Abstract
Neuroimaging biomarkers that precede cognitive decline have the potential to aid early diagnosis of Alzheimer's disease (AD). A body of diffusion tensor imaging (DTI) work has demonstrated declines in white matter (WM) microstructure in AD and its typical prodromal state, amnestic mild cognitive impairment. The present review summarizes recent evidence suggesting that WM integrity declines are present in individuals at high AD-risk, prior to cognitive decline. The available data suggest that AD-risk is associated with WM integrity declines in a subset of tracts showing decline in symptomatic AD. Specifically, AD-risk has been associated with WM integrity declines in tracts that connect grey matter structures associated with memory function. These tracts include parahippocampal WM, the cinglum, the inferior fronto-occipital fasciculus, and the splenium of the corpus callosum. Preliminary evidence suggests that some AD-risk declines are characterized by increases of radial diffusivity, raising the possibility that a myelin-related pathology may contribute to AD onset. These findings justify future research aimed at a more complete understanding of the neurobiological bases of DTI-based declines in AD. With continued refinement of imaging methods, DTI holds promise as a method to aid identification of presymptomatic AD.
Keywords: DTI, diffusion tensor imaging, APOE, presymptomatic, Alzheimer's, Alzheimer's risk
Introduction
Alzheimer's disease (AD) is the most common form of dementia. AD initially impairs memory function, but gradually progresses to affect other domains of cognition. Eventually AD results in the inability to communicate, recognize family members, or function independently. Recent estimates suggest that AD affects 4.5 million Americans and is projected to affect 13.2 million by 2050 [1]. There is now strong evidence that significant Alzheimer's neuropathology is present years before the appearance of clinical symptoms associated with amnestic mild cognitive impairment (aMCI) or AD [2, 3]. Consistent with this evidence, by the time of diagnosis, the AD brain shows widespread neural loss, cortical thinning, ventricular enlargement and demyelination.
At present, AD treatment options are limited. Currently available pharmacological therapies slow cognitive decline only for about a year. Although new interventions are under investigation, they are likely to be more successful by preventing rather than reversing neurodegneration. There is an emerging consensus that the focus of AD research should move toward identification of presymptomatic biomarkers [4, 5]. Neuroimaging is capable of playing an important role in the development of presymptomatic AD biomarkers. Indeed, newly proposed criteria for AD and its preclinical states support the use of imaging and other biomarkers to enhance early detection in those likely to develop clinical AD (http://www.alz.org/research/diagnostic_criteria/).
There is thus a strong rationale for neuroimaging studies investigating cognitively normal seniors at high AD-risk based on genetics and/or family history. As other reviews in this issue will summarize, several methodologies have been applied to asymptomatic persons to assess the brain at AD risk, including resting glucose positron emission tomography, amyloid imaging, and functional and structural magnetic resonance imaging. The majority of research conducted in presymptomatic persons to date has focused on grey matter (GM) neurodegeneration. This is because the neuropathology of AD in GM brain regions is well established: Amyloid plaques and intracellular neurofibrillary tangles initially target medial temporal lobe (MTL) GM, resulting in prominent neurodegeneration of the hippocampus and entorhinal cortex (ERC) [6, 7].
However, ever since the work of Brun and Englund [8], it has been known that microscopic WM changes are also present in AD. These authors reported a partial loss of myelin sheaths, axons, and oligodendroglial cells together with a mild glial reaction. The advent of diffusion tensor imaging (DTI) has made it possible to explore the possibility of WM microstructural alterations in vivo. DTI-based declines have been shown to contribute to age-related cognitive declines [9–11], suggesting they are functionally significant to the study of healthy and pathological forms of aging. Recent reviews have summarized evidence that WM microstructural integrity is decreased in AD and aMCI [12, 13]. The purpose of this review is to summarize evidence suggesting that WM microstructural integrity is also altered in cognitively normal individuals at high AD-risk.
We begin with a description of the two most common risk factors for late-onset AD: The epsilon-4 allele of the APOE gene, and family history of AD. We then provide a brief description of DTI and its most typical metrics used to assess WM integrity. Next, we review the patterns of WM integrity declines associated with AD-risk and compare them to declines associated with symptomatic AD. We then discuss some of the potential neurobiological bases of DTI findings. Finally, we conclude that while DTI holds promise as a contributor to early AD diagnosis, future research is required to determine the neurobiological bases of DTI changes and the validity of DTI as a biomarker of presymptomatic AD.
Risk Factors Associated with Alzheimer's Disease
Apolipoprotein E
Apolipoprotein E (APOE) confers the greatest genetic risk for late-onset AD among at least six other genes recently discovered or confirmed [14]. APOE is a glycoprotein and is involved in the transport of cholesterol and other lipids through cell membranes. In the brain, APOE is mainly produced by glial cells and is thought to be involved in neuronal growth and regeneration [15]. A polymorphism in the gene encoding APOE is known to confer an increase in the susceptibility to late-onset AD. Genetic polymorphisms are allelic variations found frequently in the population. There are at least three slightly different alleles of the APOE gene, which is located on chromosome 19. The major alleles are epsilon-2, -3, and -4. The most common allele is epsilon-3, which is found in more than half of the general population.
Possession of the APOE-4 allele is associated with an increased risk of late-onset AD [16]. In addition, there is a dose-dependency of APOE-4, such that individuals with two APOE-4 alleles (the 4/4 genotype), are at greater AD-risk than individuals with only a single copy (the 3/4 genotype) [17]. Those individuals those with two e4 alleles have up to 20 times the risk of developing AD, mainly through an effect lowering the age of onset by approximately ten years. The role of APOE in the development of AD is not yet established. One theory is that APOE-4 may result in decreased plasticity of neuronal synapses, and decreased ability to repair damaged neurons compared to other allelic variants [18].
Family History of Alzheimer's Disease
Not all people with AD have the APOE-4 allele, and not all people who have this allele will develop AD. A second significant risk factor for late-onset AD is family history. Individuals with a first-degree relative affected with AD have been found to have between a 4 to 10 fold increase in AD-risk [19, 20, 21]. Further, in those above the age of 70, the risk increases to more than 40% for those with two parents that have been diagnosed with AD. The APOE-4 gene accounts for approximately 50% of this genetic variance.
This leaves 50% of the variance in the link between family history and AD unaccounted for, suggesting the existence of other genetic risk factors for AD. Several other potential genes are currently under investigation including SORL1 on chromosome 11, A2M on chromosome 12, GST01 and GST02 on chromosome 10, and GAB2 on chromosome 11 [22]. Recently four new genes have been identified in a large genome-wide association study (MS4A, CD2AP, CD33, and EPHA1) and two more supported (BIN1 and ABCA7), confirming late-onset AD as a true polygenetic disorder [14]. At the next level of complexity significant interactions of genes CR1, CLU and PICALM with APOE genotypes have been described [23].
In addition to genetic variables, the association between environmental and lifestyle variables (e.g., diet, exercise, cognitive stimulation) and AD-risk are also being explored. Nevertheless, at present, APOE-4 and family history of dementia represent the strongest established risk factors for late-onset AD.
Diffusion Tensor Imaging
The advent of DTI has allowed for assessment of WM microstructure in vivo. DTI is an extended form of diffusion-weighted tensor trace imaging that is sensitized to the motion of water molecules as they interact within tissues, thus reflecting characteristics of their immediate structural surroundings [24, 25]. In DTI, a series of diffusion gradients are applied to determine three independent directional vectors, from which the 3-dimensional shape of diffusion can be generated for each voxel.
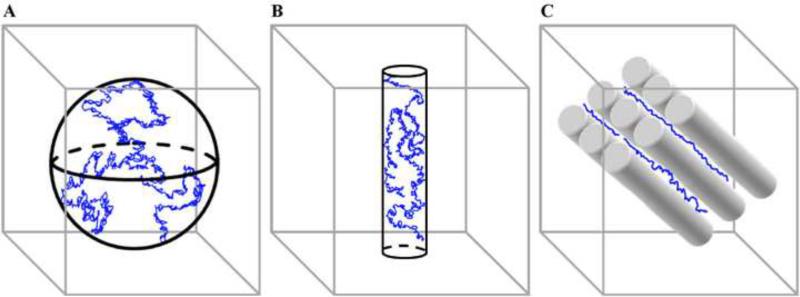
Patterns of water diffusion vary systematically across different compartments of the brain (Figure 1). Water diffusion is equal in all directions (isotropic) in the ventricles because it is unconstrained by biological barriers. In contrast, water diffusion is more directional (anisotropic) in the brain's WM due to its internal fibrous structure. For example, water diffuses up to seven times more rapidly along the direction parallel to the longitudinal axis of axonal orientation compared to the direction orthogonal to the main fiber direction [26, 27]. Structures that restrict water movement perpendicular to axonal orientation include myelin sheaths, axonal membranes, and neurofilaments within the axoplasm [28]. Within WM, water diffusion is highest in tracts that contain the largest numbers of fibers running in parallel, such as the corpus callosum [29].
Figure 1.
Schematic of DTI-based water diffusion patterns in isotropic and anisotropic environments. Water molecules are represented in blue. (A) In compartments with minimal biological barriers (e.g., ventricles; represented by a sphere), water molecules are free to diffuse equally in all directions (isotropic diffusion). (B) In compartments with an internal fibrous structure (e.g., white matter; represented here by a cylinder), biological barriers such as the axon membrane, myelin sheath, microtubules, and neurofilaments limit diffusion in one or more directions (anisotropic diffusion). (C) Within white matter, anisotropic diffusion is highest in tracts with highly organized, coherently oriented axonal bundles such as those found in the corpus callosum (represented here by cylinders). The trajectory of the water molecules is along the longitudinal axis of the axon bundles.
Measures of Diffusion Tensor Imaging
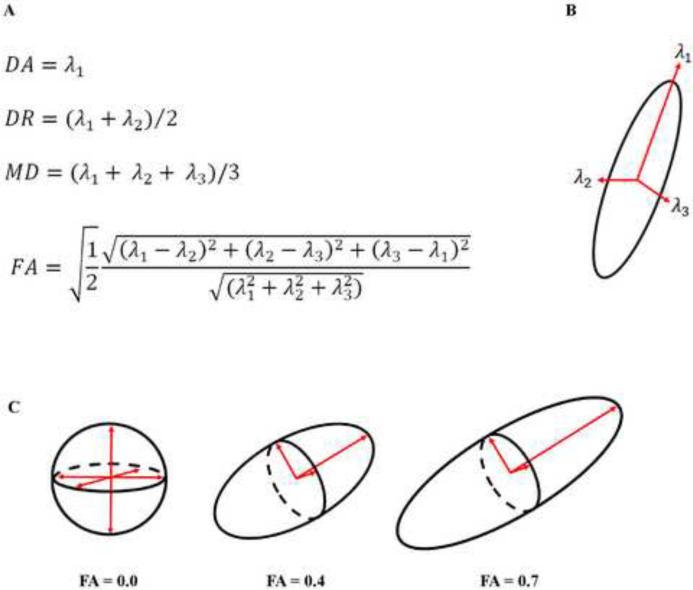
The four most commonly used measures of DTI, and the equations used to compute them, are displayed in Figure 2. These measures are derived from three eigenvectors that define the diffusion ellipsoid in each voxel. The eigenvectors are calculated for each voxel by diagonalizing each voxel's diffusion tensor. The eigenvalues are the magnitude (length) of these three eigenvectors and represent the magnitude of diffusion, diffusion coefficient, in the three principle directions. Axial diffusivity (DA) reflects the diffusion coefficient along the direction of maximal 'apparent' diffusion (λ1), whereas radial diffusivity (DR) reflects the average diffusion coefficients along the two perpendicular directions ((λ2 + λ3)/2) [30]. Mean diffusivity (MD) is a scalar measure of the total amount of diffusion within a voxel and is computed as an average of all three eigenvalues of the diffusion tensor [25]. Finally, fractional anisotropy (FA) is computed as a weighted average of the three eigenvalues of the diffusion tensor in order to represent the fraction of the tensor defined by anisotropic diffusion [25].
Figure 2.
Calculating diffusion. Axial diffusivity (DA), radial diffusivity (DR), mean diffusivity (MD), and fractional anisotropy (FA) are calculated by modeling the tensor in each voxel. Three principle eigenvectors and their associated eigenvalues are used to calculate each component. (A) Equations used to calculate DA, DR, MD, and FA. (B) The three principle eigenvectors. (C) Geometric representations of low, moderate, and high FA values.
What the Measures Reflect
Diffusion properties associated with these different DTI measures can be interpreted as reflecting different structural contributions to WM integrity. Fractional anisotropy (FA) reflects the coherence of the orientation (or directionality) of water diffusion, independent of its rate, and is thought to provide an overall assessment of WM integrity [25]. MD depends on the density of physical obstructions such as membranes and the resultant distribution of water molecules between different cell compartments [28]. Higher MD values indicate increased diffusion which suggests tissue breakdown and increased brain water content [25]. Compared to FA and MD, DTI-based component diffusivities (DA and DR) provide more specific information about the integrity of axons versus their surrounding myelin sheaths. Increased DR has been linked with loss of myelin in animal studies of experimentally induced myelin loss [30–32]. In contrast, a decrease in DA has been reported in both rodents and humans with axonal damage associated with axonal swelling, fragmentation and organelle accumulations [32, 33].
WM integrity Declines Associated with High AD-Risk
We identified seven DTI studies that have explored the role of APOE4, and/or familial history of AD, on WM microstructure [34–40]. Our search was conducted using MEDLINE and was restricted to English articles and human subjects. Search terms were 'diffusion tensor' alternatively paired with one of the following: 'APOE', 'presymptomatic Alzheimer's', 'family history Alzheimer's', or 'Alzheimer's risk'. The studies identified used a variety of different analyses including manual region-of-interest (ROI) tracing, voxel-based morphometry implemented in SPM software, or tract-based spatial statistics (TBSS) implemented in FSL software. The DTI analysis techniques, sample sizes, and reported regions showing decreased WM integrity (defined as decreased FA and/or increased MD) in individuals at high AD-risk are reported in Table 1.
Table 1.
White matter tracts showing integrity declines (FA decreases and/or MD increases) in AD risk studies.
| Study | Technique | N | WM Tract |
|---|---|---|---|
| Nierenberg et al., 2005 | ROI | 29 | PHW |
| Persson et al., 2006 | ROI, SPM-VBM | 60 | CC, CING/IFOF, HC |
| Honea et al., 2009 | FSL-TBSS | 53 | PHW |
| Gold et al., 2010 | FSL-TBSS | 57 | CING, FORNIX, CC, ILF, IFOF/UNC |
| Smith et al., 2010 | FSL-TBSS | 65 | PHW, CING, CC, ILF/IFOF |
| Bendlin et al., 2010 | SPM-VBM | 136 | HC, CING, IFOF/UNC, CC |
| Heise et al., in press | FSL-TBSS | 39 | CING, CC, SLF |
Notes: ROI, region of interest; SPM-VBM, statistical parametric mapping-voxel based morphometry; FSL-TBSS, tract-based spatial statistics; CC, corpus callosum; CING, cingulum; HC, hippocampus (grey matter); PHW, parahippocampal white matter; UNC, uncinate fasciculus; SLF, superior longitudinal fasciculus; ILF, inferior longitudinal fasciculus; IFOF, inferior fronto-occipito fasciculus.
Decisions about which analysis techniques (i.e. ROI-based versus whole-brain, or a combination of both) and which software package to use are of course based on multiple variables such as theoretical approach (i.e. hypothesis driven versus exploratory) and familiarity with specific software packages. In our studies, and those of several other groups, the tract-based spatial statistics (TBSS) technique was used in order to explore group differences in across the brain's WM. The TBSS method has several advantages compared to other whole-brain analysis techniques including improved registration of WM tracts across subjects [41] and the lack of a need to apply an arbitrary smoothing kernel, which can produce different results based on kernel size [42].
The studies conducted to date have reported decreased WM integrity predominantly in tracts with direct or secondary connections to MTL structures (Table 1). Variability in the specific affected tracts reported in different studies is likely to be due to differences in subject characteristics and analysis techniques. Nevertheless, multiple AD-risk studies have reported reduced WM integrity in parahippocampal WM [34, 36, 37], the cingulum [35, 37, 38, 39, 40], the inferior fronto-occipital fasciculus / uncinate fasciculus (IFOF/UNC) [35, 37, 38, 40] and corpus callosum [35, 37, 38, 39, 40].
Results from one of our AD-risk studies [37] are presented in Figure 3. We observed a clearly delineated decrease in FA in the inferior temporal lobe WM bilaterally (ILF / IFOF). This decrease has a strong linear spatial character suggesting involvement of a specific group of white matter fibers bridging the amygdala/hippocampal head region anteriorly and the ventral visual association areas posteriorly. We also found significant FA decreases in the splenium of the corpus callosum, the cingulum bundle, and the IFOF anteriorly. The downward curvature of the FA reductions in the IFOF is characteristic of frontal portions of this pathway, which parallels the uncinate fasciculus in part of its course. In general our findings were more robust in the temporal and frontal regions than in parietal lobes, and more spatially coherent in the ventral visual association regions, medial temporal lobe, cingulum and frontal lobe than in the parietal lobe or occipital lobe.
Figure 3.
Regions of decreased fractional anisotropy in normal, high AD-risk subjects. The anatomic underlay used for illustration is the MNI-space registered target fractional anisotropy (FA) image. The registered average FA skeleton is represented in green. Warmer colors on red-orange scale indicate higher t-values of FA decreases for the high AD-risk group compared to low AD-risk group. The patterns of FA decreases indicated by the arrowheads correspond to the inferior fronto-occipital fasciculus (A) and inferior fronto-occipital fasciculus / uncinate fasciculus (B). Decreased FA in the high AD-risk group was also evident in the splenium of the corpus callosum (C; single arrowhead) and the posterior cingulum bundle on the left (C; double arrowheads). Adapted from Smith et al. (2010) with permission.
As noted above, not all individuals at genetic/familial risk for late-onset AD will actually develop AD. In contrast, the early-onset, familial autosomal dominantly (FAD) inherited form of AD, while very rare, is nearly 100% penetrant. The future development of AD can be reliably predicted in individuals with FAD-related genetic mutations. At present, however, only one study has assessed WM integrity in presymptomatic FAD mutation carriers. Using ROI analyses, Ringman et al. [43] found decreased WM integrity in the fornix and in WM of the ventral frontal cortex. Interestingly, several of the AD-risk studies summarized above have also reported decreased WM integrity in the fornix [38] and ventral frontal cortex WM near the IFOF / UNC [35, 37, 38, 40]. Future DTI studies are required to determine the sensitivity and specificity of integrity declines in these WM tracts in predicting AD in normal seniors.
Comparison with Symptomatic AD Results
Results from DTI studies of symptomatic AD have demonstrated that reduced FA and/or increased MD is fairly widespread by the time of AD diagnosis (for a review see [12, 13]). However, the most prominent decreases have been reported in tracts with direct or secondary connections to MTL structures, including tracts showing decreases in AD-risk studies (parahippocampal WM, the cingulum, the IFOF / UNC, and the corpus callosum) [44–50]. Results from studies exploring DTI changes in aMCI have tended to report WM integrity changes which generally parallel changes in AD [12, 13]. However, the precise location of WM integrity declines tends to be more variable across aMCI studies than AD studies, which may be in part attributable to differences in diagnostic criteria used for aMCI.
A recent study that examined normal control, MCI and AD subjects found the strongest WM alterations in cingulum bundle, uncinate fasciculus, corpus callosum and the SLF. An interesting observation from this study was that increased “cigar-like” ellipsoid anisotropy was found in regions of crossing fibers in deep white matter, attributed to relative preservation of well-myelinated motor fibers with alteration of myelin in crossing association bundles [51]. This study did not examine AD risk factors in the normal control subjects.
In summary, studies of normal individuals at high AD-risk have reported WM integrity declines in a sub-set of tracts affected in symptomatic AD including parahippocampal WM, caudal portions of the cingulum, the IFOF, and posterior portions of the corpus callosum. A few studies have also reported reduced WM integrity in the fornix. Importantly, these WM tracts connect structures involved in episodic memory function, the hallmark impairment of early AD. Specifically, the caudal portion of the cingulum contains fibers that connect the posterior cingulate cortex with the entorhinal cortex and hippocampus [52]. The fornix connects the hippocampus with the septal nuclei in the basal frontal region, the mamillary bodies of the hypothalamus, and the anterior nuclei of the thalamus. The IFOF contains connections between occipital visual regions and ventrolateral prefrontal cortex, a region with an established role in memory encoding [53]. Posterior portions of the corpus callosum connect bilateral parietal regions, including the precuneus and retrosplenial regions known to contribute to memory function [54]. Finally, additional widespread WM integrity reduction in long association fibers has recently been described in MCI and AD.
Component Diffusivity Changes Associated with High AD-Risk
Of the AD-risk studies conducted to date, four have explored component diffusivities in addition to FA and MD [34, 38, 39, 40]. In each of the studies except one [39], AD-risk was associated with increased DR in many of the regions showing decreased FA. In particular, increased DR has been reported in medial temporal lobe WM [34, 40] and in the cingulum and IFOF/UNC [38, 40]. In contrast, minimal or no significant changes in DA associated with AD-risk were reported in any of these AD-risk studies.
Comparison with Symptomatic AD Results
We were able to identify four symptomatic AD studies that have explored component diffusivities [55–58]. In each of these studies except one [56] symptomatic AD was associated with increases in DA (in addition to DR). The DA increases reported were widespread, prominently affecting not simply medial temporal lobe tracts such as the fornix and cingulum, but also tracts of temporo-parietal regions such as the superior longitudinal fasciculus [55, 57, 58]. Of particular relevance was the study by Bosch et al. [55], which included an aMCI group in addition to AD and normal senior groups. These authors reported that, whereas both patient groups showed DR increases compared to the normal group, only the AD group showed DA increases.
In summary, AD-risk and symptomatic AD studies have reported different patterns of component diffusivity changes. Reported differences in DR appear to be quantitative, with AD-risk studies reporting DR increases in a subset of WM tracts showing DR increases in symptomatic AD. In contrast, reported differences in DA appear to be qualitative. Whereas AD studies have reported prominent DA alterations, AD-risk studies have reported little or no DA changes in any WM tracts. Taken together, findings from AD-risk and symptomatic AD studies are consistent with suggestions that DA increases may occur at a relatively late stage of WM damage, possibly when 'cellular debris' is cleared by microglia [32, 33].
Relationship to MTL volume
Neuropathology affects the hippocampus and entorhinal cortex (ERC) early in the course of AD [6, 7], and atrophy of these MTL structures is evident with MR imaging early in the AD disease process [59–61]. In addition, recent results suggest that smaller MTL volumes can be detected several years prior to aMCI diagnosis [62, 63]. A logical question is then whether WM microstructural changes in presymptomatic AD are independent of or secondary to classically described grey matter MTL degeneration.
Of the AD-risk studies conducted to date, three have compared GM volumes between high and low risk groups. Honea and colleagues [36] reported reduced GM volume in the hippocampus, amygdala and precuneus of a high AD-risk group. In contrast, no GM volume reductions were observed in these studies of Gold et al. [38] or Heise et al. [39]. In our study, we further explored the effects of including normalized hippocampal volume as a covariate in the group comparisons of FA/MD/DR/DA. Including normalized hippocampal volume as a covariate had minimal effects on the between-group WM microstructural results and did not alter the finding of reduced FA in the fornix in the high AD-risk group. Rather, controlling for hippocampal volume revealed an additional effect of reduced FA (and increased DR) in the high AD-risk group in the caudal portion of the left cingulum, a tract with direct connections between the MTL and posterior cingulate cortex. The available data suggest that WM integrity declines do not appear to be a direct consequence of medial temporal lobe atrophy.
Neurobiological Mechanisms Underlying WM Microstructural Declines
Little is known about the neurobiological bases of DTI-based WM integrity declines. Data related to this issue has come from analyses of DTI-based component diffusivities. The overall pattern observed in studies conducted to date suggests that AD-risk is associated with decreased FA and increased DR in the absence of prominent changes in DA. This pattern of DTI-based findings is consistent with data demonstrating that myelin and its components such as cholesterol and myelin proteins are reduced early in the AD process [64, 65] and with a recently proposed theory suggesting that disruption of myelin integrity is an early event in the course of AD [66]. Overall, AD-risk findings would appear to be inconsistent with a view that Wallerian degeneration represents the primary mechanism of decreased WM integrity preceding AD. Wallerian degeneration secondary to distal cortical atrophy would be most clearly supported by findings of significant gross tissue loss and decreased DA which have not typically been reported in AD-risk studies.
While reduced WM integrity can exist in the absence of significant grey matter atrophy, it remains possible that microstructural WM declines may in part reflect neuronal dysfunction. For example, the pattern of WM alterations reported could be due to faulty communication between neuron and oligodendrocyte. Faulty communication could be mediated from the axonal side due to neuronal dysfunction, even in the absence of gross cortical atrophy. This possibility takes into account the concept that the axon and enveloping myelin make up an integrated unit via intracellular signaling. The signaling scheme between axons and investing glia, particularly oligodendroglia in the CNS, is not yet well defined [67]. However, in the peripheral nervous system axonal signals are required for myelin maintenance including PrPc, a putative receptor for beta-amyloid [68]. Alterations in DR from myelin disruption in tracts projecting from pathologically involved areas could be reporting neuronal dysfunction through this mechanism at a very early stage, before volume changes are detectable in GM.
Future Research
There are a number of caveats related to existing DTI studies of AD-risk that highlight open questions and motivate future research. First, only a relatively small number of studies have been conducted. Future research is required to establish the most reliable DTI changes associated with AD risk. Second, DTI research of AD-risk has been limited to cross-sectional designs. Future prospective studies are required to determine which DTI based declines actually predict transition to AD. Third, the influence of vascular factors on WM integrity declines has not been systematically explored. Future studies should go beyond excluding patients with observable hyperintensities by exploring quantitative relationships between vascular factors and WM integrity. Similarly, there exists little data concerning the neurobiological bases of WM integrity declines. Existing data which speak to this issue are largely limited to DTI analyses of component diffusivities, which are only suggestive of underlying neurobiological bases (i.e. axonal or myelin decline). Future research correlating DTI declines with more direct measures of pathology are required.
Conclusions
There is promising data suggesting that WM integrity declines can be detected in individuals at high AD-risk, prior to cognitive decline. Tracts showing declines in individuals at high AD-risk involve a subset of tracts showing decline in symptomatic AD, including WM of the parahippocampal region, the cingulum, IFOF, and splenium of the corpus callosum. This network of tracts connects GM structures contributing to memory function such as the MTL, posterior parietal cortex, and inferior frontal cortex. These data suggest that disconnection of core memory structures represents an early event in the course of AD. Preliminary evidence of increased radial diffusivity in some tracts raises the possibility that a myelin-related pathology may contribute to AD onset, and there is evidence that long association tracts are affected by this pathology early in the disease process.
However, there is clearly a need for studies correlating DTI metrics with classic indices of AD pathology such as cerebrospinal fluid analytes, and brain amyloid burden. Such future studies will help determine if WM integrity changes reflect classic beta-amyloid or phospho-tau pathologies, or other pathologies more specifically related to WM. A greater understanding of the neurobiological bases of WM microstructural changes associated with AD-risk has the potential to aid the development of novel pharmacological intervention strategies for AD. For example, one reason that standard cholinergic therapies may have limited success is that they may be treating only part of the AD cascade. Future DTI findings similar to those described in this review have the potential to spur the development of additional preventative treatments intended to protect axon and myelin integrity in seniors at risk of AD.
Highlights
-
➢
Review focuses on diffusion tensor imaging (DTI) studies of normal subjects before symptoms of Alzheimer's disease (AD)
-
➢
Summarizes risk factors of AD, and DTI methodology
-
➢
Summarizes evidence that white matter integrity changes are present in normal persons at high-AD risk
-
➢
These findings have the potential to aid early diagnosis of AD
-
➢
Findings motivate future research intended to understand if white matter integrity changes reflect classic AD pathologies, or other pathologies more specifically related to white matter
Acknowledgements
We gratefully acknowledge our collaborators at the Alzheimer's Disease Research Center, and Magnetic Resonance Imaging and Spectroscopy Center at the University of Kentucky. This review was supported by NINDS Grant R01 NS-36660, NIA Grant R01 AG033036 and NSF Grant BCS-0814302.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- [2].Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- [3].Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- [4].Chong MS, Sahadevan S. Preclinical Alzheimer's disease: diagnosis and prediction of progression. Lancet Neurol. 2005;4:576–579. doi: 10.1016/S1474-4422(05)70168-X. [DOI] [PubMed] [Google Scholar]
- [5].Reiman EM, Langbaum JB, Tariot PN. Alzheimer's prevention initiative: a proposal to evaluate presymptomatic treatments as quickly as possible. Biomark Med. 2010;4:3–14. doi: 10.2217/bmm.09.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- [7].Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- [8].Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol. 1986;19:253–262. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- [9].Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA. Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci. 2009;21:289–302. doi: 10.1162/jocn.2009.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, Huettel SA. Age-related slowing of memory retrieval: contributions of perceptual speed and cerebral white matter integrity. Neurobiol Aging. 2008;29:1070–1079. doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD. Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol Aging. 2010;31:512–522. doi: 10.1016/j.neurobiolaging.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Stebbins GT, Murphy CM. Diffusion tensor imaging in Alzheimer's disease and mild cognitive impairment. Behav Neurol. 2009;21:39–49. doi: 10.3233/BEN-2009-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chua TC, Wen W, Slavin MJ, Sachdev PS. Diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease: a review. Curr Opin Neurol. 2008;21:83–92. doi: 10.1097/WCO.0b013e3282f4594b. [DOI] [PubMed] [Google Scholar]
- [14].Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD, Jarvik GP, Crane PK, Larson EB, Bird TD, Boeve BF, Graff-Radford NR, De Jager PL, Evans D, Schneider JA, Carrasquillo MM, Ertekin-Taner N, Younkin SG, Cruchaga C, Kauwe JS, Nowotny P, Kramer P, Hardy J, Huentelman MJ, Myers AJ, Barmada MM, Demirci FY, Baldwin CT, Green RC, Rogaeva E, George-Hyslop PS, Arnold SE, Barber R, Beach T, Bigio EH, Bowen JD, Boxer A, Burke JR, Cairns NJ, Carlson CS, Carney RM, Carroll SL, Chui HC, Clark DG, Corneveaux J, Cotman CW, Cummings JL, Decarli C, Dekosky ST, Diaz-Arrastia R, Dick M, Dickson DW, Ellis WG, Faber KM, Fallon KB, Farlow MR, Ferris S, Frosch MP, Galasko DR, Ganguli M, Gearing M, Geschwind DH, Ghetti B, Gilbert JR, Gilman S, Giordani B, Glass JD, Growdon JH, Hamilton RL, Harrell LE, Head E, Honig LS, Hulette CM, Hyman BT, Jicha GA, Jin LW, Johnson N, Karlawish J, Karydas A, Kaye JA, Kim R, Koo EH, Kowall NW, Lah JJ, Levey AI, Lieberman AP, Lopez OL, Mack WJ, Marson DC, Martiniuk F, Mash DC, Masliah E, McCormick WC, McCurry SM, McDavid AN, McKee AC, Mesulam M, Miller BL, Miller CA, Miller JW, Parisi JE, Perl DP, Peskind E, Petersen RC, Poon WW, Quinn JF, Rajbhandary RA, Raskind M, Reisberg B, Ringman JM, Roberson ED, Rosenberg RN, Sano M, Schneider LS, Seeley W, Shelanski ML, Slifer MA, Smith CD, Sonnen JA, Spina S, Stern RA, Tanzi RE, Trojanowski JQ, Troncoso JC, Van Deerlin VM, Vinters HV, Vonsattel JP, Weintraub S, Welsh-Bohmer KA, Williamson J, Woltjer RL, Cantwell LB, Dombroski BA, Beekly D, Lunetta KL, Martin ER, Kamboh MI, Saykin AJ, Reiman EM, Bennett DA, Morris JC, Montine TJ, Goate AM, Blacker D, Tsuang DW, Hakonarson H, Kukull WA, Foroud TM, Haines JL, Mayeux R, Pericak-Vance MA, Farrer LA, Schellenberg GD. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat Genet. 2011;43:436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- [16].Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- [17].Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- [18].Laws SM, Hone E, Gandy S, Martins RN. Expanding the association between the APOE gene and the risk of Alzheimer's disease: possible roles for APOE promoter polymorphisms and alterations in APOE transcription. J Neurochem. 2003;84:1215–1236. doi: 10.1046/j.1471-4159.2003.01615.x. [DOI] [PubMed] [Google Scholar]
- [19].Cupples LA, Farrer LA, Sadovnick AD, Relkin N, Whitehouse P, Green RC. Estimating risk curves for first-degree relatives of patients with Alzheimer's disease: the REVEAL study. Genet Med. 2004;6:192–196. doi: 10.1097/01.gim.0000132679.92238.58. [DOI] [PubMed] [Google Scholar]
- [20].Green RC, Cupples LA, Go R, Benke KS, Edeki T, Griffith PA, Williams M, Hipps Y, Graff-Radford N, Bachman D, Farrer LA. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287:329–336. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- [21].Silverman JM, Ciresi G, Smith CJ, Marin DB, Schnaider-Beeri M. Variability of familial risk of Alzheimer disease across the late life span. Arch Gen Psychiatry. 2005;62:565–573. doi: 10.1001/archpsyc.62.5.565. [DOI] [PubMed] [Google Scholar]
- [22].Bird TD. Genetic aspects of Alzheimer disease. Genet Med. 2008;10:231–239. doi: 10.1097/GIM.0b013e31816b64dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jun G, Naj AC, Beecham GW, Wang LS, Buros J, Gallins PJ, Buxbaum JD, Ertekin-Taner N, Fallin MD, Friedland R, Inzelberg R, Kramer P, Rogaeva E, St George-Hyslop P, Cantwell LB, Dombroski BA, Saykin AJ, Reiman EM, Bennett DA, Morris JC, Lunetta KL, Martin ER, Montine TJ, Goate AM, Blacker D, Tsuang DW, Beekly D, Cupples LA, Hakonarson H, Kukull W, Foroud TM, Haines J, Mayeux R, Farrer LA, Pericak-Vance MA, Schellenberg GD. Meta-analysis confirms CR1, CLU, and PICALM as alzheimer disease risk loci and reveals interactions with APOE genotypes. Arch Neurol. 2010;67:1473–1484. doi: 10.1001/archneurol.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- [26].Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- [27].Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- [28].Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- [29].Shimony JS, McKinstry RC, Akbudak E, Aronovitz JA, Snyder AZ, Lori NF, Cull TS, Conturo TE. Quantitative diffusion-tensor anisotropy brain MR imaging: normative human data and anatomic analysis. Radiology. 1999;212:770–784. doi: 10.1148/radiology.212.3.r99au51770. [DOI] [PubMed] [Google Scholar]
- [30].Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- [31].Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- [32].Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn Reson Med. 2006;55:302–308. doi: 10.1002/mrm.20774. [DOI] [PubMed] [Google Scholar]
- [33].Concha L, Gross DW, Wheatley BM, Beaulieu C. Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. Neuroimage. 2006;32:1090–1099. doi: 10.1016/j.neuroimage.2006.04.187. [DOI] [PubMed] [Google Scholar]
- [34].Nierenberg J, Pomara N, Hoptman MJ, Sidtis JJ, Ardekani BA, Lim KO. Abnormal white matter integrity in healthy apolipoprotein E epsilon4 carriers. Neuroreport. 2005;16:1369–1372. doi: 10.1097/01.wnr.0000174058.49521.16. [DOI] [PubMed] [Google Scholar]
- [35].Persson J, Lind J, Larsson A, Ingvar M, Cruts M, Van Broeckhoven C, Adolfsson R, Nilsson LG, Nyberg L. Altered brain white matter integrity in healthy carriers of the APOE epsilon4 allele: a risk for AD? Neurology. 2006;66:1029–1033. doi: 10.1212/01.wnl.0000204180.25361.48. [DOI] [PubMed] [Google Scholar]
- [36].Honea RA, Vidoni E, Harsha A, Burns JM. Impact of APOE on the healthy aging brain: a voxel-based MRI and DTI study. J Alzheimers Dis. 2009;18:553–564. doi: 10.3233/JAD-2009-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Smith CD, Chebrolu H, Andersen AH, Powell DA, Lovell MA, Xiong S, Gold BT. White matter diffusion alterations in normal women at risk of Alzheimer's disease. Neurobiol Aging. 2010;31:1122–1131. doi: 10.1016/j.neurobiolaging.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gold BT, Powell DK, Andersen AH, Smith CD. Alterations in multiple measures of white matter integrity in normal women at high risk for Alzheimer's disease. Neuroimage. 2010;52:1487–1494. doi: 10.1016/j.neuroimage.2010.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Heise V, Filippini N, Ebmeier KP, Mackay CE. The APOE varepsilon4 allele modulates brain white matter integrity in healthy adults. Mol Psychiatry. doi: 10.1038/mp.2010.90. [DOI] [PubMed] [Google Scholar]
- [40].Bendlin BB, Ries ML, Canu E, Sodhi A, Lazar M, Alexander AL, Carlsson CM, Sager MA, Asthana S, Johnson SC. White matter is altered with parental family history of Alzheimer's disease. Alzheimers Dement. 2010;6:394–403. doi: 10.1016/j.jalz.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- [42].Jones DK, Symms MR, Cercignani M, Howard RJ. The effect of filter size on VBM analyses of DT-MRI data. Neuroimage. 2005;26:546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- [43].Ringman JM, O'Neill J, Geschwind D, Medina L, Apostolova LG, Rodriguez Y, Schaffer B, Varpetian A, Tseng B, Ortiz F, Fitten J, Cummings JL, Bartzokis G. Diffusion tensor imaging in preclinical and presymptomatic carriers of familial Alzheimer's disease mutations. Brain. 2007;130:1767–1776. doi: 10.1093/brain/awm102. [DOI] [PubMed] [Google Scholar]
- [44].Firbank MJ, Blamire AM, Krishnan MS, Teodorczuk A, English P, Gholkar A, Harrison R, O'Brien JT. Atrophy is associated with posterior cingulate white matter disruption in dementia with Lewy bodies and Alzheimer's disease. Neuroimage. 2007;36:1–7. doi: 10.1016/j.neuroimage.2007.02.027. [DOI] [PubMed] [Google Scholar]
- [45].Huang J, Friedland RP, Auchus AP. Diffusion tensor imaging of normal-appearing white matter in mild cognitive impairment and early Alzheimer disease: preliminary evidence of axonal degeneration in the temporal lobe. AJNR Am J Neuroradiol. 2007;28:1943–1948. doi: 10.3174/ajnr.A0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Medina D, DeToledo-Morrell L, Urresta F, Gabrieli JD, Moseley M, Fleischman D, Bennett DA, Leurgans S, Turner DA, Stebbins GT. White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiol Aging. 2006;27:663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- [47].Rose SE, McMahon KL, Janke AL, O'Dowd B, de Zubicaray G, Strudwick MW, Chalk JB. Diffusion indices on magnetic resonance imaging and neuropsychological performance in amnestic mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2006;77:1122–1128. doi: 10.1136/jnnp.2005.074336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sydykova D, Stahl R, Dietrich O, Ewers M, Reiser MF, Schoenberg SO, Moller HJ, Hampel H, Teipel SJ. Fiber connections between the cerebral cortex and the corpus callosum in Alzheimer's disease: a diffusion tensor imaging and voxel-based morphometry study. Cereb Cortex. 2007;17:2276–2282. doi: 10.1093/cercor/bhl136. [DOI] [PubMed] [Google Scholar]
- [49].Teipel SJ, Stahl R, Dietrich O, Schoenberg SO, Perneczky R, Bokde AL, Reiser MF, Moller HJ, Hampel H. Multivariate network analysis of fiber tract integrity in Alzheimer's disease. Neuroimage. 2007;34:985–995. doi: 10.1016/j.neuroimage.2006.07.047. [DOI] [PubMed] [Google Scholar]
- [50].Xie S, Xiao JX, Gong GL, Zang YF, Wang YH, Wu HK, Jiang XX. Voxel-based detection of white matter abnormalities in mild Alzheimer disease. Neurology. 2006;66:1845–1849. doi: 10.1212/01.wnl.0000219625.77625.aa. [DOI] [PubMed] [Google Scholar]
- [51].Douaud G, Jbabdi S, Behrens TE, Menke RA, Gass A, Monsch AU, Rao A, Whitcher B, Kindlmann G, Matthews PM, Smith S. DTI measures in crossing-fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer's disease. Neuroimage. 2011;55:880–890. doi: 10.1016/j.neuroimage.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mufson EJ, Pandya DN. Some observations on the course and composition of the cingulum bundle in the rhesus monkey. J Comp Neurol. 1984;225:31–43. doi: 10.1002/cne.902250105. [DOI] [PubMed] [Google Scholar]
- [53].Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- [54].Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- [55].Bosch B, Arenaza-Urquijo EM, Rami L, Sala-Llonch R, Junque C, Sole-Padulles C, Pena-Gomez C, Bargallo N, Molinuevo JL, Bartres-Faz D. Multiple DTI index analysis in normal aging, amnestic MCI and AD. Relationship with neuropsychological performance. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2010.02.004. [DOI] [PubMed] [Google Scholar]
- [56].Stricker NH, Schweinsburg BC, Delano-Wood L, Wierenga CE, Bangen KJ, Haaland KY, Frank LR, Salmon DP, Bondi MW. Decreased white matter integrity in late-myelinating fiber pathways in Alzheimer's disease supports retrogenesis. Neuroimage. 2009;45:10–16. doi: 10.1016/j.neuroimage.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Acosta-Cabronero J, Williams GB, Pengas G, Nestor PJ. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer's disease. Brain. 2010;133:529–539. doi: 10.1093/brain/awp257. [DOI] [PubMed] [Google Scholar]
- [58].Salat DH, Tuch DS, van der Kouwe AJ, Greve DN, Pappu V, Lee SY, Hevelone ND, Zaleta AK, Growdon JH, Corkin S, Fischl B, Rosas HD. White matter pathology isolates the hippocampal formation in Alzheimer's disease. Neurobiol Aging. 2010;31:244–256. doi: 10.1016/j.neurobiolaging.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Jack CR, Jr., Petersen RC, Xu YC, Waring SC, O'Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Convit A, De Leon MJ, Tarshish C, De Santi S, Tsui W, Rusinek H, George A. Specific hippocampal volume reductions in individuals at risk for Alzheimer's disease. Neurobiol Aging. 1997;18:131–138. doi: 10.1016/s0197-4580(97)00001-8. [DOI] [PubMed] [Google Scholar]
- [61].de Leon MJ, DeSanti S, Zinkowski R, Mehta PD, Pratico D, Segal S, Clark C, Kerkman D, DeBernardis J, Li J, Lair L, Reisberg B, Tsui W, Rusinek H. MRI and CSF studies in the early diagnosis of Alzheimer's disease. J Intern Med. 2004;256:205–223. doi: 10.1111/j.1365-2796.2004.01381.x. [DOI] [PubMed] [Google Scholar]
- [62].Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Jicha GA, Cooper G, Markesbery WR. Brain structural alterations before mild cognitive impairment. Neurology. 2007;68:1268–1273. doi: 10.1212/01.wnl.0000259542.54830.34. [DOI] [PubMed] [Google Scholar]
- [63].Martin SB, Smith CD, Collins HR, Schmitt FA, Gold BT. Evidence that volume of anterior medial temporal lobe is reduced in seniors destined for mild cognitive impairment. Neurobiol Aging. 2010;31:1099–1106. doi: 10.1016/j.neurobiolaging.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Han X, D MH, McKeel DW, Jr., Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer's disease: potential role in disease pathogenesis. J Neurochem. 2002;82:809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- [65].Roher AE, Weiss N, Kokjohn TA, Kuo YM, Kalback W, Anthony J, Watson D, Luehrs DC, Sue L, Walker D, Emmerling M, Goux W, Beach T. Increased A beta peptides and reduced cholesterol and myelin proteins characterize white matter degeneration in Alzheimer's disease. Biochemistry. 2002;41:11080–11090. doi: 10.1021/bi026173d. [DOI] [PubMed] [Google Scholar]
- [66].Bartzokis G. Alzheimer's disease as homeostatic responses to age-related myelin breakdown. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535–561. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- [68].Bremer J, Baumann F, Tiberi C, Wessig C, Fischer H, Schwarz P, Steele AD, Toyka KV, Nave KA, Weis J, Aguzzi A. Axonal prion protein is required for peripheral myelin maintenance. Nature Neuroscience. 2010;13:310–U319. doi: 10.1038/nn.2483. [DOI] [PubMed] [Google Scholar]