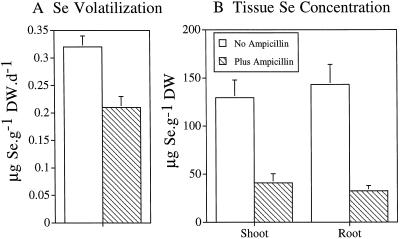
Our results suggest that bacteria in the rhizosphere facilitate Se accumulation and volatilization by plants. The rate of Se volatilization from ampicillin-supplied plants (with inhibited rhizosphere bacteria) was significantly lower than that of plants with their naturally occurring rhizosphere bacterial populations (P < 0.05; Fig. 1A). Furthermore, the Se concentrations in roots and shoots of ampicillin-treated plants were lower than those in untreated plants (P < 0.05; Fig. 1B). Ampicillin added to the nutrient solution of plants inhibited Se volatilization by about 35% and tissue Se accumulation by about 70%. It is evident that ampicillin inhibited the growth of bacteria because acridine-orange direct counts of total microbes in the nutrient solution of ampicillin-treated plants were 4-fold lower than microbe counts of plants without ampicillin (data not shown).
Figure 1.
Role of bacteria in Se volatilization (A) and accumulation (B) from plants supplied with 20 μm selenate. Ampicillin at 100 mg L−1 was used as an antibiotic to inhibit rhizosphere bacteria. The mean and sd values of three replicates are shown. Differences in Se accumulation and volatilization between ampicillin-treated and untreated plants were significant (P < 0.05). DW, Dry weight.
Use of Axenic Plant Experiments to Determine the Role of Bacteria in Se Accumulation and Volatilization
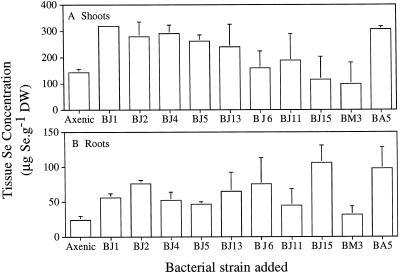
Several strains of rhizosphere bacteria were isolated from Indian mustard plants grown in soil to determine the effect of pure cultures on Se accumulation into plant tissues. When individual strains of bacteria isolated from Indian mustard (Fig. 2, the BJ strains) were added to the rhizosphere of axenic Indian mustard plants supplied with selenate, most of the bacterial isolates significantly enhanced the ability of axenic plants to accumulate Se in their roots and shoots (P < 0.05). One of the rhizosphere bacterial strains (BA5), which was isolated from salt-marsh bulrush (Scirpus robustus) roots, also enhanced Se accumulation in Indian mustard. Therefore, the rhizosphere bacteria are not plant specific in enhancing tissue Se accumulation. Different bacterial strains had different abilities to enhance Se accumulation in plants. For example, strains BJ1 and BJ2 were much better than strain BM3 at enhancing Se uptake into axenic Indian mustard.
Figure 2.
Inoculation of bacterial isolates into the rhizosphere of axenic plants leads to increased Se accumulation in shoots (A) and roots (B) of plants supplied with 20 μm selenate. The mean and sd values of three replicates are shown. All bacterial strains were isolated from the rhizosphere of Indian mustard plants except for strains BM3 and BA5, which were isolated from the rhizosphere of salt-marsh bulrush plants. The differences in tissue Se accumulation for axenic plants compared with plants inoculated with bacteria were significant in most cases (P < 0.05). The following bacteria-treated plants showed nonsignificant differences from axenic tissue Se accumulation: shoot Se accumulation for strains BJ13, BJ6, BJ11, and BJ15, and both root and shoot Se accumulation for strain BM3. DW, Dry weight.
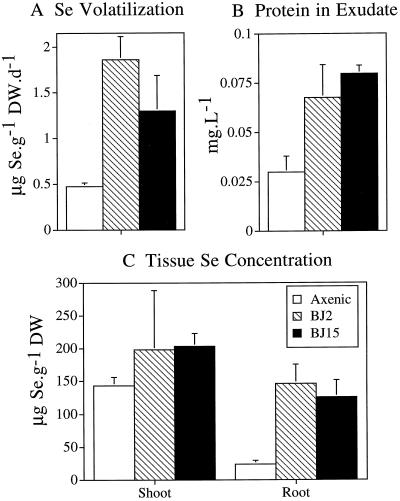
In addition to facilitating Se accumulation into plant tissues, rhizosphere bacteria also enhanced Se volatilization by plants (Fig. 3). Bacterial strains BJ2 and BJ15 were identified as good candidates for enhancing Se accumulation into plants (Fig. 2). When these bacterial strains were inoculated into the rhizosphere of axenic Indian mustard plants, the rate of Se volatilization from selenate was 4-fold higher than that of axenic control plants (P < 0.05; Fig. 3A). Furthermore, similar to the data presented in Figure 2, plants inoculated with strains BJ2 and BJ15 accumulated more Se in their tissues (Fig. 3C); compared with axenic controls, both bacterial strains increased the Se concentrations in shoots and roots 1.4- and 5-fold, respectively (P < 0.05). The protein content of bacteria-free root exudate was also significantly increased in plants with rhizosphere bacteria compared with axenic plants (P < 0.05; data shown in the legend to Fig. 3B).
Figure 3.
Inoculation of bacterial strains BJ2 and BJ15 into the rhizosphere of axenic plants leads to increased Se volatilization (A). Inoculation of these bacteria into the rhizosphere of axenic plants also increases the protein content of the root exudate (B) and Se accumulation in plant tissues (C). Bacterial strains BJ2 and BJ15 were identified as superior strains for plant tissue Se accumulation (Fig. 2). The mean and sd values of three replicates are shown. Differences in Se accumulation and volatilization between bacteria-treated and untreated plants were significant (P < 0.05). The amount of Se volatilized during a 24-h period by strains BJ2 and BJ15 alone (0.08 and 0.37 μg d−1) was subtracted from the amount volatilized by the plant-bacteria combination during the same period. DW, Dry weight.
Effect of Bacteria and Se on Plant Growth
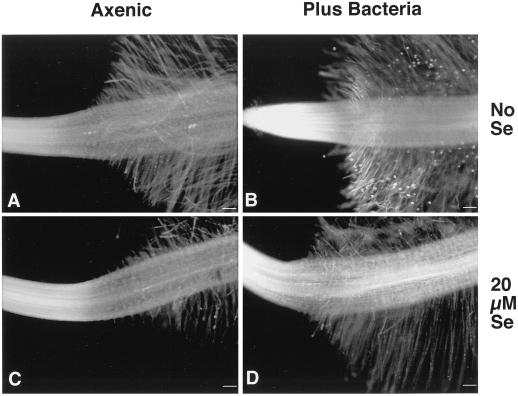
Initially, bacteria were thought to enhance Se uptake into Indian mustard by stimulating root-hair production. Plants that germinated in media containing 20 μm selenate from seeds coated with bacteria had more and longer root hairs than plants that germinated from axenic seeds (Fig. 4). Compared with axenic plants, root-hair production in bacteria-supplied plants began closer to the root apical meristem. Scanning electron micrographs showed that bacteria colonized all areas of the roots of seedlings that germinated from bacteria-coated seeds; no bacteria were detectable on axenic seedlings (data not shown). As the Se concentration in the medium increased from 0 to 20 μm, the length of the root hairs of bacteria-supplied plants appeared to be unaffected by Se, whereas that of axenic plants decreased. At Se concentrations of 50 μm or higher, plants with bacteria still had increased root surface area compared with axenic controls, although the roots and root hairs were much shorter (data not shown).
Figure 4.
Axenic plants infected with bacterial strain BJ2 (A and C) had increased root-hair production compared with axenic plants (B and D) supplied with 0 and 20 μm selenate in agar medium. Seedlings were germinated from surface-sterilized seeds coated with or without bacteria in a methylcellulose paste, stained with acridine orange, and observed at 5× magnification. Similar results were observed when strain BJ15 was used and when the root tips were observed at 10× magnification by confocal microscopy. Bars = 100 μm.
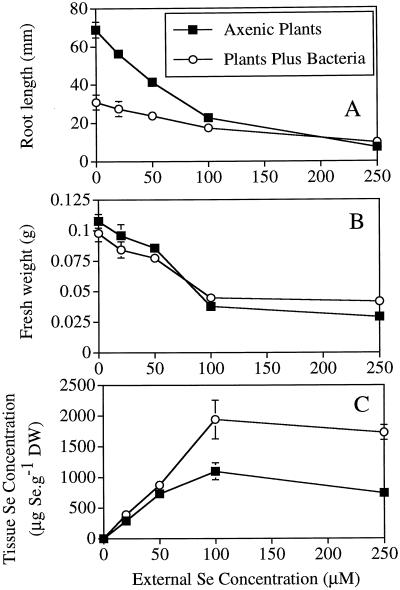
As the Se concentration in the medium increased, there was a decrease in the root length of seedlings with or without bacterial strains BJ2 or BJ15 in their rhizosphere. Seedlings that germinated from seeds coated with bacterial strain BJ2 or BJ15 had significantly shorter roots than axenic seedlings at most external Se concentrations (P < 0.05; Fig. 5A); at 250 μm there was no significant difference in root length between seedlings with and without bacteria. The fresh weights of seedlings with and without bacteria were similar and decreased with increasing Se concentrations in the growth medium (Fig. 5B). At a Se concentration of 250 μm, the seedlings that germinated from bacteria-coated seeds had significantly higher fresh weights than axenic seedlings (P < 0.05). The seedlings with bacteria in their rhizosphere had significantly higher tissue Se concentrations (1.3- to 2-fold) compared with axenic plants at all external Se concentrations tested (P < 0.05; Fig. 5C).
Figure 5.
Effect of bacteria and different Se concentrations on growth and Se accumulation by axenic plants grown in agar. A, The length of the longest root; B, fresh weight of individual seedlings; and C, Se concentration in seedling tissue (root plus shoot). All differences between bacteria-treated and untreated axenic plants were statistically significant (P < 0.05) except for root lengths at 250 μm Se and fresh weights at 0, 20, and 100 μm Se. DW, Dry weight.
Experiments with Root Exudate
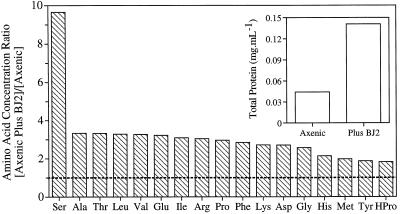
Compared with axenic plants, plants with bacteria in their rhizosphere had increased levels of amino acids in their growth medium, which was considered as diluted root exudate and had been filter sterilized to remove the bacteria (Fig. 6). The concentration of all of the amino acids measured was at least 2-fold higher in the root exudate of plants with bacteria compared with that of axenic plants, and Ser levels were even enhanced 9-fold. The total protein content of the filter-sterilized root exudate from plants with bacteria was 2.8-fold higher than that of plants without bacteria (Fig. 6, inset).
Figure 6.
The amino acid and protein contents of 0.22-μm filtered root exudate from plants inoculated with strain BJ2 were higher than those from axenic plants; all were supplied with 20 μm selenate. The amino acid concentration in root exudate from bacteria-supplied plants (0.5–23.6 nmol mL−1) was divided by that of axenic plants (0.2–9.2 nmol mL−1). The protein content shown in the inset was calculated from the amino acid content.
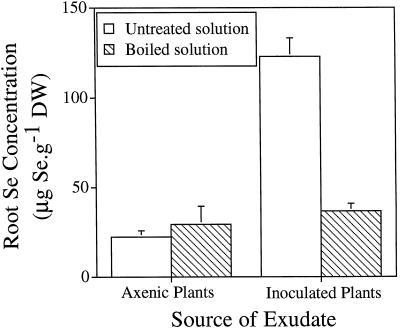
To determine if a bioactive compound in the root exudate of the plant-bacterial combination facilitated Se accumulation into plant tissues, bacteria-free root exudate from a plant-microbe combination was either left untreated or boiled, and then added to a fresh batch of axenic plants. This bacteria-free root exudate from a plant-microbe combination enhanced Se accumulation in axenic plant roots 5.5-fold (P < 0.05; Fig. 7). Boiled root exudate had no such effect. Se accumulation in shoots was 2-fold higher in plants that received root exudate from a plant-bacteria combination compared with the axenic controls (data not shown); however, this difference was not statistically significant. The negative control for this experiment was untreated or boiled root exudate from axenic plants added to a fresh batch of axenic plants. The negative controls did not have higher tissue Se concentrations than axenic plants (compare Fig. 7, bars on left, and Fig. 2, first bar). When root exudate was concentrated 1000-fold by freeze drying, resuspended in buffer, dialyzed, and analyzed by native and SDS-PAGE, no proteins in the exudate were visible on the gel.
Figure 7.
The bacteria-plant combination produced a heat-labile compound in diluted root exudate that, when supplied to axenic plants, enhanced the Se concentration in roots 5-fold. The mean and sd values of three replicates are shown. Differences in root Se concentrations of axenic plants treated with growth medium (diluted root exudate) supplied with bacteria and axenic plants treated with root exudate or boiled exudate were significant (P < 0.05). The root exudate from axenic plants or plants grown with strain BJ2 was filtered through a 0.22-μm filter to remove bacteria, and then added to a fresh batch of axenic plants with 20 μm selenate.
DISCUSSION
This study showed that bacteria in the rhizosphere are necessary to achieve optimum rates of Se accumulation and volatilization by plants. The rate of Se accumulation and volatilization from plants is also dependent on the chemical form of Se, competition due to sulfate, concentration of Se, the plant species, the age of the plant, and other environmental factors such as pH and temperature (Terry et al., 1992; Zayed and Terry, 1992; Terry and Zayed, 1994; de Souza et al., 1998; Pilon-Smits et al., 1998). Recent kinetic studies have shown that selenate uptake and maintenance in the root may be rate limiting for Se assimilation and volatilization by Indian mustard (de Souza et al., 1998). The present study showed that rhizosphere bacteria can overcome part of the rate limitation, i.e. Se uptake, and thereby increase Se accumulation in tissues and Se volatilization. The reduction of selenate inside the plant was also shown to be rate limiting for Se volatilization (de Souza et al., 1998). Bacteria in the rhizosphere should have little effect on Se reduction within plant tissue, and should not, therefore, have as great an effect on Se volatilization as on Se uptake. This contention is supported by the results from the antibiotic experiment (Fig. 1), which showed that bacteria played a larger role in plant Se accumulation (70%) than in volatilization (35%). This disproportional increase in tissue Se accumulation compared with Se volatilization may also be attributable to the rapid translocation of selenate into shoots (de Souza et al., 1998; Zayed et al., 1998) away from the root, which is the site of volatilization (Zayed and Terry, 1994).
The bacterial strains used in this study were isolated from roots and selected from the general rhizosphere population based on their ability to tolerate 20 μm Se; therefore, the isolates obtained were very likely to be involved in Se transformations in the rhizosphere. Indeed, the ability to increase plant tissue Se accumulation and volatilization was a characteristic shared by most of these Se-tolerant bacterial strains (Fig. 2); strain BM3 was an exception. The Se-tolerant bacterial strains were not plant specific; for example, strain BA5, which was isolated from the rhizosphere of bulrush, also enhanced Se accumulation in Indian mustard. Similar results were found with axenic broccoli (Brassica oleracea botrytis L.) plants, in which rhizosphere bacteria isolated from Indian mustard or salt-marsh bulrush also enhanced Se uptake. This information is beneficial because it shows that a bacterium from a certain plant species may be used in conjunction with other plant species to improve their phytoremediation potential. Many of the bacteria tested in this study can also accumulate, precipitate, and volatilize Se on their own (M. de Souza, D. Chu, M. Zhao, and N. Terry, unpublished). The contribution of bacteria to Se volatilization (when nonaxenic plants were used) was minimized by rinsing the plants in sterile water to remove loosely bound bacteria from the roots before the Se-volatilization measurements, and by using controls to estimate the rate of Se volatilization by bacteria alone.
Bacteria in the rhizosphere interact in numerous ways with plants to improve their growth (Kapulnik, 1996). Aside from the well-characterized nitrogen-fixing legume symbioses and plant-pathogen host interactions, rhizosphere bacteria can stimulate plant growth by producing phytohormones (Fallik et al., 1994), enhancing mineral and water uptake (Lin et al., 1983), producing antibiotics to inhibit pathogens (Lesinger and Margraff, 1979), and altering root morphology (Lin et al., 1983; Kapulnik, 1996). The present study showed that bacteria appear to increase the plant's potential for Se phytoremediation because they facilitate Se accumulation and volatilization.
In the presence of high Se concentrations, rhizosphere bacteria caused plants to increase their root-hair production (Fig. 4). When rhizosphere bacteria were inoculated onto surface-sterilized Indian mustard seeds and the seeds were germinated in tissue-culture medium, the bacteria colonized the roots and enhanced their surface area. Initially, it was assumed that the increased root surface area of plants with bacteria caused their increased tissue Se uptake and accumulation. However, when other chemical forms of Se, selenite and SeMet, were supplied to plants, the tissue Se concentrations were not enhanced in plants inoculated with bacteria compared with axenic plants (data not shown). Therefore, it is unlikely that the stimulation of root-hair production by bacteria is responsible for enhancing Se accumulation from selenate.
A heat-labile compound was shown to enhance Se accumulation in axenic plants. This compound has not yet been identified, and it is not clear whether it is produced by the plant or by the rhizosphere bacteria. Because the protein content of root exudate from plants with bacteria in their rhizosphere was higher than that of axenic plants (Figs. 3 and 6), and because these plants also had higher rates of Se accumulation and volatilization (Fig. 3), the heat-labile compound could be proteinaceous in nature and could also enhance Se volatilization. The root exudate of axenic plants inoculated with bacteria had a higher amino acid content than that of axenic plants; in particular, Ser levels were greatly enhanced. It is possible that adding Ser or its derivatives to axenic plants will enhance Se volatilization because o-acetylserine is an intermediate in the proposed Se-volatilization pathway (Terry and Zayed, 1994), where it is involved in the incorporation of inorganic Se into selenocysteine
The heat-labile compound produced by the plant-bacteria interaction may be involved in stimulating the selenate transporter. Alternatively, the heat-labile compound produced by the plant-bacteria interaction may be a reductant or enzyme produced by the bacteria, which is capable of converting selenate to organic Se compounds such as SeMet. Earlier studies with Indian mustard and broccoli plants have shown that plants take up SeMet at faster rates than selenate, and that the root-to-shoot ratio for tissue Se concentration is higher when SeMet is supplied, compared with selenate (Zayed et al., 1998). Figure 3 provides some evidence to support the contention that some of the bacterial strains used in this study may enhance selenate uptake by converting selenate to organic Se in the rhizosphere. Compared with axenic plants, plants supplied with bacterial strains BJ2 and BJ15 had 4- to 6-fold higher root Se concentrations (consistent with faster rates of uptake into roots), and 3- to 4-fold higher root-to-shoot ratios for tissue Se concentration, consistent with lower rates of translocation from root to shoot, a characteristic shown by SeMet-amended plants (Zayed et al., 1998). It is unlikely that bacterial reduction of selenate to selenite (Macy et al., 1993; Oremland et al., 1994; Losi and Frankenberger, 1997) contributes greatly to the enhanced Se uptake in bacteria-supplied plants treated with selenate, compared with axenic plants. This is because selenite is taken up at slower rates than selenate, which, in turn, is taken up at a slower rate than SeMet (de Souza et al., 1998; Zayed et al., 1998).
The fact that bacteria only enhance Se accumulation in plant tissues when supplied with selenate (but not selenite or SeMet) is interesting because selenate is the major form of Se in soils in the San Joaquin Valley and in agricultural drainage water resulting from irrigation (McNeal and Balisteri, 1989). Furthermore, this result suggests that different uptake mechanisms exist for the different chemical forms of Se. Selenate, a chemical analog of sulfate, is thought to be taken up into plants by active uptake via the sulfate transporter protein located in the root plasma membrane (Leggett and Epstein, 1956), selenite is thought to be taken up passively (Arvy, 1993), whereas SeMet is thought to be taken up actively in a manner similar to that of S-containing amino acids (Sandholm et al., 1973; Abrams et al., 1990). The results from the present study suggest that bacteria in the rhizosphere may stimulate only the sulfate transporter protein and not the SeMet transporter protein. This would explain why bacteria stimulate only selenate accumulation and not selenite or SeMet accumulation in plants (data not shown).
Bacteria that were identified in the laboratory as being superior for enhancing Se phytoextraction and phytovolatilization (Figs. 2 and 3) could be tested under field conditions. For example, germinating bacteria-coated seeds in Se-contaminated soil could make the phytoremediation of selenate-contaminated sites more efficient if the selected bacteria can compete favorably with the resident populations. In addition to being an excellent species for Se phytoremediation (Banuelos and Meek, 1990; Banuelos et al., 1995; Zayed and Terry, 1998), Indian mustard is an excellent candidate for the phytoremediation of metals from contaminated soil and water through processes such as phytoextraction and rhizofiltration (Dushkenov et al., 1995; Kumar et al., 1995). Also, Indian mustard used in conjunction with rhizosphere bacteria that are superior at enhancing plant Se accumulation and volatilization may be used to remove Se from contaminated soils and agricultural drainage water in the San Joaquin Valley and other places where Se contamination is a problem.
Abbreviation:
- SeMet
selenomethionine
Footnotes
This work was supported by the Electric Power Research Institute (grant nos. W08021-30 and W04163 to N.T.).
LITERATURE CITED
- Abrams MM, Shennan C, Zasoski RJ, Burau RG. Selenomethionine uptake by wheat seedlings. Agron J. 1990;82:1127–1130. [Google Scholar]
- Anderson TA, Guthrie EA, Walton BT. Bioremediation in the rhizosphere: plant roots and associated microbes clean contaminated soil. Environ Sci Technol. 1993;27:2630–2636. [Google Scholar]
- Arvy MP. Selenate and selenite uptake and translocation in bean plants (Phaseolus vulgaris) J Exp Bot. 1993;44:1083–1087. [Google Scholar]
- Atkinson R, Aschmann SM, Hasegawa D, Thompson-Eagle ET, Frankenberger WT., Jr Kinetics of the atmospherically important reactions of dimethyl selenide. Environ Sci Technol. 1990;24:1326–1332. [Google Scholar]
- Aubert H, Pinta M (1977) Trace Elements in Soils. Elsevier Science Publishers, New York, pp 5–11
- Azaizeh H, Gowthaman S, Terry N. Microbial selenium volatilization in rhizosphere and bulk soils from a constructed wetland. J Environ Qual. 1997;26:666–672. [Google Scholar]
- Banuelos GS, Cardon G, Mackey B, Ben-Asher J, Wu L, Beuselinck P, Akohoue S, Zambrzuski S. Boron and selenium removal in boron laden soils by four sprinkler irrigated plant species. J Environ Qual. 1993;22:786–792. [Google Scholar]
- Banuelos GS, Meek DW. Accumulation of selenium in plants grown on selenium-treated soil. J Environ Qual. 1990;19:772–777. [Google Scholar]
- Banuelos GS, Terry N, Zayed A, Wu L (1995) Managing high soil Se with phytoremediation. In GE Schuman, GF Vance, eds, Selenium: Mining, Reclamation, and Environmental Impact. Proceedings of the 12th Annual National Meeting of the American Society for Surface Mining and Reclamation. June 3–8, 1995, Gillete, WY. American Society for Surface Mining and Reclamation, Princeton, WV, pp 394–405
- de Souza MP, Pilon-Smits EAH, Lytle CM, Hwang S, Tai J, Honma TSU, Yeh L, Terry N. Rate-limiting steps in selenium assimilation and volatilization by Indian mustard. Plant Physiol. 1998;117:1487–1494. doi: 10.1104/pp.117.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza MP, Yoch DC. Purification and characterization of dimethyl-sulfoniopropionate (DMSP) lyase from an Alcaligenes-like dimethyl sulfide-producing marine isolate. Appl Environ Microbiol. 1995;61:21–26. doi: 10.1128/aem.61.1.21-26.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushkenov V, Kumar PBAN, Motto H, Raskin I. Rhizofiltration: the use of plants to remove heavy metals from aqueous streams. Environ Sci Technol. 1995;29:1239–1245. doi: 10.1021/es00005a015. [DOI] [PubMed] [Google Scholar]
- Evans CS, Asher CJ, Johnson CM. Isolation of dimethyl diselenide and other volatile selenium compounds from Astragalus racemosus (Pursh.) Aust J Biol Sci. 1968;21:13–20. [Google Scholar]
- Fallik E, Sarig S, Okon Y. Morphology and physiology of plant roots associated with Azospirillum. In: Okon Y, editor. Azospirillum/Plant Associations. London: CRC Press; 1994. pp. 77–86. [Google Scholar]
- Frankenberger WT Jr, Karlson U (1994) Microbial volatilization of selenium from soils and sediments. In WT Frankenberger Jr, S Benson, eds, Selenium in the Environment. Marcel Dekker, New York, pp 369–387
- Ganther HE, Levander OA, Saumann CA. Dietary control of selenium volatilization in the rat. J Nutr. 1966;88:55–60. doi: 10.1093/jn/88.1.55. [DOI] [PubMed] [Google Scholar]
- Hansen D, Duda P, Zayed AM, Terry N. Selenium removal by constructed wetlands: role of biological volatilization. Environ Sci Technol. 1998;32:591–597. doi: 10.1021/es0260216. [DOI] [PubMed] [Google Scholar]
- Hoagland D, Arnon DI (1938) The water culture method for growing plants without soil. Bull Calif Agric Stat 346
- Hobbie JE, Daley RJ, Jasper S. Use of nucleopore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977;33:1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapulnik Y (1996) Plant growth promotion by rhizosphere bacteria. In Y Waisel, A Eshel, U Kafkazi, eds, Plant Roots: The Hidden Half. Marcel Dekker, New York, pp 769–781
- Kumar PBAN, Dushkenov V, Motto H, Raskin I. Phytoextraction: the use of plants to remove heavy metals from soils. Environ Sci Technol. 1995;29:1232–1238. doi: 10.1021/es00005a014. [DOI] [PubMed] [Google Scholar]
- Leggett JE, Epstein E. Kinetics of sulfate adsorption by barley roots. Plant Physiol. 1956;31:222–226. doi: 10.1104/pp.31.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesinger T, Margraff R. Secondary metabolites of the fluorescent pseudomonads. Microbiol Rev. 1979;43:422–442. doi: 10.1128/mr.43.3.422-442.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BG. Volatile selenium in higher plants: the production of dimethyl selenide in cabbage leaves by the enzymatic cleavage of methylselenomethionine selenonium salt. PhD thesis. Berkeley: University of California; 1971. [Google Scholar]
- Lewis BG, Johnson CM, Delwiche CC. Release of volatile selenium compounds by plants: collection procedures and preliminary observations. J Agric Food Chem. 1966;14:638–640. [Google Scholar]
- Lin W, Okon Y, Hardy RWF. Enhanced mineral uptake by Zea mays and Sorghum bicolor roots inoculated with Azospirillum brasilense. Appl Environ Microbiol. 1983;45:1775–1779. doi: 10.1128/aem.45.6.1775-1779.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan TJ, Chang AC, Page AL, Ganje TJ. Accumulation of selenium in crops grown on sludge-treated soil. J Environ Qual. 1987;16:349–352. [Google Scholar]
- Losi ME, Frankenberger WT., Jr Reduction of selenium oxyanions by Enterobacter cloacae SLD1a-1: isolation and growth of the bacterium and its expulsion of selenium particles. Appl Environ Microbiol. 1997;63:3079–3084. doi: 10.1128/aem.63.8.3079-3084.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy JM, Lawson S, DeMoll-Decker H. Bioremediation of selenium oxyanions in San Joaquin drainage water using Thauera selenatis in a biological reactor system. Appl Microbiol Biotechnol. 1993;40:588–594. [Google Scholar]
- Manceau A, Gallup DL. Removal of selenocyanate in water by precipitation: characterization of copper-selenium precipitate by x-ray diffraction, infrared, and x-ray absorption spectroscopy. Environ Sci Technol. 1997;31:968–976. [Google Scholar]
- Martin TD. Determining selenium in wastewater sediment and sludge by flameless atomic absorption. Atomic Absorption Newslett. 1975;14:109–116. [Google Scholar]
- McConnell KP, Portman OW. Toxicity of dimethyl selenide in the rat and mouse. Proc Soc Exp Biol Med. 1952;79:230–231. doi: 10.3181/00379727-79-19333. [DOI] [PubMed] [Google Scholar]
- McNeal JM, Balisteri LS (1989) Geochemistry and Occurrence of Selenium: An Overview. Selenium in Agriculture and the Environment. Soil Science Society of America special publication no. 23, Madison, WI
- Ohlendorf HM, Hoffman DJ, Saiki MK, Aldrich TW. Embryonic mortality and abnormalities of aquatic birds: apparent impacts of selenium from irrigation drain water. Sci Total Environ. 1986;52:49–63. [Google Scholar]
- Oremland RS, Switzer-Blum J, Culbertson CW, Visscher PT, Miller LG, Dowdle P, Strohmaier FE. Isolation, growth, and metabolism of an obligately anaerobic, selenate-respiring bacteria, strain SES-3. Appl Environ Microbiol. 1994;60:3011–3019. doi: 10.1128/aem.60.8.3011-3019.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon-Smits EAH, de Souza MP, Hong G, Amini A, Bravo RC, Payabyab ST, Terry N (1998) Selenium volatilization and accumulation by twenty aquatic plant species. J Environ Qual (in press)
- Presser TS, Ohlendorf HM. Biogeochemical cycling of selenium in the San Joaquin Valley, California, USA. Environ Manage. 1987;11:805–821. [Google Scholar]
- Saiki MK, Lowe TP. Selenium in aquatic organisms from subsurface agricultural drainage water, San Joaquin Valley, California. Arch Environ Contam Toxicol. 1987;19:496–499. doi: 10.1007/BF01055416. [DOI] [PubMed] [Google Scholar]
- Sandholm M, Oksanen HE, Pesonen L. Uptake of selenium by aquatic organisms. Limnol Oceanogr. 1973;18:496–498. [Google Scholar]
- Sorensen J (1997) The rhizosphere as a habitat for soil microorganisms. In JD van Elsas, JT Trevors, EMH Wellington, eds, Modern Soil Microbiology, Marcel Dekker, New York, pp 21–45
- Terry N, Carlson C, Raab TK, Zayed AM. Rates of selenium volatilization among crop species. J Environ Qual. 1992;21:341–344. [Google Scholar]
- Terry N, Zayed AM (1994) Selenium volatilization by plants. In WT Frankenberger Jr, S Benson, eds, Selenium in the Environment. Marcel Dekker, New York, pp 343–369
- Terry N, Zayed AM (1998) Phytoremediation of selenium. In WT Frankenberger Jr, RA Engberg, eds, Environmental Chemistry of Selenium. Marcel Dekker, New York, pp 633–657
- Wilber CG. Toxicology of selenium: a review. Clin Toxicol. 1980;17:171–230. doi: 10.3109/15563658008985076. [DOI] [PubMed] [Google Scholar]
- Zayed AM, Lytle CM, Terry N. Accumulation and volatilization of different chemical species of selenium by plants. Planta. 1998;206:284–292. [Google Scholar]
- Zayed AM, Terry N. Selenium volatilization in broccoli as influenced by sulfate supply. J Plant Physiol. 1992;140:646–652. [Google Scholar]
- Zayed AM, Terry N. Selenium volatilization in roots and shoots: effects of shoot removal and sulfate level. J Plant Physiol. 1994;143:8–14. [Google Scholar]