Abstract
In response to the increasingly evident need for herpes simplex virus (HSV) serotype-specific serologic assays that rely on proteins other than glycoprotein-G (gG), we developed a rapid serologic assay that is based on type-specific epitopes within the large subunit of HSV ribonucleotide reductase (R1). The assay (Au-2 enzyme-linked immunosorbent assay [ELISA]) uses an HSV type 2 (HSV-2) R1 peptide antigen. It provides a reliable method for detecting serotype-specific antibody to a protein other than gG-2. The Au-2 ELISA has high sensitivity and specificity as determined by direct comparison to Western blotting, a widely accepted “gold standard,” and to ELISA with an HSV-1 R1 peptide (Au-1). The use of the Au-2 ELISA in conjunction with the gG-2-based assays will improve the sensitivity and specificity of serologic diagnosis and patient management.
Humans are infected by two serotypes of herpes simplex virus (HSV) that share a high degree of genetic homology (19). HSV type 2 (HSV-2) is the primary cause of genital herpes, one of the most common sexually transmitted diseases. Worldwide studies indicate that HSV-2 infection has reached epidemic proportions (1, 15, 24, 26, 58, 65). Virus infection can cause severe generalized disease in the immunocompromised and in neonates, and it increases the risk of infection with human immunodeficiency virus (7, 49). Because virus isolation and typing and/or PCR can only be done during the acute phase of the infection, serological screening is needed for the identification of asymptomatic individuals with past HSV-2 infection (2, 6, 16). Serology-based discrimination between HSV-2 and HSV-1 infection is also important in clinical settings. This includes alerting asymptomatic patients to the risk of frequent recurrences (more frequent for HSV-2 [61]) or acquisition of infection with the HSV serotype for which the patient is negative; confirmation of clinical diagnosis, particularly in patients whose viral cultures are negative; atypical presentation of genital herpes (33); discordance in HSV infection in a sexual partnership; and the identification of patients at risk for adverse sequelae. For example, a pregnant woman, particularly if she is HSV-2 negative but has an HSV-2-infected sexual partner (34), or the asymptomatic patient at risk of complications of the central nervous system (HSV-1 infection can cause encephalitis in adults [18, 32, 62], while HSV-2 infection of the central nervous system is commonly restricted to a self-limiting, nonfatal meningitis [12, 53]). However, detection of type-specific antibody has traditionally been hampered by the extensive homology between the HSV-2 and HSV-1 proteins (19) and the resulting cross-reactivity of the viral antibodies (2, 6, 23, 41).
The presence in HSV-2 glycoprotein G (gG-2) of epitopes that are not cross-reactive with counterparts in HSV-1 gG-1 (38, 40) has led to the development of type-specific serologic assays that are based on the gG protein. Overall, commercially available gG-2-based assays have good levels of sensitivity and specificity (4). However, the use of a single antigen for type-specific serologic diagnosis suffers from a number of limitations, including loss of relevant epitopes during antigen preparation, preferential recognition of type-common immunodominant epitopes by sera from various individuals, variable timing of seroconversion to distinct epitopes, occurrence of gG-2-negative HSV-2 strains, variability of the gG-1 or gG-2 genes among wild-type virus strains, and loss of gG-1 and gG-2 antibodies in a significant percentage of individuals (3, 13, 20, 21, 37, 47, 55). Some studies have reported false positivity, which was attributed to the use of specific assay configurations and/or unique study groups (21, 35, 41, 46). Nonetheless, it is widely accepted that improved specificity and sensitivity will require the exploitation of assays based on type-specific epitopes in HSV proteins other than gG (20, 21, 31, 55). One such protein is the large subunit of HSV ribonucleotide reductase (R1, also known as ICP10 and ICP6 for HSV-2 and HSV-1, respectively), the amino terminus of which has a relatively high proportion of type-specific epitopes (19, 42) and is structurally and functionally distinct in HSV-2 and HSV-1 (5, 6, 9, 44, 45, 56). Here, we report that an enzyme-linked immunosorbent assay (ELISA) based on type-specific epitopes in the amino-terminal domain of the HSV-2 R1 protein can detect serotype-specific antibodies in human sera, and we compare the results to those obtained by Western blotting.
MATERIALS AND METHODS
Viruses, cells, and virus infection.
HSV-2 (strain G) and HSV-1 (strain F) were previously described (6, 45, 56). Vero (African green monkey kidney) cells were grown in minimum essential medium with 10% fetal bovine serum and used for virus growth, as previously described (6).
Patients and sera.
A total of 214 sera were included in these studies. Of these, 192 were from human immunodeficiency virus-negative patients seen in a Baltimore sexually transmitted disease clinic during the 1980s as part of a National Institutes of Health-supported study of the role of T-cell immunity in recurrent HSV outbreaks, and 22 were from patients seen in the dermatology clinics of the University of Maryland Hospital during the last 4 years as part of a National Institutes of Health-supported study of the role of HSV in erythema multiforme. This latter group of patients was diagnosed with recurrent HSV-2 (13 patients) or HSV-1 (9 patients) disease confirmed clinically and by virus isolation and typing. All sera were aliquoted at the time of collection and stored at −80°C until use. The aliquots used in these studies had not been thawed before. Paired sera were obtained from HSV-2-infected mice and consisted of a sample drawn before inoculation and two samples drawn at 2 and 4 weeks after inoculation (seroconversion panels) (10). The generation and specificity of the rabbit antibody to the Au-2 peptide (Au-2 antibody) were previously described (9, 45, 56). Preimmune rabbit serum served as an additional control.
Antigens.
The Au-2 peptide, ARSPSERQEPREPE, was synthesized and conjugated to biotin at the amino terminus. It was purified by high-performance liquid chromatography, and the sequence was verified by microsequencing. Au-2 represents amino acids 13 to 26 of the HSV-2 R1 protein, and it is not present in the HSV-1 R1 homologue (9, 19, 42). The solid-phase synthesis of the Au-2 peptide, its conjugation to keyhole limpet hemocyanin, a known promoter of antigenicity, via an additional Cys residue, and polyclonal antibody production in rabbits were previously described (9). The Au-1 peptide, LAHGHHVYGQRVNG, represents amino acids 38 to 51 within the HSV-1 R1 protein. It is not present in HSV-2 R1. The peptides were used as antigens in the ELISA. Optimal concentrations were determined for each reagent of the test based on results from checkerboard titrations.
Au-2 and Au-1 ELISAs.
The peptides (5 and 500 μg/ml for Au-2 and Au-1, respectively) were adsorbed to MaxiSorp plates (Nalge Nunc International) in 0.1 M sodium bicarbonate buffer (pH 9.2) by incubation at room temperature (RT) for 16 h (overnight) with shaking. The plates were washed three times with distilled water (250 μl/well) to remove unattached peptide and blocked by incubation (30 min) with 1% bovine serum albumin (BSA fraction V) in calcium- and magnesium-free phosphate-buffered saline (1% BSA-CMF PBS; Gibco-BRL). Selected wells were blocked without peptide and used as controls; wells containing antigen, but no serum, were used to normalize absorbance values. Sera were centrifuged at 16,000 × g in an Eppendorf 5415C microcentrifuge in order to remove particulate and lipid contaminants, diluted in 1% BSA-CMF PBS, and added to the plates (100 μl/well). After incubation for 30 min at RT with shaking, the plates were washed three times with CMF PBS-0.005% Tween 20 (250 μl/well) and treated with goat anti-human immunoglobulin G (IgG) conjugated to horseradish peroxidase (diluted 1:10,000 in 1% BSA-CMF PBS; 100 μl/well; Jackson Immunochemicals). They were incubated for 30 min at RT with shaking. The plates were washed three times, and 100 μl of 2,2′-azinobis(3-ethylbenzothiazolinesulfonic acid) substrate (Kirkegaard & Perry Laboratories, Gaithersburg, Md.)/well was added. Following incubation for 30 min at RT, the absorbance was read at 405 nm with a Biotek ELX800 microplate reader blanked on air.
Characterization of the assay.
The analytical response curve was determined by results obtained from twofold serial dilutions of the Au-2 antibody and six human sera, three from HSV-2- and three from HSV-1-infected patients. In addition, the following quality control parameters were used to evaluate the test. For intraassay precision, two sera that represented two levels of reactivity (negative and low-positive) were tested in several microtiter plates; for each one, n = 3 to 8 wells. The intraassay precision was defined as the coefficient of variation (CV) obtained from each set of wells. Briefly, the mean and standard deviation (SD) were calculated and the CV was determined by dividing each SD by each mean and converting to a percentage. Mean CVs were calculated per individual and for the entire data set. For interassay precision, aliquots of these samples were tested in separate microtiter plates (n = 4 to 9). The interassay CV was determined from the mean and the SD. The interassay precision data were used to determine acceptable ranges. Acceptability of each plate reading was decided according to the quality control results. Sensitivity was calculated as follows: [1 − (number of false negatives/total number tested by Western blotting)]. Specificity was defined in the same manner, but the number of false positives was used in place of the false negatives.
Development of a cutoff equation and borderline sera.
Samples presumed to be negative for HSV-2-specific antibody were assayed with the Au-2 peptide. Using the raw optical density (OD) of these samples, a target cutoff value above the mean was arbitrarily determined. Negative and positive human sera were chosen next, based on the mouse seroconversion panels. Finally, a cutoff point was derived to approximate the target cutoff OD based on the HSV-2-negative and -positive samples.
Western blotting.
The Western blot assay was as previously described (56). Vero cells were infected with 10 PFU of HSV-2 or HSV-1/cell, and cell extracts were prepared at 13 h postinfection, when R1 levels are maximal (56). Briefly, infected cells were lysed with radioimmunoprecipitation buffer (20 mM Tris-HCl [pH 7.4], 0.15 mM NaCl, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate) supplemented with phosphatase and protease inhibitor cocktails (Sigma) and sonicated twice for 30 s at 25% output power using the Sonicator/Ultrasonic processor (Misonix, Inc., Farmingdale, N.Y.). Total protein was determined by the bicinchoninic assay (Pierce, Rockford, Ill.), and proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 7% acrylamide gels and transferred to nitrocellulose membranes. The blots were incubated (1 h, RT) in TN-T buffer (0.01 M Tris-HCl [pH 7.4], 0.15 M NaCl, 0.05% Tween 20) containing 5% nonfat dried milk to block nonspecific binding and exposed (1 h, RT) to the appropriate antibodies (diluted in TN-T buffer with 5% milk). After three washes with TN-T buffer, the blots were incubated with anti-human IgG-peroxidase (diluted 1:10,000) for 1 h at RT. In some experiments, protein A-peroxidase (diluted 1:500) was used instead of anti-IgG-peroxidase. Detection was with ECL reagents (Amersham Life Science, Arlington Heights, Ill.) and exposure (1 to 4 min) to high-performance chemiluminescence film (Hyperfilm ECL; Amersham). If the R1 or gG bands were not visualized and fewer than four bands appeared that could be identified as HSV specific, an equivocal profile was reported.
gG-2 based ELISA (Gull Laboratories, Inc., Salt Lake City, Utah).
The assay was performed according to the manufacturer's instructions, using the supplied plates coated with affinity-purified gG-2, reference sera, and all diluents. Absorbance was read at 405 nm, and OD values were considered positive, negative, or borderline according to the instructions.
RESULTS
ELISA with Au-2 peptide.
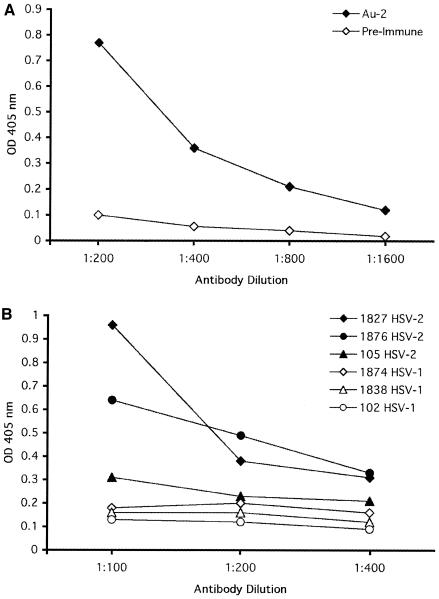
Au-2-coated plates were assayed with twofold-increasing dilutions of IgG purified from the Au-2 antibody and preimmune rabbit serum, used as a control. The absorbance (OD) at a 405-nm wavelength was linear up to a value of 1.9. The highest Au-2 antibody dilution that gave rise to a twofold higher absorbance value above the maximal response seen in the absence of Au-2 peptide or with preimmune serum was 1:1,600 (Fig. 1A). The Au-2 IgG did not react with the Au-1 peptide (OD = 0.03 to 0.04). The ELISA was then repeated with Au-2 peptide and twofold dilutions of nine human sera, five from HSV-2-infected patients (no. 1876, 1827, 105, 1875, and 1880) and four from HSV-1-infected patients (no. 102, 1838, 1874, and 358). The OD values for the HSV-2 sera ranged between 0.3 and 0.95 at a dilution of 1:100. The highest dilution with an OD value higher than 0.25 was 1:400. The highest OD values for the HSV-1 sera ranged between 0.1 and 0.21 at dilutions of 1:100 to 1:400 (Fig. 1B).
FIG. 1.
ELISA with Au-2 antibody and human sera. (A) Increasing dilutions of IgG purified from rabbit Au-2 antibody and preimmune rabbit serum were assayed in the Au-2 ELISA as described in Materials and Methods. (B) Increasing dilutions of sera from patients with HSV-2 or HSV-1 infections confirmed clinically and by virus isolation were assayed in the Au-2 ELISA. Results are shown for representative sera.
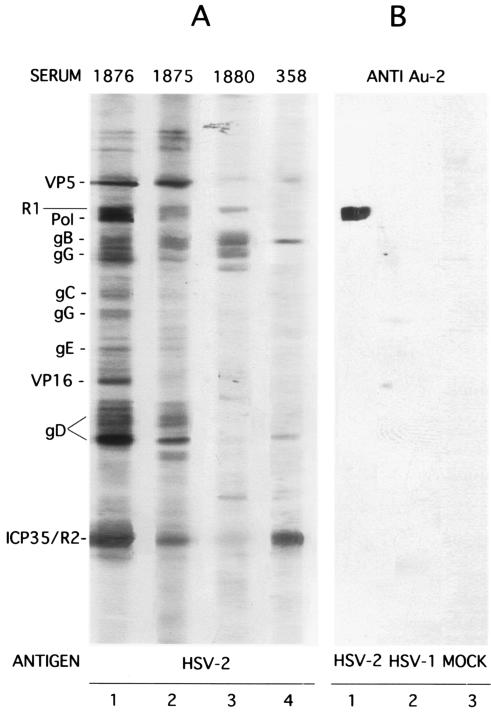
Specificity was confirmed by Western blotting. The Au-2 antibody recognized the R1 protein in extracts from HSV-2-infected (Fig. 2B, lane 1) but not from HSV-1-infected (Fig. 2B, lane 2) or mock-infected (Fig. 2B, lane 3) cells. The human sera, studied in parallel with extracts of HSV-2-infected cells, recognized at least four bands that could be identified as HSV proteins. Representative results shown in Fig. 2A indicate that the sera from HSV-2-infected patients recognized R1 and gG-2 as well as various other, type-common proteins (Fig. 2A, lanes 1 to 3). The sera from HSV-1-infected patients did not recognize the R1 and gG-2 proteins, but they were positive for type-common proteins (Fig. 2A, lane 4).
FIG. 2.
Sera from HSV-2 patients recognize HSV-2 R1 in Western blot analysis. HSV-2 (no. 1876, 1875, and 1880) and HSV-1 (no. 358) human sera and rabbit Au-2 antibody (anti-Au-2) were assayed by Western blotting with extracts of HSV-2-, HSV-1-, or mock-infected cells. Recognized HSV bands are listed in the left margin.
Multiple additional determinations were performed in order to examine the reproducibility of the Au-2 ELISA in four to nine separate assays and on different plates. The absorbance values for three HSV-2 sera ranged between 0.33 and 0.5, with a median value of 0.37 and a mean value of 0.38. Absorbance values for three HSV-1 sera were 0.17 to 0.2, with a median value of 0.16 and a mean value of 0.17. The intraassay precision was less than 5%, with an interassay precision of less than 10%. Essentially similar results (7 to 8% interassay precision) were obtained using different aliquots of the Au-2 peptide stored at −80°C as 1-mg/ml stock solutions, or with antigen-coated plates stored at −20°C for up to 1 month. Additional controls consisted of three to six wells lacking various components of the ELISA in each assay. The mean OD for wells containing all components except for patient serum was 0.09 ± 0.005 (n = 26 plates). The mean OD of wells containing antigen alone was 0.08 ± 0.003 (n = 5 plates). Wells containing conjugate alone had a mean OD value of 0.09 ± 0.005 (n = 20 plates), and wells that contained sera (from HSV-2-positive or -negative patients) and conjugate, but no antigen, had mean OD values of 0.11 ± 0.01 (n = 17 plates). Collectively, these controls support the conclusion that the sera from HSV-2-infected patients specifically bind to the Au-2 peptide antigen.
Definition of the Au-2 and Au-1 ELISA protocols.
The following variables were examined in a checkerboard fashion using 22 sera with Western blotting-confirmed HSV-2 or HSV-1 specificity: (i) Au-2 peptide concentration (5, 25, and 50 μg/ml); (ii) serum dilution (1:50, 1:100, 1:200, and 1:400); (iii) time of incubation with substrate (30, 50, 60, and 70 min); and (iv) reaction temperature (RT and 37°C). Absorbance values were normalized by subtracting the mean control (no-serum) value plus 2 SD of the mean. Sensitivity was calculated as follows: [1 − (number of false negatives/total number of sera tested by Western blotting)]. Specificity was defined in the same manner, but the number of false positives was used in place of false negatives. For all these conditions, sensitivity ranged between 86.3 and 100% and specificity ranged between 82 and 100%. The highest sensitivity and specificity were obtained with 5 μg of Au-2 peptide/ml, a 1:50 serum dilution, and 30 min of incubation with substrate at RT. Consequently, this protocol was adopted for the Au-2 ELISA. Similar analyses using the Au-1 peptide led to the adoption of a protocol in which the peptide was used at 500 μg/ml and the serum dilution was 1:200.
Au-2 ELISA: determination of cutoff value.
In order to define a cutoff value, it was necessary to test sera negative for HSV-2, even when positive for HSV-1 antibody. They included rabbit and mouse preimmune sera and sera from nine patients with HSV-1 infection confirmed clinically and by virus isolation who denied a history of HSV-2 infection. The mean OD value of the preimmune rabbit and mouse sera was 0.12. The target cutoff value (0.17) was arbitrarily set at 10 SD above this mean. A cutoff equation [(negative control OD/2) + (positive control OD/4)] was formulated using negative and positive sera from the mouse seroconversion series (obtained before infection and at 2 and 4 weeks after infection). It yielded cutoff values of 0.18, 0.19, and 0.19 for three plates used to test these sera. The highest OD value of the human sera (0.17) was lower than the calculated cutoff values. Nonetheless, in order to increase reproducibility, OD values ranging between 0.19 and 0.21 were considered borderline and not specifically attributable to either HSV-1 or HSV-2 infection.
Reactivity of human sera with Au-2 and Au-1 peptides.
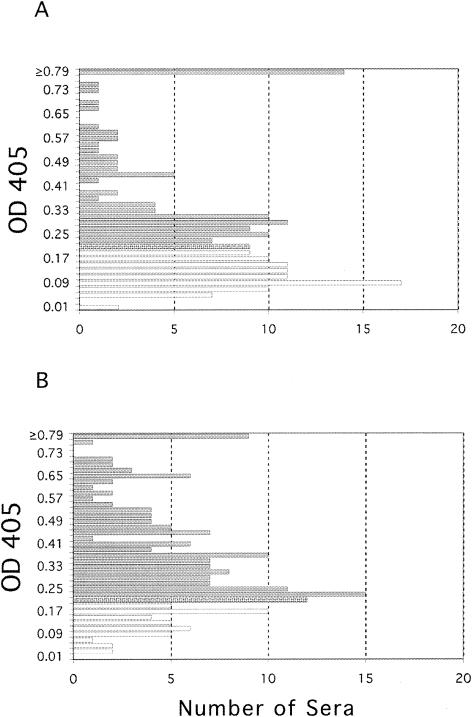
In order to examine the specificity of the ELISA, we compared the reactivity profiles of our sera with the Au-2 and Au-1 peptide antigens. Sera collected from 192 subjects with an unknown history of HSV-2 infection were assayed by Au-2 ELISA, and the results are summarized in the histogram shown in Fig. 3A. Sera with normalized OD values below 0.19 were considered HSV-2 negative (n = 88). Values between 0.19 and 0.21 were considered borderline (n = 9), and values above 0.21 were considered HSV-2 positive (n = 95). Most of the sera had OD values ranging between 0.25 and 0.4. OD values of ≥0.79 were seen in 14 sera. Overall, 49.5% of the sera were positive for HSV-2, 45.8% were negative for HSV-2, and 4.7% were inconclusive. As a control for specificity in the ELISA itself, the same sera were then assayed with the Au-1 peptide (Au-1 ELISA), and the results are summarized in the histogram shown in Fig. 3B. Sera with normalized absorbance values below 0.19 (n = 40) were considered negative, values between 0.19 and 0.21 (n = 12) were considered borderline, and values greater than 0.21 (n = 138) were considered positive. Overall, 72.6% of the sera were positive for HSV-1, 21% were HSV-1 negative, and 6.3% were inconclusive. The patterns of reactivity for both the Au-2 peptide (49% positive) and the Au-1 peptide (73% positive) reflected the well-established proportions of HSV-2- and HSV-1-infected individuals in similar populations (58, 64, 65), suggesting that the peptides specifically bind to HSV-2 or HSV-1 antibodies in the patient sera. We interpret the data to suggest that the assay is functional and capable of differentiating between the Au-2 and Au-1 antigens.
FIG. 3.
Reactivity of human sera with Au-2 and Au-1 peptides. (A) Histogram summarizing the reactivity of human sera with Au-2 peptide. (B) Histogram summarizing reactivity of the same sera with Au-1 peptide.
Western blotting confirmation of the Au-2 ELISA with human sera.
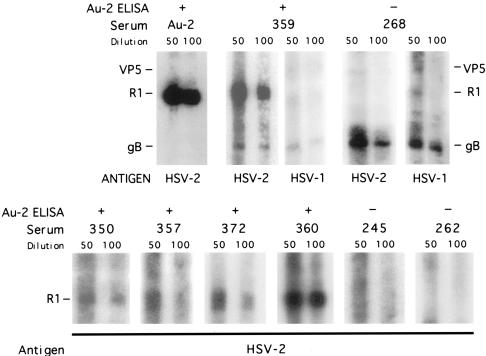
The specificity of the Au-2 ELISA was next confirmed by Western blotting, which is the accepted specificity standard. All the available sera (a total of 131) were assayed both by ELISA with Au-2 and Au-1 antigens and by Western blotting with extracts of HSV-2-infected cells. Results are shown in Fig. 4 for representative sera and are summarized for all sera in Table 1. Sera positive in the Au-2 ELISA recognized the HSV-2, but not the HSV-1, R1 protein, independent of the ability to react with other type-common proteins. Sera negative in the Au-2 ELISA did not recognize the HSV-2 R1 protein, but they could recognize the HSV-1 R1 protein, as shown for one such serum (no. 268). The overall correlation between the results obtained by Au-2 ELISA and Western blotting is summarized in Table 1. Using the Western blotting results as the standard, 66 sera (50.4%) were positive for HSV-2 R1 and 57 of these were also positive by Au-2 ELISA, for a sensitivity of 94.6%. Sixty-two sera were negative for HSV-2 R1 by Western blotting and 52 of these were also negative by Au-2 ELISA, for a specificity of 95.4%.
FIG. 4.
Correlation of Au-2 ELISA with Western blotting. Western blotting results are shown for eight representative human sera, compared to Au-2 ELISA results. Data for all sera are summarized in Table 1.
TABLE 1.
Direct comparison of Au-2 ELISA to Western blotting results
| Western blotting result (no.) | No. with ELISA resulta
|
||
|---|---|---|---|
| Positive | Negative | Borderline | |
| Positive (66) | 57 | 7 | 2 |
| Negative (62) | 6 | 52 | 4 |
| Borderline positive (3) | 3 | 0 | 0 |
| Total (131) | |||
Sensitivity, 94.7%; specificity, 95.4%.
Comparison of the Au-2 and Gull gG-2 ELISAs.
The performance of the Au-2 ELISA was directly compared to that of the Gull gG-2 ELISA for 112 sera, using Western blotting as the standard. Of the 54 sera positive by Western blotting, 47 were positive by Au-2 ELISA and 44 were positive by Gull ELISA. Of the 55 Western blotting-negative sera, 47 were also negative by the Au-2 ELISA, but only 11 were negative by the Gull ELISA. Of three sera considered borderline positive by Western blotting, all were positive by Au-2 ELISA and two were positive by Gull ELISA. Four of six sera considered borderline by Au-2 ELISA were negative by Western blotting, and three of the six Gull borderline sera were also negative by Western blotting. The data suggest that (i) the Gull assay has relatively low specificity and (ii) the Au-2 ELISA identifies a distinct patient subset.
DISCUSSION
Laboratory diagnosis of clinically overt HSV infections is based on virus isolation and PCR. However, serotype-specific assays are needed for the diagnosis of latently infected patients. Because Western blotting identifies antibody reactivity with separated protein bands, it is considered the “gold standard” for detection of type-specific antibodies (4, 20, 50). However, the assay is complex and time-consuming, it requires a specialized laboratory and it is not cost-effective. Accordingly, simpler assays have been developed, and all are based on the amino-terminal, cell-associated, domain of gG-2 that contains type-specific epitopes (27, 36). These assays include immunodot and various ELISA configurations with gG-2 purified from virus-infected cells, recombinant truncated gG-2 produced in baculovirus, or synthetic peptides that represent type-specific epitopes (4, 25, 28, 29, 31, 36, 40, 43, 52, 59, 63). Three assays are commercially available. They use lectin-purified gG (54, 59) or recombinant gG produced in baculovirus (46), and they distinguish between HSV-1 and HSV-2 antibodies (4). However, the reliability of a diagnostic assay that is based on type-specific epitopes in a single viral protein (viz. gG-2) suffers from a number of potential limitations. These result from (i) decreased gG-2 antibody production from acyclovir therapy (13, 61), (ii) infection with gG-2-negative HSV-2 strains (37), (iii) general genetic variability of gG in wild-type HSV strains (37, 48), (iv) false-positive results in some populations (30, 35), (v) positive-to-negative shifts in gG-1- and gG-2-based seroassays in a significant percentage of individuals independent of the test format (55), and (vi) discordant interpretation of the results (54). A relatively low concordance between the results obtained by virus isolation and gG-based serology was also reported and ascribed to the late time of gG antibody appearance relative to antibodies that target type-common antigens (21, 48, 55). While gG-2-based diagnosis is reliable overall, and at least some of the same problems might also apply to assays based on other viral proteins, data interpretation would greatly benefit from the concomitant use of assays that are based on serotype-specific epitopes in proteins other than gG-2 (20, 31, 55) and/or type-common antigens (48). This is particularly true for patients lacking symptoms or a history of genital HSV, for whom confirmation of a positive test is particularly desirable (4).
The Au-2 ELISA uses serotype-specific peptides in the R1 protein to selectively capture type-specific antibody in human sera. It is based on previous findings that the N-terminal domain of the HSV-2 R1 protein is unique and contains predominant type-specific epitopes (5, 6, 9, 19, 44, 45, 56, 57). Cutoff values used in various ELISA configurations were determined by adding a constant factor to the mean of the negative controls or adding a specified number of SD to the OD of the negative samples (17). In order to achieve optimum specificity, the target cutoff value of the Au-2 ELISA was arbitrarily set at 10 SD above the mean OD of preimmune rabbit and mouse sera. After this target cutoff value was established, a cutoff value was developed using positive and negative sera from a mouse seroconversion panel. Both negative and positive controls were tested in each assay, and these tailored cutoff values were confirmed by testing of sera from patients with infection confirmed by virus isolation. Nonetheless, in order to increase reproducibility, we recommend that cutoff values between 0.19 and 0.21 be considered borderline or inconclusive and subjected to Western blot confirmation. Using these criteria, and with Western blotting as the gold standard, the sensitivity of the Au-2 ELISA was 94.6% and its specificity was 95.4%. Contrary to premarket evaluations (4), the Gull gG-2 ELISA had a relatively high level of false positivity, consistent with recent findings that the specificity of this assay is only 48% for HSV-2 (35). Significantly, the Gull assay is no longer available, and its problems are probably not shared by other gG-2-based assays. Notwithstanding, these difficulties underscore the need for a confirmatory assay that is based on other serotype-specific proteins, such as that provided by the Au-2 ELISA.
The Au-2 ELISA developed in this study provides a reliable method for detecting serotype-specific antibody to a protein other than gG-2. The assay demonstrated high sensitivity and specificity as determined by direct comparison to Western blotting, which is a widely accepted standard. It is based on type-specific epitopes in the HSV-2 R1 protein, which seems to be a particularly good target for serologic assays because its expression is associated with reactivation of latent virus (8, 60) and there is some evidence of continuous virus reactivation (22) and, therefore, frequent immunological boosting. Previous studies described a level of antigenic variability in HSV-2 isolates by using antibodies that recognize sequences within the carboxy-terminal domain of the R1 protein (11), which interacts with the small RR subunit (14). Antigenic variability was not reported for the amino-terminal domain of the HSV-2 R1 protein in which the Au-2 peptide is located, at least to the extent to which it was investigated (44). While such variability cannot be excluded, its risk is reduced by the small size of the peptide compared to a full-length protein, such as gG-2. Arguing in favor of its conservation, the peptide is located within a stretch of 69 amino acids which are required for R1 protein expression (39) and, thereby, virus growth (57). Additional studies are needed in order to verify the validity of these interpretations. We advocate the use of the Au-2 assay in conjunction with, rather than instead of, the gG-2 assay. Such use should improve data interpretation, the sensitivity and specificity of serologic diagnosis, and patient management.
REFERENCES
- 1.Armstrong, G. L., J. Schillinger, L. Markowitz, A. J. Nahmias, R. E. Johnson, G. M. McQuillan, and M. E. St. Louis. 2001. Incidence of herpes simplex virus type 2 infection in the United States. Am. J. Epidemiol. 153:912-920. [DOI] [PubMed] [Google Scholar]
- 2.Ashley, R. L., and A. Wald. 1999. Genital herpes: review of the epidemic and potential use of type-specific serology. Clin. Microbiol. Rev. 12:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashley, R. L., M. Eagleton, and N. Pfeiffer. 1999. Ability of a rapid serology test to detect seroconversion to herpes simplex virus type 2 glycoprotein G soon after infection. J. Clin. Microbiol. 37:1632-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashley, R. L. 2002. Performance and use of HSV type-specific serology test kits. Herpes 9:38-45. [PubMed] [Google Scholar]
- 5.Aurelian, L. 1998. Herpes simplex virus type 2: unique biological properties include neoplastic potential mediated by the PK domain of the large subunit of ribonucleotide reductase. Front. Biosci. 3:237-249. [DOI] [PubMed] [Google Scholar]
- 6.Aurelian, L. 2000. Herpes simplex viruses, p. 384-409. In S. Specter, R. L. Hodinka, S. A. Young (ed.), Clinical virology manual, 3rd ed. ASM Press, Washington, D.C.
- 7.Aurelian, L. (ed). 1990. Herpesviruses, the immune system and AIDS. Developments in medical virology 6. Kluwer Academic Publishers, Boston, Mass.
- 8.Aurelian, L., and C. C. Smith. 2000. Herpes simplex virus type 2 growth and latency reactivation by co-cultivation are inhibited with antisense oligonucleotides complementary to the translation initiation site of the large subunit of ribonucleotide reductase (RR1). Antisense Nucleic Acid Drug Dev. 10:77-85. [DOI] [PubMed] [Google Scholar]
- 9.Aurelian, L., P. Terzano, C. C. Smith, T. D. Chung, A. Shamsuddin, S. Costa, and C. Orlandi. 1989. Amino terminal epitope of herpes simplex virus type 2 ICP10 protein as a molecular diagnostic marker for cervical intraepithelial neoplasia. Cancer Cells 7:187-191. [Google Scholar]
- 10.Aurelian, L., S. Yasumoto, and C. C. Smith. 1988. Antigen-specific immune suppressor factor in herpes simplex virus type 2 infections of UVB-irradiated mice. J. Virol. 62:2520-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bapat, A. R., S. P. Grill, L. M. Nutter, and Y.-C. Cheng. 1987. Study of ribonucleotide reductase in cells infected with six clinical isolates of herpes simplex virus type 2 (HSV-2) with mutations in its larger subunit. Virology 161:249-251. [DOI] [PubMed] [Google Scholar]
- 12.Bergstrom, T., A. Vahlne, K. Alestig, S. Jeansson, M. Forsgren, and E. Lycke. 1990. Primary and recurrent herpes simplex virus type 2-induced meningitis. J. Infect. Dis. 162:322-330. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein, D. L., M. A. Lovett, and Y. J. Bryson. 1984. The effects of acyclovir on antibody response to herpes simplex virus in primary genital herpetic infections. J. Infect. Dis. 150:7-13. [DOI] [PubMed] [Google Scholar]
- 14.Chung, T. D., J. Luo, J. P. Wymer, C. C. Smith, and L. Aurelian. 1991. Leucine repeats in the large subunit of herpes simplex virus type 2 (HSV-2) ribonucleotide reductase (ICP10) are involved in RR activity and subunit complex formation. J. Gen. Virol. 72:1139-1144. [DOI] [PubMed] [Google Scholar]
- 15.Corey, L. 2002. Challenges in genital herpes simplex virus management. J. Infect. Dis. 186:S29-S33. [DOI] [PubMed] [Google Scholar]
- 16.Cowan, F. M. 2000. Testing for type-specific antibody to herpes simplex virus—implications for clinical practice. J. Antimicrob. Chemother. 45:S9-S13. [DOI] [PubMed] [Google Scholar]
- 17.Dawson, G. J., K. H. Chau, C. M. Cabal, P. O. Yarbough, G. R. Reyes, and I. K. Mushahwar. 1992. Solid phase enzyme-linked immunosorbent assay for hepatitis E virus IgG and IgM antibodies utilizing recombinant antigens and synthetic peptides J. Virol. Methods 38:175-186. [DOI] [PubMed] [Google Scholar]
- 18.Dennett, C., G. M. Cleator, and P. E. Klapper. 1997. HSV-1 and HSV-2 in herpes simplex encephalitis: a study of sixty-four cases in the United Kingdom. J. Med. Virol. 53:1-3. [DOI] [PubMed] [Google Scholar]
- 19.Dolan, A., F. E. Jamieson, C. Cunningham, B. C. Barnett, and D. J. McGeogh. 1998. The genome sequence of herpes simplex virus type 2. J. Virol. 72:2010-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eing, B. R., L. Lippelt, E. U. Lorentzen, W. Hafezi, W. Schlumberger, K. Steinhagen, and J. E. Kuhn. 2002. Evaluation of confirmatory strategies for detection of type-specific antibodies against herpes simplex virus type 2. J. Clin. Microbiol. 40:407-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eis-Hubinger, A. M., M. Daumer, B. Matz, and K. E. Schneweis. 1999. Evaluation of three glycoprotein G2-based enzyme immunoassays for detection of antibodies to herpes simplex virus type 2 in human sera. J. Clin. Microbiol. 37:1242-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman, L. T., A. R. Ellison, C. C. Voytek, L. Yang, P. Krause, and T. P. Margolis. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc. Natl. Acad. Sci. USA 99:978-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Field, R. R., D. W. T. Ho, W. L. Irving, I. D. Isaacs, and A. C. Cunningham. 1993. The reliability of serological tests for the diagnosis of genital herpes: a critique. Pathology 25:175-179. [DOI] [PubMed] [Google Scholar]
- 24.Fisman, D. N., M. Lipsitch, E. W. Hook III, and S. J. Goldie. 2002. Projection of the future dimensions and costs of the genital herpes simplex type 2 epidemic in the United States. Sex. Transm. Dis. 29:608-622. [DOI] [PubMed] [Google Scholar]
- 25.Gopal, R., T. Gibbs, M. J. Slomka, J. Whitworth, L. M. Carpenter, A. Vyse, and D. W. Brown. 2000. A monoclonal blocking EIA for herpes simplex virus type 2 antibody: validation for seroepidemiological studies in Africa. J. Virol. Methods 87:71-80. [DOI] [PubMed] [Google Scholar]
- 26.Gottlieb, S. L., J. M. Douglas, Jr., D. S. Schmid, G. Bolan, M. Iatesta, C. K. Malotte, J. Zenilman, M. Foster, A. E. Barón, J. F. Steiner, T. A. Peterman, M. L. Kamb, et al. 2002. Seroprevalence and correlates of herpes simplex virus type 2 infection in five sexually transmitted-disease clinics. J. Infect. Dis. 186:1381-1389. [DOI] [PubMed] [Google Scholar]
- 27.Grabowska, A., C. Jameson, P. Laing, S. Jeansson, E. Sjogren-Jansson, J. Taylor, A. Cunningham, and W. L. Irving. 1999. Identification of type-specific domains within glycoprotein G of herpes simplex virus type 2 (HSV-2) recognized by the majority of patients infected with HSV-2, but not by those infected with HSV-1. J. Gen. Virol. 80:1789-1798. [DOI] [PubMed] [Google Scholar]
- 28.Hashido, M., F. K. Lee, S. Inouye, and T. Kawana. 1997. Detection of herpes simplex virus type specific antibodies by an enzyme-linked immunosorbent assay based on glycoprotein G. J. Med. Virol. 53:319-323. [DOI] [PubMed] [Google Scholar]
- 29.Ho, D. W., P. R. Field, W. L. Irving, D. R. Packham, and A. L. Cunningham. 1993. Detection of immunoglobulin M antibodies to glycoprotein G-2 by Western blot (immunoblot) for diagnosis of initial herpes simplex virus type 2 genital infections. J. Clin. Microbiol. 31:3157-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogrefe, W., X. Su, J. Song, R. Ashley, and L. Kong. 2002. Detection of herpes simplex virus type 2-specific immunoglobulin G antibodies in African sera by using recombinant gG2 Western blotting and gG2 inhibition. J. Clin. Microbiol. 40:3635-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikoma, M., J.-A. Liljeqvist, J. Groen, K. L. Glazenburg, T. T. Hauw, and S. Welling-Wester. 2002. Use of a fragment of glycoprotein G-2 produced in the baculovirus expression system for detecting herpes simplex virus type 2-specific antibodies. J. Clin. Microbiol. 40:2526-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleinschmidt-Demasters, B. K., and D. H. Gilden. 2001. The expanding spectrum of herpesvirus infections of the nervous system. Brain Pathol. 11:440-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koutsky, L. A., R. L. Ashley, K. K. Holmes, C. E. Stevens, C. W. Critchlow, N. Kiviat, C. M. Lipinski, P. Wolner-Hanssen, and L. Corey. 1990. The frequency of unrecognized type 2 herpes simplex virus infection among women: implications for the control of genital herpes. Sex. Transm. Dis. 17:90-94. [DOI] [PubMed] [Google Scholar]
- 34.Kulhanjian, J. A., V. Soroush, D. S. Au, R. N. Bronzan, L. L. Yasukawa, L. E. Weylman, A. M. Arvin, and C. G. Prober. 1992. Identification of women at unsuspected risk of primary infection with herpes simplex virus type 2 during pregnancy. N. Engl. J. Med. 326:916-920. [DOI] [PubMed] [Google Scholar]
- 35.Leach, C. T., R. L. Ashley, J. Baillargeon, and H. B. Jenson. 2002. Performance of two commercial glycoprotein G-based enzyme immunoassays for detecting antibodies to herpes simplex viruses 1 and 2 in children and young adolescents. Clin. Diagn. Lab. Immunol. 9:1124-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levi, M., U. Ruden, H. Carlberg, and B. Wahren. 1999. The use of peptides from glycoproteins G-2 and D-1 for detecting herpes simplex virus type 2 and type-common antibodies. J. Clin. Virol. 12:243-252. [DOI] [PubMed] [Google Scholar]
- 37.Liljeqvist, J. Å., B. Svennerholm, and T. Bergström. 1999. Herpes simplex virus type 2 glycoprotein G-negative clinical isolates are generated by single frameshift mutations. J. Virol. 73:9796-9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liljeqvist, J. Å., E. Trybala, B. Svennerholm, S. Jeansson, E. Sjogren-Jannson, and T. Bergström. 1998. Localization of type-specific epitopes of herpes simplex virus type 2 glycoprotein G recognized by human and mouse antibodies. J. Gen. Virol. 79:1215-1224. [DOI] [PubMed] [Google Scholar]
- 39.Luo, J. H., C. C. Smith, M. Kulka, and L. Aurelian. 1991. A truncated protein kinase domain of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) expressed in Escherichia coli. J. Biol. Chem. 266:20976-20983. [PubMed] [Google Scholar]
- 40.Marsden, H. S., K. MacAulay, J. Murray, and I. W. Smith. 1998. Identification of an immunodominant sequential epitope in glycoprotein G of herpes simplex virus type 2 that is useful for serotype-specific diagnosis. J. Med. Virol. 56:79-84. [DOI] [PubMed] [Google Scholar]
- 41.Martins, T. B., R. D. Woolstenhulme, T. D. Jaskowski, H. R. Hill, and C. M. Litmin. 2001. Comparison of four enzyme immunoassays with a Western blot assay for the determination of type-specific antibodies to herpes simplex virus. Am. J. Clin. Pathol. 115:272-277. [DOI] [PubMed] [Google Scholar]
- 42.Nikas, I., J. McLauchlan, A. J. Davison, W. R. Taylor, and J. B. Clements. 1986. Structural features of ribonucleotide reductase. Proteins Struct. Funct. Genet. 1:376-384. [DOI] [PubMed] [Google Scholar]
- 43.Oladepo, D. K., P. E. Klapper, and H. S. Marsden. 2000. Peptide based enzyme-linked immunoassays for detection of anti-HSV-2 IgG in human sera. J. Virol. Methods 87:63-70. [DOI] [PubMed] [Google Scholar]
- 44.Peng, T., J. R. C. Hunter, and J. W. Nelson. 1996. The novel protein kinase and the RR1 subunit of herpes simplex virus has autophosphorylation and transphosphorylation activity that differs in its ATP requirements for HSV-1 and HSV-2. Virology 216:184-196. [DOI] [PubMed] [Google Scholar]
- 45.Perkins, D., E. F. R. Pereira, and L. Aurelian. 2003. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) functions as a dominant regulator of apoptosis in hippocampal neurons by activating the ERK survival pathway and upregulating the antiapoptotic protein Bag-1. J. Virol. 77:1292-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prince, H. E., C. E. Ernst, and W. R. Hogrefe. 2000. Evaluation of an enzyme immunoassay system for measuring herpes simplex virus (HSV) type 1-specific and HSV type 2-specific IgG antibodies. J. Clin. Lab. Anal. 14:13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rekabdar, E., P. Tunback, J. A. Liljeqvist, and T. Bergstrom. 1999. Variability of the glycoprotein G gene in clinical isolates of herpes simplex virus type 1. Clin. Diagn. Lab. Immunol. 6:826-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rekabdar, E., P. Tunback, J.-A. Liljeqvist, M. Lindh, and T. Bergstrom. 2002. Dichotomy of glycoprotein G gene in herpes simplex virus type 1 isolates J. Clin. Microbiol. 40:3245-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Renzi, C., J. M. Douglas, Jr., M. Foster, C. W. Critchlow, R. Ashley-Morrow, S. P. Buchbinder, B. A. Koblin, D. J. McKirnan, K. H. Mayer, and C. L. Celum. 2003. Herpes simplex virus type 2 infection as a risk factor for human immunodeficiency virus acquisition in men who have sex with men. J. Infect. Dis. 187:19-25. [DOI] [PubMed] [Google Scholar]
- 50.Safrin, S., A. Arvin, J. Mills, and R. Ashley. 1992. Comparison of the Western immunoblot assay and a glycoprotein G enzyme immunoassay for detection of serum antibodies to herpes simplex virus type 2 in patients with AIDS. J. Clin. Microbiol. 30:1312-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samarai, A., A. Shareef, G. Kinghorn, and C. Potter. 1989. Sequential genital infections with herpes simplex virus types 1 and 2. Genitourin. Med. 65:39-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sánchez-Martínez, D., D. S. Schmid, W. Whittington, D. Brown, W. C. Reeves, S. Chatterjee, R. J. Whitley, and P. E. Pellett. 1991. Evaluation of a test based on baculovirus-expressed glycoprotein G for detection of herpes simplex virus type-specific antibodies. J. Infect. Dis. 164:1196-1199. [DOI] [PubMed] [Google Scholar]
- 53.Sauerbrei, A., U. Eichhorn, G. Hottenrott, and P. Wutzler. 2000. Virological diagnosis of herpes simplex encephalitis. J. Clin. Virol. 17:31-36. [DOI] [PubMed] [Google Scholar]
- 54.Saville, M., D. Brown, C. Burgess, K. Perry, S. Barton, F. Cowan, G. Palu, and C. Mengoli. 2000. An evaluation of near patient tests for detecting herpes simplex virus type-2 antibody. Sex. Transm. Infect. 76:381-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmid, D. S., D. R. Brown, R. Nisenbaum, R. L. Burke, D. Alexander, R. Ashley, P. E. Pellett, and W. C. Reeves. 1999. Limits in reliability of glycoprotein G-based type-specific serologic assays for herpes simplex virus types 1 and 2. J. Clin. Microbiol. 37:376-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith, C. C., J. Nelson, L. Aurelian, M. Gober, and B. B. Goswami. 2000. Ras-GAP binding and phosphorylation by herpes simplex virus type 2 RR1 PK (ICP10 PK) and activation of the Ras/MEK/MAPK mitogenic pathway are required for timely onset of virus growth. J. Virol. 74:10417-10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith, C. C., T. Peng, M. Kulka, and L. Aurelian. 1998. The PK domain of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) is involved in IE gene transcription and virus growth. J. Virol. 72:9131-9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith, J. S., and N. J. Robinson. 2002. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J. Infect. Dis. 186:S3-S28. [DOI] [PubMed] [Google Scholar]
- 59.Svennerholm, B., S. Olofsson, S. Jeansson, A. Vahlne, and E. Lycke. 1984. Herpes simplex virus type-selective enzyme linked immunosorbent assay with Helix pomatia lectin-purified antigens. J. Clin. Microbiol. 19:235-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wachsman, M., M. Kulka, C. C. Smith, and L. Aurelian. 2001. A growth and latency defective herpes simplex virus type 2 mutant (ICP10ΔPK) has prophylactic and therapeutic protective activity in guinea pigs. Vaccine 19:1879-1890. [DOI] [PubMed] [Google Scholar]
- 61.Wald, A., L. Corey, R. Cone, A. Hobson, G. Davis, and J. Zeh. 1997. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J. Clin. Investig. 99:1092-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whitley, R. J., and F. Lakeman. 1995. Herpes simplex virus infections of the central nervous system: therapeutic and diagnostic considerations. Clin. Infect. Dis. 20:414-420. [DOI] [PubMed] [Google Scholar]
- 63.Whittingon, W. L., C. L. Celum, A. Cent, and R. L. Ashley. 2001. Use of a glycoprotein G-based type specific assay to detect antibodies to herpes simplex virus type 2 among persons attending sexually transmitted clinics. Sex. Transm. Dis. 28:99-104. [DOI] [PubMed] [Google Scholar]
- 64.Wutzler, P., H. W. Doerr, I. Färber, U. Eichhorn, B. Helbig, A. Sauerbrei, A. Brandstädt, and H. F. Rabenau. 2000. Seroprevalence of herpes simplex virus type 1 and type 2 in selected German populations—relevance for the incidence of genital herpes. J. Med. Virol. 61:201-207. [DOI] [PubMed] [Google Scholar]
- 65.Xu, F., J. A. Schillinger, M. R. Sternberg, R. E. Johnson, F. K. Lee, A. J. Nahmias, and L. E. Markowitz. 2002. Seroprevalence and coinfection with herpes simplex virus type 1 and type 2 in the United States, 1988-1994. J. Infect. Dis. 185:1019-1024. [DOI] [PubMed] [Google Scholar]