Abstract
The transcription factor Krüppel-like factor 5 (KLF5) is primarily expressed in the proliferative zone of the mammalian intestinal epithelium where it regulates cell proliferation. Studies showed that inhibition of KLF5 expression reduces proliferation rates in human colorectal cancer cells and intestinal tumor formation in mice. To identify chemical probes that decrease levels of KLF5, we used cell-based ultrahigh-throughput screening (uHTS) to test compounds in the NIH’s public domain, the Molecular Libraries Probe Production Centers Network (MLPCN) library. The primary screen involved luciferase assays in the DLD-1/pGL4.18hKLF5p cell line, which stably expressed a luciferase reporter driven by the human KLF5 promoter. A cytotoxicity counterscreen was performed in the rat intestinal epithelial cell line, IEC-6. We identified 97 KLF5-selective compounds with EC50<10 µM for KLF5 inhibition and EC50>10 µM for IEC-6 cytotoxicity. The two most potent compounds, CIDs (PubChem Compound IDs) 439501 and 5951923, were further characterized based on computational, Western blot, and cell viability analyses. Both of these compounds and two newly-synthesized structural analogs of CID 5951923 significantly reduced endogenous KLF5 protein levels and decreased viability of several colorectal cancer cell lines without any apparent impact on IEC-6 cells. Finally, when tested in the NCI-60 panel of human cancer cell lines, compound CID 5951923 was selectively active against colon cancer cells. Our results demonstrate the feasibility of uHTS in identifying novel compounds that inhibit colorectal cancer cell proliferation by targeting KLF5.
Keywords: Colorectal cancer, KLF5, Ultrahigh-throughput screen, Luciferase, Cell viability, Small-molecule compounds
INTRODUCTION
Krüppel-like factor 5 (KLF5; also known as intestinal Krüppel-like factor, IKLF) is a member of the highly conserved zinc finger transcription factor family (1–3). Studies indicate that KLF5 regulates numerous biological processes including growth, proliferation, differentiation, development, stem cell renewal, inflammation, angiogenesis, and vascular remodeling (4–12). In adults, KLF5 is highly expressed in the rapidly dividing crypt epithelial cells of the intestine (13). The expression pattern of KLF5 is similar to some components of the Wnt pathway, which regulates intestinal crypt epithelial cell proliferation and is frequently perturbed during tumorigenesis (14, 15). Interestingly, KLF5 expression can be modulated by Wnt signaling (16) and conversely, KLF5 modulates activity of β-catenin, a critical mediator of Wnt signaling (17). Moreover, ectopic expression of KLF5 increases the rate of proliferation of cultured cells, leading to anchorage-independent growth (4, 18). These studies indicate that KLF5 has a crucial role in regulating intestinal epithelial cell proliferation.
Expression of KLF5 is frequently increased in transformed cells. For example, KLF5 was shown to be a target of oncogenic HRAS and KRAS, and mediates their transforming activity by targeting key components of the cell cycle (3, 19, 20). In vivo, intestinal tumors derived from mice transgenic for oncogenic KRAS and human primary colorectal cancers with mutated KRAS contain high levels of KLF5 (3). Importantly, genetic reduction of Klf5 in transgenic mice reduces intestinal tumor formation in mice harboring a germline mutation in the colon cancer tumor suppressor gene, Apc, or combined Apc and KRAS mutations (1, 17). These studies underscore an essential role of KLF5 in promoting intestinal tumorigenesis.
Expression and activity of KLF5 can be regulated at transcriptional and posttranslational levels (21). Earlier studies identified several compounds or stimuli that modulate KLF5 expression, with consequent alteration in growth behavior, in either a positive (examples include phorbol ester, fetal bovine serum, epidermal growth factor, and lipopolysaccharide) (4, 12, 22) or negative (all-trans retinoic acid [ATRA] and mitogen-activated protein kinase [MAPK] inhibitors, PD98059 and U0125) (18, 20) manner.
In a proof-of-principle effort to identify novel compounds that inhibit KLF5 expression, we recently conducted a screen of 1,280 compounds in the Library of Pharmacologically Active Compounds (LOPAC1280) and identified several small molecules that inhibit the KLF5 promoter activity (23). Importantly, many of these inhibitors, which reduce KLF5 protein levels, also inhibit proliferation of colorectal cancer cell lines that exhibit high levels of endogenous KLF5. These results provided the rationale for conducting an additional high-throughput screen of a much larger compound library belonging to NIH’s MLPCN with the intention of identifying additional novel and potent small-molecule inhibitors of KLF5 expression. We anticipated that optimized screening leads could help understand the in vitro and in vivo consequences of knocking down KLF5 protein levels. Moreover, identified molecular probes could potentially be developed as novel therapeutic agents for treating colorectal cancer.
MATERIALS AND METHODS
Cell Lines
The human colorectal cancer cell line, DLD-1, and rat intestinal epithelial cell line, IEC-6, were purchased from the American Type Culture Collection (ATCC). DLD-1 cells were maintained in RPMI1640, supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. IEC-6 cells were grown in DMEM, supplemented with 5% FBS, 1% penicillin/streptomycin and 4µg/ml of human recombinant insulin. The DLD-1/pGL4.18hKLF5p cell line (23) was maintained in RPMI1640 with 10% FBS and 1% penicillin/streptomycin supplemented with 800 µg/ml of geneticin. We routinely performed morphology checks on all cell lines. Additionally, the cell lines were tested at Bionique Testing Laboratories for mycoplasma contamination. Furthermore, each experiment had controls conditions to assure the behavior of tested cell lines.
Reagents
Cell culture media, FBS, and geneticin were purchased from Invitrogen. The SteadyLite HTS assay kit was purchased from Perkin Elmer. Control compounds LY294002 and doxorubicin were purchased from Calbiochem and Sigma Aldrich, respectively. A cell-Titer Glo luciferase viability assay kit was purchased from Promega. A rabbit polyclonal antibody generated against amino acid positions 95–111 of the KLF5 protein was manufactured by QCB. Rabbit antibodies against EGFR, pEGFR, EGR1, pERK, p38, p-p38 were purchased from Cell Signaling. Rabbit antibodies against ERK were purchased from Millipore. Mouse monoclonal antibodies against β-actin were purchased from Sigma-Aldrich.
Ultrahigh-Throughput Screen (uHTS)
A. KLF5 Luciferase Cell-Based Screen
Prior to the start of the assay, 2,500 DLD-1/pGL4.18hKLF5p cells in 5 µl media per well were dispensed into 1,536-well plates. The assay was started immediately by dispensing 20 nl of the test compounds in DMSO (final DMSO concentration, 0.4%), DMSO alone (0% inhibition control), or LY294002 (final concentration, 200 µM, 100% inhibition control) to the appropriate wells. The plates were then incubated for 27 h at 37°C and equilibrated to room temperature for 30 minutes. The assay was stopped by dispensing 5 µl of SteadyLite HTS luciferase substrate to each well, followed by incubation at room temperature for 15 m. Well luminescence was measured on the ViewLux plate reader. The percent inhibition for each compound was calculated as follows: % Inhibition=[1−((Test_Compound − Median_High_Control)/(Median_Low_Control−Median__High_Control))]*100, where: Test_Compound is defined as luminescence of wells containing test compound. Low_Control is defined as luminescence of wells containing DMSO. High_Control is defined as luminescence of wells containing LY294002.
B. IEC-6 Cytotoxicity Counterscreen
Prior to the start of the assay, 1,250 IEC-6 cells in 5 µl media per well were dispensed into 1,536-well plates. The assay was started immediately by dispensing 20 nL of test compound in DMSO (final DMSO concentration, 0.4%), DMSO alone (0% inhibition control), or doxorubicin (final concentration, 150 µM, 100% inhibition control) to the appropriate wells. The plates were then incubated for 48 h at 37°C and equilibrated to room temperature for 30 minutes The assay was stopped by dispensing 5 µl of CellTiter-Glo reagent to each well, followed by incubation at room temperature for 15 minutes. Well luminescence was measured on the ViewLux plate reader. The percent inhibition for each compound was calculated as follows: % Inhibition=(1−((Test_Compound-Median_High_Control) / (Median_Low_Control − Median_High_Control)))*100, where: Test_Compound is defined as luminescence of wells containing test compound. Low_Control is defined as luminescence of wells containing DMSO. High_Control is defined as luminescence of wells containing doxorubicin.
Biological Activity and Chemical Structure Data
To determine nominally active compounds (“hits”) a cutoff algorithm was used, as described previously (24). We aggregated and analyzed the primary, confirmatory, and dose-response screening results of the human KLF5 promoter-luciferase inhibition assays and the IEC-6 cytotoxicity counterscreen, as well as data for luciferase inhibition data (to identify possible assay artifacts). Data for these assays are available online (http://pubchem.ncbi.nlm.nih.gov/): KLF5 (PubChem Assay IDs (AIDs) 1700 and 1835) and IEC-6 (PubChem AIDs 1825 and 1905) primary and confirmatory HTS data as well as dose-response data (PubChem AIDs 1973 and 1975). Luciferase inhibition data from PubChem AIDs 411 and 1379 was aggregated prior to use. For all compounds, a global PubChem promiscuity Index (PCIdx) was computed as previously described (25). Briefly, PCIdx is the ratio of the number of assays reported in PubChem in which a compound is found active and the number of assays in which it was tested. All chemical structures associated with the screening results were downloaded from PubChem using Substance Identifier number (SID).
Structure-Activity Response (SAR) Clustering and Reporting
For the most active confirmed hits (those with KLF5 dose response IC50<10 µM) we identified all structurally-related compounds screened. This information provides insight into substructural features associated with KLF5 inhibition and regions of hit molecule amenable to structural variability. Briefly, we employed ECFP6 fingerprints (26) and the Tanimoto similarity threshold of 0.6 using Accelrys Pipeline Pilot (27). The resulting set of compounds were then clustered by maximum common substructure (MCS) with a minimum MCS size of 15 using LibMCS Clustering from ChemAxon (28). Only clusters that had at least one seed compound (i.e. a confirmed screening hits with KLF5 IC50<10 µM) were kept.
All results including chemical structures obtained after clustering, their biological activity data, promiscuity data, and cluster information were then assembled into an interactive SAR cluster report using the Pipeline Pilot reporting collection (Supplementary Figure 3). MCS cores were highlighted for easier review of SAR in each series. The report displays the most active compounds of each cluster in overview section, which links to each cluster series. Compounds are displayed as a matrix of chemical structure with the numerical and textual data shown underneath.
Western Blot Analysis
Prior to the start of the assay, 2 × 105 cells in 1 ml media per well were seeded into 12-well plates. The assay was started 24 h post-seeding by dispensing 2 µl of test compounds in DMSO (final DMSO concentration, 0.2%) or DMSO alone to the appropriate wells. After 24 h incubation the cells were lysed in 200 µl Laemmli buffer and subjected to electrophoresis in polyacrylamide gel. The proteins were then transferred to a nitrocellulose membrane and developed with appropriate antibodies. The developed films were scanned and densitometry was performed using Scion Image software (NIH Image site: http://rsb.info.nih.gov/nih-image/).
Cell Viability Assay
Prior to the start of the assay, 104 cells in 100 µl media per well were dispensed into 96-well plates. The assay was started immediately by dispensing 0.5 µl of test compounds in DMSO (final DMSO concentration, 0.4%) or DMSO alone to the appropriate wells. The plates were incubated for 48 h and well luminescence was measured on the Biotek Synergy 2 plate reader according to manufacturer’s protocol.
Statistical Analysis
Nonlinear regression (curve fit) test with sigmoidal dose-response was performed using GraphPad Prism for Macintosh, GraphPad Software, San Diego California USA, www.graphpad.com.
RESULTS
Ultrahigh-Throughput Screen for KLF5 Inhibitors
We conducted a primary screen of the NIH small molecule library, which consisted of 290,735 compounds, using the DLD-1/pGL4.18hKLF5p reporter cell line (Figure 1; PubChem AID 1700). This cell line stably expresses a luciferase reporter gene under the control of 1,959-nucleotides of the human KLF5 promoter (23). The compounds were added at a final concentration of 5 µM in 0.4% DMSO. LY294002 (23) at 200 µM concentration was used as a positive control with the ability to inhibit 100% luciferase activity. DMSO at 0.4% was used as negative control (Supplementary Figure 1A). The mean Z′-factor of the primary screen was 0.58 ± 0.05 (n=240 plates). The range in percent inhibition of luciferase activity for all compounds tested in the screen exhibits a broad distribution in which the majority of compounds were inactive (Supplementary Figure 1A).
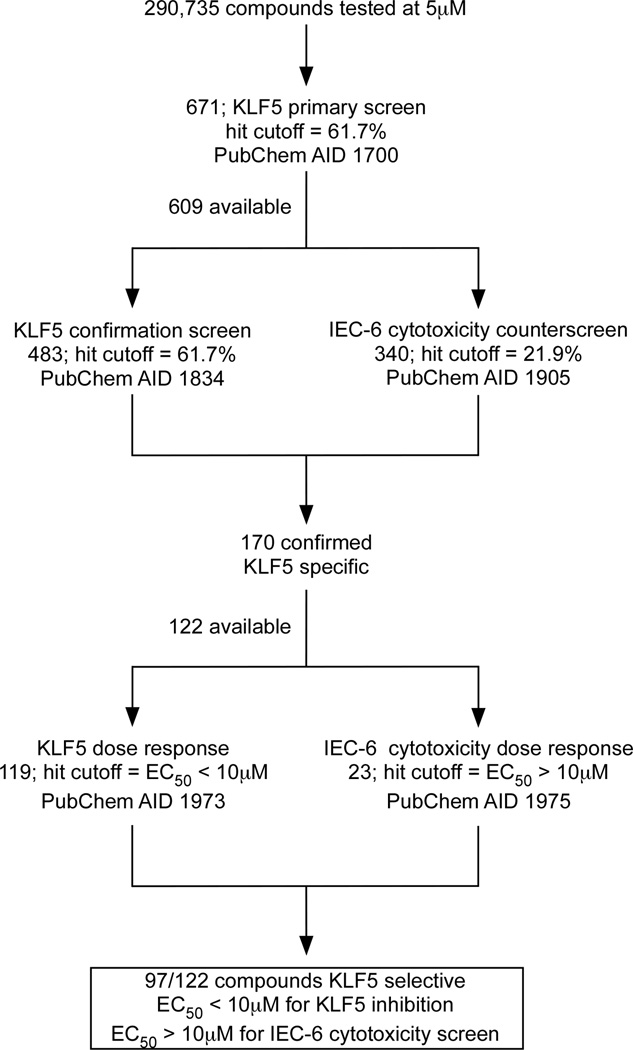
Figure 1.
An outline of the ultrahigh-throughput screen strategy to identify KLF5 inhibitors. Summaries of the probe development efforts are deposited in the NIH database (PubChem BioAssays http://pubchem.ncbi.nlm.nih.gov/). Each assay has an ascribed PubChem assay identification (AID) number. The hit cutoff rate for KLF5 primary and confirmatory screens and for IEC-6 cytotoxicity counterscreen were calculated based on the average percentage of inhibition of all tested compounds with the hit threshold selection of μ±3σ, where μ is the mean value and σ is the standard deviation of the entire assay (24).
Six hundred and seventy one library members (0.23%) achieved the active criteria of > 61.7% inhibition of luciferase activity at 5 µM (Supplementary Figure 1A and 1B). Six hundred and nine of these compounds were available for KLF5 confirmatory screen and IEC-6 cytotoxicity counterscreen (Figure 1). The KLF5 confirmatory screen was performed in the same fashion as the primary screen and yielded 483 hits with a hit cutoff at 61.7% (Figure 1; PubChem AID 1834). At the same time, IEC-6 cytotoxicity screen was performed and yielded 340 positive hits using a viability cutoff of 21.9% (Figure 1; PubChem AID 1905).
The KLF5 confirmatory assay and IEC-6 conuterscreen identified 170 compounds that were KLF5-specific, i.e. inhibited luciferase activity in DLD-1/pGL4.18hKLF5p reporter cell line > 61.7% and were not cytotoxic to IEC-6 cells, i.e. exhibited < 21.9% inhibition in the counterscreen. One hundred and twenty-two available compounds in this group were then subjected to dose-response assays in the DLD-1/pGL4.18hKLF5p and IEC-6 cell lines. One hundred nineteen compounds had EC50<10 µM in luciferase dose-response assay and 23 had EC50>10 µM in IEC-6 cytotoxicity dose-response assay (Figure 1; PubChem AIDs 1973 and 1975, respectively). This yielded 97 KLF5-selective compounds with EC50<10 µM for KLF5 inhibition and EC50>10 µM for IEC-6 cytotoxicity (Figure 1).
Structure-Activity Response (SAR) Cluster Analysis of KLF5 Inhibitors
Prior to data analysis all screening results from primary, confirmatory, and dose-response assays of KLF5 inhibition and IEC-6 cytotoxicity counterscreen were aggregated. In addition, to exclude possible artifacts, luciferase inhibition data and global PubChem promiscuity was also obtained. Clusters of chemical series related to the most active KLF5 inhibitors were then generated and all information was assembled into an interactive SAR cluster report. One hundred and fifteen structural clusters were identified with cluster size ranging from 1 – 25 compounds (Supplementary Figure 2). The interactive cluster report is provided as Supplementary Figure 3. After medicinal chemistry review of the active clusters, 19 compounds were selected for further follow-up (Supplementary Figure 4).
Validation of Hit Compounds
Primary Western Blot Analysis
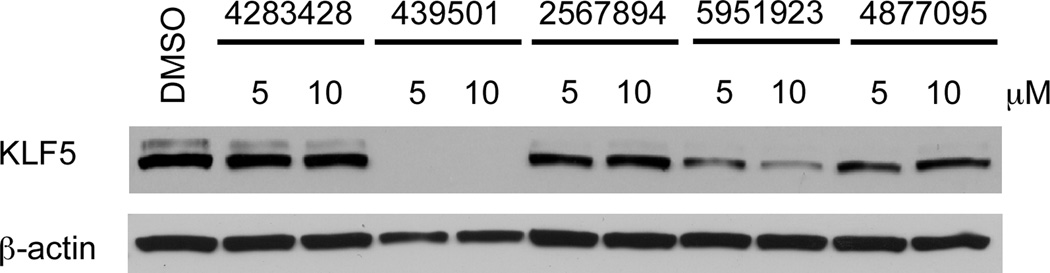
We tested 19 selected compounds in DLD-1 cell line with respect to their ability to decrease endogenous levels of KLF5 protein. The cells were treated for 24 h with 5 and 10 µM of each compound. Western blot analysis revealed that five compounds, CIDs 4283428, 439501, 2567894, 5951923, and 4877095, reduced endogenous KLF5 levels to the control level (Figure 2; Supplementary Table 1). The inhibitory effects of compounds CIDs 439501 and 5951923 were more pronounced than were those of the other three tested compounds (Figure 2; Supplementary Table 1).
Figure 2.
Western blot analyses of the effects of select compounds from the uHTS campaign on KLF5 protein levels in DLD-1 cells. DLD-1 cells were treated with 5 or 10 µM of the compounds in 0.4% DMSO or DMSO alone for 24 h before extraction of proteins for Western blotting. The membranes were probed with antibodies against KLF5 and β-actin. The ID numbers of the compounds correspond to their PubChem Compound ID (CID).
Primary Cell Viability Analysis
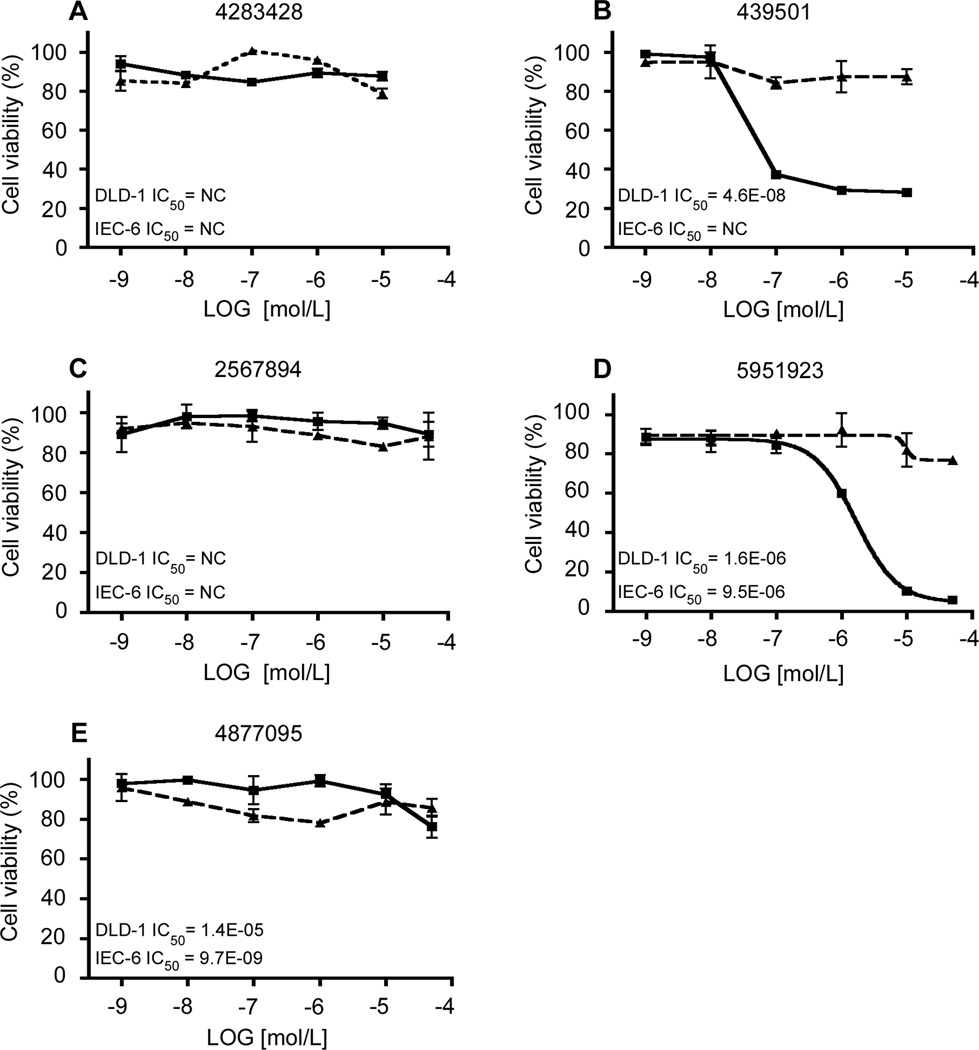
We then determined the effect of the 5 compounds in Figure 2 on the rate of proliferation of DLD-1 and IEC-6 cells. We treated each cell line for 48 h with various concentrations of the compounds. This was followed by Cell-Titer Glo assay to measure the rate of cell proliferation. As seen in Figure 3, while none of the compounds affected the viability of IEC-6 cells, two, CIDs 439501 and 5951923, inhibited proliferation of DLD-1 cells in a dose-dependent manner (Figure 3D and 3E, respectively). These two compounds had an IC50 of 0.046 µM and 1.6 µM, respectively. The potency of inhibition correlated with each compound’s ability to reduce endogenous KLF5 levels (Figure 4). In contrast, neither CIDs 4395011 nor 5951923 reduced endogenous KLF5 levels or viability of IEC-6 cells over a wide range of concentrations (Supplementary Figure 5).
Figure 3.
Cell viability assays of DLD-1 and IEC-6 cells treated with the same compounds assayed in Figure 2. Cells were seeded in 96-well plates with medium containing DMSO or increasing concentrations of CIDs 4283428 (A), 439501 (B), 2567894 (C), 5951923 (D) and 4877095 (E), for 2 d before the measurement of luciferase activity with Cell-Titer Glo assays. The control (cells with medium containing only DMSO) was defined as 100% and the results from other measurements were calculated accordingly. IC50 was calculated for each compound, NC – not converged. Each experiment was performed in triplicates. DLD-1 – solid lines, IEC-6 – dashed lines.
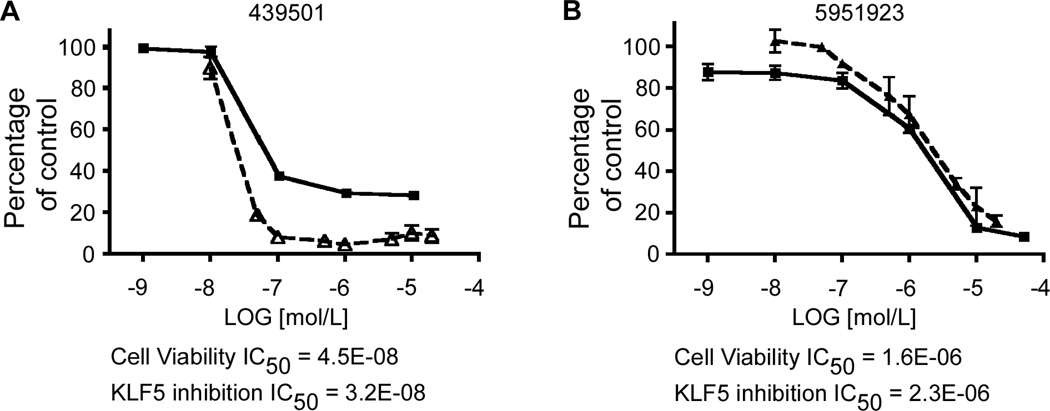
Figure 4.
Cell viability and KLF5 inhibition assays performed in DLD-1 cells. For the cell viability assay, DLD-1 cells were seeded in 96-well plates with medium containing DMSO or increasing concentrations of the compounds: CIDs 439501 (A) and 5951923 (B), for 2 d before the measurement of luciferase with Cell-Titer Glo assays. For KLF5 inhibition, DLD-1 cells were treated with DMSO or increasing concentrations of the compounds, CIDs 439501 (A) and 5951923 (B), and protein extracts were collected for Western blotting analyses with KLF5 and β-actin antibodies. The control (cells with medium containing DMSO) was defined as 100% and the results from other measurements were calculated accordingly. IC50 was calculated for both compounds. Each experiment was performed in triplicate. Cell viability – solid lines, KLF5 levels – dashed lines.
CID 439501 is known as ouabain, a cardiac glycoside that inhibits Na+/K+ ATPase and is used to treat congestive heart failure (29). Cardiac glycosides have been shown to have anti-tumor properties in a number of model systems, but clinical utility has not yet been demonstrated (30). Ouabain has a history of showing promise in antitumor assays, yet several efforts to find structural analogs with specificity against certain cancer cell lines and acceptable levels of off-target cytotoxicity have not succeeded (Daniel Zaharevitz, personal communication). For this reason our attention was focused on the second compound, CID 5951923, with a structure previously not linked to antitumor activity and with the sufficiently low level of off-target activity. With regard to its activity in other assays, CID 5951923 has to date been tested in 342 high-throughput bioassays reported in PubChem and was identified as an active compound in only 14 of these assays (4.1%) (Supplementary Table 2; PubChem BioAssays: http://pubchem.ncbi.nlm.nih.gov/). Six of the fourteen assays were directly connected with our efforts to identify KLF5 inhibitors (Supplementary Table 2).
The PubChem assay selectivity analysis and the data showing selective KLF5 activity led us to design and synthesize analogs of CID 5951923. Our aim was to identify more potent compounds with respect to decreasing KLF5 protein levels and viability of colon cancer cell lines, while also determining whether potentially problematic substructure features of CID 5951923, such as the ester group, the nitroaryl group, and the conjugated double bond, could be replaced without loss of KLF5-specific activity.
The Effects of CID 5951923 and its Derivatives on Cell Signaling
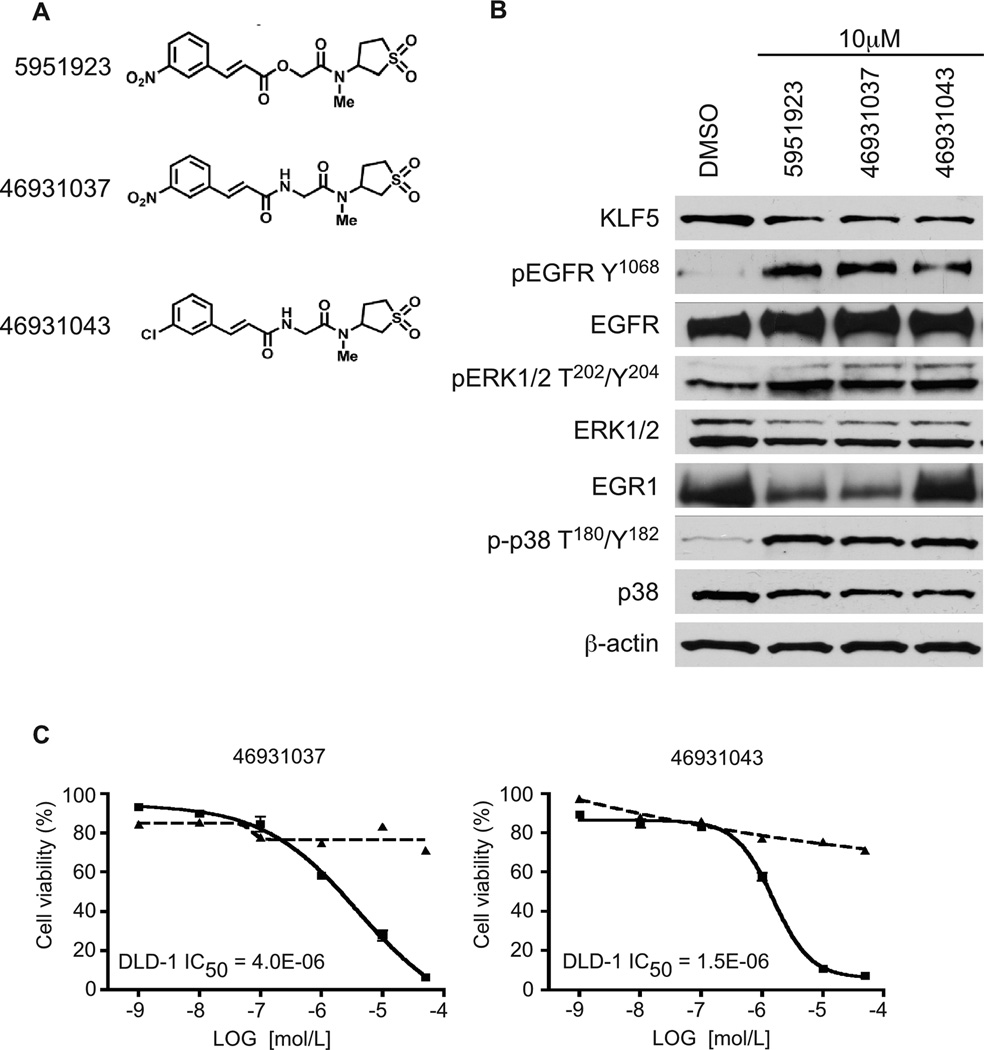
We synthesized 22 structural analogs of CID 5951923 and performed luciferase assays using the DLD-1/pGL4.18hKLF5p reporter cell line at 10 µM concentration for each compound. Based on the results of this experiment (data not shown), we selected two compounds with similar KLF5 activity to CID 5951923. The structures of CID 5951923 and synthesis pathways and the structures of the two most active analogs, CIDs 469301037 and 469301043, are shown in Figure 5A, Supplementary Figures 6, 7 and 8. Notably the ester group maybe replaced with an amide group and additionally the aromatic group can be replaced with a chlorine atom without erosion of activity (CID 46931043).
Figure 5.
Western blot and cell viability analyses of CID 5951923 and its two derivatives, CIDs 469301037 and 469301043, on DLD-1 cells. (A) Chemical structures of CIDs 5951923, 469301037, and 469301043. (B) DLD-1 cells were treated with 10 µM of the compounds in 0.4% DMSO or DMSO alone for 24 h before extraction of proteins for Western blotting against the indicated proteins. (C) For cell viability assay, DLD-1 cells were seeded in 96-well plates with medium containing DMSO or increasing concentrations of the compounds, CIDs 469301037 and 469301043, for 2 d before the measurement of luciferase activity with Cell-Titer Glo assays. The control (cells with medium containing only DMSO) was defined as 100% and the results from other measurements were calculated accordingly. IC50 was calculated for both compounds. Each experiment was performed in triplicate. DLD-1 – solid lines, IEC-6 – dashed lines.
We examined the effects of these compounds on several signaling pathways. As seen in Figure 5B, at 10 µM, all three compounds significantly decreased endogenous KLF5 levels in the DLD-1 cell line when compared to control. We noticed that phosphorylation levels of pEGFFpY1068 were increased after treatment with the three compounds. There was an upregulation of phosphorylation of pERK1/2pT202/Y204. Two of the compounds, CIDs 5951923 and 46931037, downregulated EGR1, which was shown to directly modulate KLF5 expression at a transcriptional level (20, 22). All three compounds increased phosphorylation levels of p-p38 T180/Y182, without any impact on the unphosphorylated form of p38. There were no changes in activity of the AKT, JNK and p70 S6 kinase pathways after treatment with tested compounds (data not shown). Both CIDs 469301037 and 469301043, inhibited proliferation of DLD-1 cells to a similar extent as their parent compound (Figure 5C). Neither had any appreciable effect on the viability of IEC-6 cells (Figure 5C).
Effects of Compound 5951923 on Proliferation of Cancer Cell Lines in the NCI-60 Panel
The National Cancer Institute offers as part of the Developmental Therapeutics Program the possibility of testing the activity of promising antitumor agents versus a panel of 60 different cancer cell lines (NCI-60 panel, http://dtp.cancer.gov/docs/common_files/submit_compounds.html). A screen was performed with a concentration of 10 µM of CID 5951923 on the NCI-60 panel by the Drug Synthesis and Chemistry Branch at National Cancer Institute. The results are presented in Supplementary Figure 9C. Although potency was weak, what inhibition there is seems to be mainly in cell lines that highly express KLF5 (Supplementary Figure 9B). The exceptions were poor inhibition in the highly expressing KM12 colon cancer cell line and inhibition in a number of leukemia cell lines. These results are consistent with the notion that KLF5 is the primary mediator for the inhibitory effect of compound CID 5951923 on proliferation on colon cancer cells. Microarray analysis of the NCI-60 panel showed that colon cancer cell lines had the highest KLF5 mRNA levels compared to cancer cells of other tissue origins (Supplementary Figure 9). These results suggest that KLF5 is the primary mediator responsible for the effects of CID 5951923 in slowing the proliferation of colon cancer cells.
DISCUSSION
To identify novel KLF5 inhibitors, we performed an ultrahigh-throughput screen of the MLPCN library of small molecules using cell-based luciferase reporter assay in the DLD-1/pGL4.18hKLF5p cell line. To identify true positives we performed confirmatory KLF5 screens as well as cytotoxicity and luciferase counterscreens to select for compounds that decrease luciferase reporter activity driven by the human KLF5 promoter in DLD-1 cells but have no impact on the viability of IEC-6 cells. Both the confirmatory screen and counterscreen were repeated at a dose range. From these efforts, 97 compounds were identified that were KLF5 selective in DLD-1 cells but had little effect on viability of IEC-cells. The number of inhibitors was further narrowed down using a combination of computational, Western blot, and cell viability analyses. This led to the identification of two potent KLF5-selective inhibitors, CIDs 439501 and 5951923.
CID 439501 is ouabain that was originally found in the seeds of African plant Strophanthus gratus. It belongs to the family of cardiac glycosides and has been used for treatment of congestive heart failure. Due to the narrow therapeutic window, ouabain is not in use in other applications (29). Moreover, its proposed mechanism of action which includes disruption of mitochondrial membrane potential, increase Ca2+ uptake, sustain reactive oxygen species production, and selective protein kinase C activation through its binding to the subunit of Na+/K+ ATPase (31–34) seems disconnected to KLF5 activity. Ouabain preferentially binds the α3 subunit over the α1 subunit of Na+/K+ ATPase and the lack of expression of the α3 subunit confers resistance to ouabain, as is the case for rodent cells. It is of interest to note that colon cancer cells have a higher ratio of α3 to α1 subunit compared to normal colonic epithelial cells (35). This may explain the exquisite sensitivity of colon cancer cells to ouabain as observed in the current study. There have been numerous attempts to modify the structure of ouabain, but any altered structural changes caused loss of its activity. This compound was tested in the NCI-60 panel and is growth inhibitory to all tested human cancer cell lines (http://dtp.cancer.gov/dtpstandard/servlet/MeanGraphSummary?searchtype=NSC&outputformat=html&searchlist=25485). As potential use of ouabain in cancer treatment is controversial, additional studies are needed to demonstrate efficacy. We should mention that during our previous proof-of-principle screen with the LOPAC1280 library, ouabain and dihydroouabain, were identified as potential KLF5 inhibitors (23).
The second compound, identified in this uHTS screen, CID 5951923, is a novel agent and is more appealing due to its structural novelty and lack of assay promiscuity, with activity in fourteen of 342 high-throughput assays deposited in PubChem database (Supplementary Table 2). Six of the listed assays are related to this KLF5 inhibitor effort. Of the remaining eight assays, three (PubChem AIDs 2717, 449748, and 463074) were designed to identify inhibitors of breast cancer stem cells. It is of interest to note that KLF5 plays a role in embryonic stem cell renewal by maintaining the cells in an undifferentiated state (7, 8). Our lab is currently examining the role of KLF5 in regulating proliferation of adult intestinal stem cells and colon cancer stem cells. Another screen (PubChem AID 1578) that led to the identification of CID 5951923 as an active compound used luciferase reporter assay driven by a NFκB promoter. The purpose of this screen was to identify compounds that inhibit NOD1, which is involved in the transmission of signal during inflammation and is responsible for activation of NFκB. Our group previously showed that activation of NFκB is KLF5-dependent and that downregulation of KLF5 abrogates the activation of NFκB upon lipopolysaccharide stimulation (12).
Given the evidence linking CID 5951923 to KLF5-specific inhibition, structural analogs were prepared and two of these compounds, CIDs 46931037 and 46931043, similarly affect KLF5 protein levels and improve upon one or more unwanted structural aspects of the parent, CID 5951923. We found that the parent compound and both of these analogs activate the p38 pathway as seen by phosphorylation of p38 at T180/Y182 sites (Figure 5). Increase of p38 phosphorylation is associated primarily with inflammation and stress induction (36). Other findings demonstrate a role for p38 in cell proliferation, differentiation, and tumorigenesis. For example, recent results suggest that activation of p38 leads to a decrease of cell proliferation, induction of cell differentiation, and suppression of tumorigenesis (37, 38). Moreover, p38 has been shown to negatively regulate cell proliferation by stimulating EGFR phosphorylation and internalization as well as through induction of p53 in response to stress stimuli (39, 40). The exact molecular mechanism of interaction of p38 and KLF5 needs to be however determined.
We found that the three lead compounds also activate the EGFR/ERK pathway by stimulating phosphorylation of EGFR at Y1068 and ERK1/2 at T202/Y204 sites (Figure 5). Moreover, CIDs 5951923 and 46931037 decreased EGR1 protein levels. It has been shown that KLF5 expression is induced by the mitogen-activated protein kinases (MAPKs), MEK/ERK, through activation of EGR1 (22). As a downstream target of the MAPK pathway, we demonstrated that KLF5 mediates the transforming effect of oncogenic HRAS in fibroblasts in an EGR1-dependent manner (19, 20) and oncogenic KRAS in intestinal epithelial cells (1). In squamous epithelial cells of the esophagus, KLF5 influences cell proliferation by activating EGFR transcription, leading to the induction of the MEK/ERK pathway (41). Conversely, EGFR and MEK/ERK stimulate expression of KLF5, forming a positive feedback loop (41). Given that EGR1 is situated in a critical location, downstream of EGFR/RAS/MEK/ERK and upstream of KLF5, it is possible that EGR1 provides a negative feedback of its upstream events. Alternatively, the induction of EGFR/MEK/ERK pathway in drug-treated cells represents a compensatory mechanism by which the cells attempt to offset the inhibitory effects of the compounds. The exact molecular mechanisms by which CIDs 59551923, 46931037, and 46931043 affect KLF5 expression, including crosstalk between different signaling pathways such as that of p38 and EGFR/MEK/ERK, remain to be established.
An important observation of our study is the correlation between the ability of the compounds identified by the uHTS, including CIDs 5951923 and 439501, to reduce KLF5 expression and the viability of colon cancer cells (see, for example, Figure 4). Moreover, neither compound affects KLF5 levels and viability of the non-transformed intestinal epithelial cells, IEC-6 (Supplementary Figure 5). These findings indicate that the compounds are selective for colon cancer cells over non-transformed cells, a feature critically important for the development of cancer therapeutics. It is also interesting to note that among the cancer cell lines in the NCI-60 panel, colorectal cancer cells have the highest basal KLF5 mRNA levels and are most likely to be inhibited by CID 5951923 (Supplementary Figure 9). These results suggest that CID 5951923 or a close structural analog may be a useful probe to study KLF5 function. A similarly target-specific more potent analog may offer promise as a colon cancer-specific therapeutic agent.
In summary, we used an uHTS approach to identify novel compounds with potential therapeutic benefits by targeting KLF5 expression, a therapeutically relevant target commonly over-expressed in colon cancer cells. The screen yielded several potential candidates, one of which (CID 5951923), appears to be highly selective for colon cancer cells. Two synthetic analogues of CID 5951923 also appeared to be equally effective. We are now in the process of generating additional derivatives of CID 5951923 with the hope of further increasing KLF5-specific activity and drug-likeness. We believe that this lead will prove useful as a probe for understanding KLF5 function and that its analogs may be suitable for the preclinical evaluation in animal models of colon cancer.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Dr. Haian Fu and Yuhong Du for their help with using screening facility equipment at Chemical Biology Discovery Center at Emory University, and Pierre Baillargeon, Lina DeLuca, and Dr. Louis Scampavia at The Scripps Research Institute Molecular Screening Center for compound management and quality control of compounds. Dr. Hugh Rosen, Dr. Steven Brown, and Dr. William R. Roush for their help with shaping the screening project, and Becky A. Mercer for her work towards submission of the final report of Chemical Probe Development Plan (CPDP) to PubChem. We would also like to thank Katharine Emery for help with facilitating the collaboration among the involved Institutions. The National Institutes of Health Molecular Library Screening Center Network (MLPCN, Grant# MH084512) supported the research efforts of TB, PC, SC, MC, YH, PH, FM, SS, and TS.
Financial Support: NIH DA26215, DK052230, DK64399, CA084179 and MH084512.
Abbreviations
- AID
assay ID
- CID
compound ID
- IEC-6
intestinal epithelial cell-6
- KLF5
Krüppel-like factor 5
- LOPAC
library of pharmacologically active compounds
- MAPK
mitogen-activated protein kinase
- MCS
maximum common substructure
- SAR
structure-activity relationship
- SID
substance ID
- uHTS
ultrahigh-throughput screen
REFERENCES
- 1.Nandan MO, Ghaleb AM, McConnell BB, Patel NV, Robine S, Yang VW. Kruppel-like factor 5 is a crucial mediator of intestinal tumorigenesis in mice harboring combined ApcMin and KRASV12 mutations. Mol Cancer. 2010;9:63. doi: 10.1186/1476-4598-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghaleb AM, Nandan MO, Chanchevalap S, Dalton WB, Hisamuddin IM, Yang VW. Kruppel-like factors 4 and 5: the yin and yang regulators of cellular proliferation. Cell Res. 2005;15:92–96. doi: 10.1038/sj.cr.7290271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nandan MO, McConnell BB, Ghaleb AM, Bialkowska AB, Sheng H, Shao J, et al. Kruppel-like factor 5 mediates cellular transformation during oncogenic KRAS-induced intestinal tumorigenesis. Gastroenterology. 2008;134:120–130. doi: 10.1053/j.gastro.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun R, Chen X, Yang VW. Intestinal-enriched Kruppel-like factor (Kruppel-like factor 5) is a positive regulator of cellular proliferation. J Biol Chem. 2001;276:6897–6900. doi: 10.1074/jbc.C000870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sur I, Rozell B, Jaks V, Bergstrom A, Toftgard R. Epidermal and craniofacial defects in mice overexpressing Klf5 in the basal layer of the epidermis. J Cell Sci. 2006;119:3593–36601. doi: 10.1242/jcs.03070. [DOI] [PubMed] [Google Scholar]
- 6.Oishi Y, Manabe I, Tobe K, Tsushima K, Shindo T, Fujiu K, et al. Kruppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 2005;1:27–39. doi: 10.1016/j.cmet.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Jiang J, Chan YS, Loh YH, Cai J, Tong GQ, Lim CA, et al. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10:353–360. doi: 10.1038/ncb1698. [DOI] [PubMed] [Google Scholar]
- 8.Parisi S, Passaro F, Aloia L, Manabe I, Nagai R, Pastore L, et al. Klf5 is involved in self-renewal of mouse embryonic stem cells. J Cell Sci. 2008;121:2629–2634. doi: 10.1242/jcs.027599. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Tetreault MP, Yermolina YA, Goldstein BG, Katz JP. Kruppel-like factor 5 controls keratinocyte migration via the integrin-linked kinase. J Biol Chem. 2008;283:18812–18820. doi: 10.1074/jbc.M801384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagai R, Suzuki T, Aizawa K, Shindo T, Manabe I. Significance of the transcription factor KLF5 in cardiovascular remodeling. J Thromb Haemost. 2005;3:1569–1576. doi: 10.1111/j.1538-7836.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 11.Dong JT, Chen C. Essential role of KLF5 transcription factor in cell proliferation and differentiation and its implications for human diseases. Cell Mol Life Sci. 2009;66:2691–2706. doi: 10.1007/s00018-009-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chanchevalap S, Nandan MO, McConnell BB, Charrier L, Merlin D, Katz JP, et al. Kruppel-like factor 5 is an important mediator for lipopolysaccharide-induced proinflammatory response in intestinal epithelial cells. Nucleic Acids Res. 2006;34:1216–1223. doi: 10.1093/nar/gkl014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conkright MD, Wani MA, Anderson KP, Lingrel JB. A gene encoding an intestinal-enriched member of the Kruppel-like factor family expressed in intestinal epithelial cells. Nucleic Acids Res. 1999;27:1263–1270. doi: 10.1093/nar/27.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregorieff A, Pinto D, Begthel H, Destree O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. 2005;19:877–890. doi: 10.1101/gad.1295405. [DOI] [PubMed] [Google Scholar]
- 16.Ziemer LT, Pennica D, Levine AJ. Identification of a mouse homolog of the human BTEB2 transcription factor as a beta-catenin-independent Wnt-1-responsive gene. Mol Cell Biol. 2001;21:562–574. doi: 10.1128/MCB.21.2.562-574.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McConnell BB, Bialkowska AB, Nandan MO, Ghaleb AM, Gordon FJ, Yang VW. Haploinsufficiency of Kruppel-like factor 5 rescues the tumor-initiating effect of the Apc(Min) mutation in the intestine. Cancer Res. 2009;69:4125–4133. doi: 10.1158/0008-5472.CAN-08-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chanchevalap S, Nandan MO, Merlin D, Yang VW. All-trans retinoic acid inhibits proliferation of intestinal epithelial cells by inhibiting expression of the gene encoding Kruppel-like factor 5. FEBS Lett. 2004;578:99–105. doi: 10.1016/j.febslet.2004.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nandan MO, Chanchevalap S, Dalton WB, Yang VW. Kruppel-like factor 5 promotes mitosis by activating the cyclin B1/Cdc2 complex during oncogenic Ras-mediated transformation. FEBS Lett. 2005;579:4757–4762. doi: 10.1016/j.febslet.2005.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nandan MO, Yoon HS, Zhao W, Ouko LA, Chanchevalap S, Yang VW. Kruppel-like factor 5 mediates the transforming activity of oncogenic H-Ras. Oncogene. 2004;23:3404–3413. doi: 10.1038/sj.onc.1207397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu R, Zheng HQ, Zhou Z, Dong JT, Chen C. KLF5 promotes breast cell survival partially through fibroblast growth factor-binding protein 1-pERK-mediated dual specificity MKP-1 protein phosphorylation and stabilization. J Biol Chem. 2009;284:16791–16798. doi: 10.1074/jbc.M808919200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawai-Kowase K, Kurabayashi M, Hoshino Y, Ohyama Y, Nagai R. Transcriptional activation of the zinc finger transcription factor BTEB2 gene by Egr-1 through mitogen-activated protein kinase pathways in vascular smooth muscle cells. Circ Res. 1999;85:787–795. doi: 10.1161/01.res.85.9.787. [DOI] [PubMed] [Google Scholar]
- 23.Bialkowska AB, Du Y, Fu H, Yang VW. Identification of novel small-molecule compounds that inhibit the proproliferative Kruppel-like factor 5 in colorectal cancer cells by high-throughput screening. Mol Cancer Ther. 2009;8:563–570. doi: 10.1158/1535-7163.MCT-08-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodder P, Cassaday J, Peltier R, Berry K, Inglese J, Feuston B, et al. Identification of metabotropic glutamate receptor antagonists using an automated high-throughput screening system. Anal Biochem. 2003;313:246–254. doi: 10.1016/s0003-2697(02)00608-5. [DOI] [PubMed] [Google Scholar]
- 25.Schurer SC, Vempati U, Smith R, Southern M, Lemmon V. BioAssay Ontology Annotations Facilitate Cross-Analysis of Diverse High-Throughput Screening Data Sets. J Biomol Screen. 2011;16:415–426. doi: 10.1177/1087057111400191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers D, Hahn M. Extended-connectivity fingerprints. J Chem Inf Model. 2010;50:742–754. doi: 10.1021/ci100050t. [DOI] [PubMed] [Google Scholar]
- 27.Pipeline Pilot 8.0. San Diego, CA: Accelrys; 2010. [Google Scholar]
- 28.ChemAxon JChem Software Suite. Budapest, HU: ChemAxon; 2010. [Google Scholar]
- 29.Newman RA, Yang P, Pawlus AD, Block KI. Cardiac glycosides as novel cancer therapeutic agents. Mol Interv. 2008;8:36–49. doi: 10.1124/mi.8.1.8. [DOI] [PubMed] [Google Scholar]
- 30.Vaklavas C, Chatzizisis YS, Tsimberidou AM. Common cardiovascular medications in cancer therapeutics. Pharmacol Ther. 2011;130:177–190. doi: 10.1016/j.pharmthera.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol. 2007;293:C509–C536. doi: 10.1152/ajpcell.00098.2007. [DOI] [PubMed] [Google Scholar]
- 32.Chen JQ, Contreras RG, Wang R, Fernandez SV, Shoshani L, Russo IH, et al. Sodium/potassium ATPase (Na+, K+-ATPase) and ouabain/related cardiac glycosides: A new paradigm for development of anti- breast cancer drugs? Breast Cancer Res Treat. 2006;96:1–15. doi: 10.1007/s10549-005-9053-3. [DOI] [PubMed] [Google Scholar]
- 33.Nesher M, Shpolansky U, Rosen H, Lichtstein D. The digitalis-like steroid hormones: new mechanisms of action and biological significance. Life Sci. 2007;80:2093–2107. doi: 10.1016/j.lfs.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Tian J, Haas M, Shapiro JI, Askari A, Xie Z. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J Biol Chem. 2000;275:27838–27844. doi: 10.1074/jbc.M002950200. [DOI] [PubMed] [Google Scholar]
- 35.Sakai H, Suzuki T, Maeda M, Takahashi Y, Horikawa N, Minamimura T, et al. Up-regulation of Na(+),K(+)-ATPase alpha 3-isoform and down-regulation of the alpha1-isoform in human colorectal cancer. FEBS Lett. 2004;563:151–154. doi: 10.1016/S0014-5793(04)00292-3. [DOI] [PubMed] [Google Scholar]
- 36.Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 37.Hui Lx, Bakiri L, Stepniak E, Wagner EF. p38alpha: a suppressor of cell proliferation and tumorigenesis. Cell Cycle. 2007;6:2429–2433. doi: 10.4161/cc.6.20.4774. [DOI] [PubMed] [Google Scholar]
- 38.Han J, Sun P. The pathways to tumor suppression via route p38. Trends Biochem Sci. 2007;32:364–371. doi: 10.1016/j.tibs.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Bulavin DV, Fornace AJ., Jr p38 MAP kinase's emerging role as a tumor suppressor. Adv Cancer Res. 2004;92:95–118. doi: 10.1016/S0065-230X(04)92005-2. [DOI] [PubMed] [Google Scholar]
- 40.Zwang Y, Yarden Y. p38 MAP kinase mediates stress-induced internalization of EGFR: implications for cancer chemotherapy. Embo J. 2006;25:4195–4206. doi: 10.1038/sj.emboj.7601297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y, Goldstein BG, Nakagawa H, Katz JP. Kruppel-like factor 5 activates MEK/ERK signaling via EGFR in primary squamous epithelial cells. Faseb J. 2007;21:543–550. doi: 10.1096/fj.06-6694com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.