Abstract
Marine waste is an abundant renewable source for the recovery of several value added metabolites with potential industrial applications. This study describes the production of chitinase on marine waste, with the subsequent use of the same marine waste for the extraction of antioxidants. A chitinase-producing bacterium isolated from seafood effluent was identified as Alcaligenes faecalis AU02. Optimal chitinase production was obtained in culture conditions of 37°C for 72 h in 100 ml medium containing 1% shrimp and crab shell powder (1:1) (w/v), 0.1% K2HPO4, and 0.05% MgSO4·7H2O. The molecular weight of chitinase was determined by SDS-PAGE to be 36 kDa. The optimum pH, temperature, pH stability, and thermal stability of chitinase were about 8, 37°C, 5–12, and 40–80°C, respectively. The antioxidant activity of A. faecalis AU02 culture supernatant was determined through scavenging ability on 1,1-diphenyl-2-picrylhydrazyl (DPPH) as 84%, and the antioxidant compound was characterized by TLC and its FT-IR spectrum. The present study proposed that marine wastes can be utilized to generate a high-value-added product and that pharmacological studies can extend its use to the field of medicine.
Keywords: Chitinase, Marine waste, Alcaligenes faecalis, Antioxidant
Introduction
Chitin—a polysaccharide consisting of 1,4-linked N-acetyl-d-glucosamine moieties—is the second most abundant biopolymer on Earth and a constant source of renewable raw materials. Recent studies have focused on converting chitin to oligosaccharides as these are not only water-soluble but also possess versatile functional properties such as anti-tumor and antimicrobial activities (Suzuki et al. 1986; Wang et al. 2008a; Liang et al. 2007). Traditionally, the preparation of chitin involves demineralization and deproteinization of shellfish waste with the use of strong acids or bases (Synowiecki and Al-Khateeb 2000; Wang et al. 2008b). There are several problems with such existing chemical processes, including the large amount of short-chain oligosaccharides produced, low yields of oligosaccharides, the high costs of separation, and also environmental pollution. Alternatively, chitinase hydrolysis, with its advantages in environmental compatibility, low cost, and reproducibility, has become increasingly popular in recent years (Kadokura et al. 2007).
Chitinases are a group of enzymes that hydrolyze the β-1,4-linkages in chitin to low-molecular-weight products, and have been shown to be produced by a number of microorganisms. Generally, chitinase-producing strains will use chitin or colloidal chitin as a carbon source and produce a mixture of chitinases (EC 3.2.1.14) and N-acetylglucosaminidase (EC 3.2.1.52).
Bioconversion of chitinous materials has been proposed as a waste treatment alternative to the disposal of shellfish wastes. To further enhance the utilization of chitin-containing marine crustacean waste, some of recent studies have focused on the bioconversion of shellfish chitin wastes for the production of proteases and/or chitinases (Wang et al. 2006; Wang and Yeh 2006). The utilization of shellfish waste not only solves environmental problems but also decreases the production costs of microbial chitinases (Liu et al. 2003).
In the present study, an attempt was made to optimize the culture conditions of Alcaligenes faecalis AU02 for chitinase production using cheaper carbon sources such as shrimp and crab shell powder. In addition, chitinase from A. faecalis AU02 was purified and characterized, and the antioxidant activity of the culture supernatant was analyzed.
Materials and methods
Materials
The marine waste used in this study was composed of shrimp shells and crab shells obtained from processing units and local marine food suppliers. The shrimp shell powder (SSP) and crab shell powder (CSP) used in these experiments were prepared according to the method of Wang et al. (2006). In the preparation of SSP and CSP, the shrimp shells and crab shells were washed thoroughly with tap water and then dried. The dried materials obtained were milled to a powder and this fine powdered waste was used directly as the carbon source for chitinase production.
Microorganism and enzyme production
Alcaligenes faecalis AU02 was isolated from sea food industrial effluent and maintained on nutrient agar plates at 37°C. To determine optimal culture conditions, growth as well as enzyme production was carried out in a basal medium containing 0.1% K2HPO4 and 0.05% MgSO4·7H2O (pH 8) and supplemented with 0.5–2% (w/v) of the various carbon sources (SSP, CSP and different proportions of S/CSP) to be investigated. For chitinase production, 100 mL of the resultant medium in a 500-mL Erlenmeyer flask was cultured aerobically at 37°C for 72 h on a rotary shaker (150 rpm). After centrifugation (12,000 g, 4°C, for 20 min), the supernatant was collected for measurement of chitinase activity.
Purification of chitinase enzyme
Shake flask cultures were harvested after 72 h and the cells were removed by centrifugation at 6,000 g for 30 min at 4°C. The cell-free supernatant was used as crude enzyme. The crude chitinase was precipitated with ammonium sulfate at 60% saturation and allowed to stand overnight at 4°C. The precipitates were collected by centrifugation and dialyzed against 100 mM Tris-HCl buffer (pH 8.0) for 24 h at 4°C. Dialyzed enzyme solution was loaded onto a DEAE-cellulose column (2.0 × 25 cm) equilibrated with Tris-HCl buffer (pH 8.0). The enzyme was eluted with a linear gradient of NaCl (0–1 M in 100 mM Tris-HCl buffer) at a flow rate of 25 ml/h. The eluted fractions were assayed for enzyme activity.
Sephadex G-50 gel filtration chromatography
Enzyme solution prepared as above was loaded onto a Sephadex G-50 gel filtration column (2.5 × 120 cm) pre-equilibrated with 100 mM Tris-HCl buffer (pH 8), then eluted with the same buffer. The protein concentration was estimated by measuring the absorbance at 280 nm. Peaks exhibiting chitinase activity were pooled together and used as purified enzyme. This purified enzyme solution was used to investigate the effects of temperature and pH on enzyme activity and stability.
Analytical methods
Chitinase activity was determined by incubating 1.0 ml each of colloidal chitin and enzyme solution at 40°C for 1 h. The mixture was centrifuged at 5,000 g for 5 min and reducing sugars in the supernatant were estimated by the 3,5-dinitrosalicylic acid (DNS) method (Miller 1959). One unit of chitinase activity is defined as the amount of enzyme required to release 1 μmol N-acetylglucosamine (GlcNAc) in 1 min under the above described assay conditions.
The amount of protein in the crude and purified enzyme was measured by the method of Lowry et al. (1951) with bovine serum albumin as a standard.
The molecular mass of the purified chitinase was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to the method of Laemmli (1970). Molecular weight was estimated by comparing the mobility of the sample with that of standard molecular weight markers (29–205 kDa).
Effect of temperature on enzyme activity and stability
The effect of temperature on enzyme activity was determined by incubating the mixture (enzyme + 1% colloidal chitin) for 60 min at temperatures ranging from 30 to 95°C. Thermostability studies were performed by incubating the purified enzyme at temperatures from 30 to 95°C for 24 h. The residual activity was quantified at 40°C for 20 min with the DNS method.
Effect of pH on enzyme activity and stability
The effect of pH on enzyme activity was determined by incubating the mixture for 60 min at 40°C with appropriate buffers; buffers used were (0.1 mol l −1) sodium citrate (pH 3–6), sodium phosphate (pH 6–8), glycine-NaOH (9–11), and dilute NaOH for pH 12–13. pH stability studies were performed by incubating the purified enzyme with different pH buffers for 24 h at 4°C, then quantifying the residual activity by the DNS method.
Scavenging ability of culture supernatant on DPPH radicals
The culture supernatant (150 μl) of Alcaligenes faecalis AU02 was mixed with 37.5 μl methanolic solution containing 0.75 mM 1,1-diphenyl-2-picrylhydrazyl (DPPH) (HiMedia, Mumbai, India) radicals. The mixture was shaken vigorously and left to stand for 30 min in the dark, and the absorbance was then measured at 517 nm against a blank (Shimada et al. 1992). The scavenging ability was calculated as follows: scavenging ability (%) = [(ΔA 517 control − ΔA 517 sample) /ΔA 517 control] × 100.
Thin layer chromatography analysis
The antioxidant materials of the culture supernatant from Alcaligenes faecalis AU02 produced by utilizing marine wastes were analyzed by silica gel TLC using 5:4:3 (v/v/v) n-butanol/methanol/16% aqueous ammonia as the mobile phase (Kadokura et al. 2007). Silica gel TLC plates (0.25 mm) were obtained from Merck (Darmstadt, Germany). After developing the TLC plates, the compounds were visualized by spraying with ethanol containing 0.5% (w/v) ninhydrin, followed by heating. The antioxidant material was identified based on the Rf value in comparison with standard chitooligosaccharides (HiMedia). Functional groups in the culture supernatant exhibiting antioxidant activity were determined by Fourier transform-infra red (FT-IR) spectra recorded on an FT-IR spectrometer (Biorad-40 model, Bio-Rad, Richmond, CA) using the test compound and KBr pellets.
Results and discussion
Isolation, identification and screening of a chitinase-producing strain
Alcaligenes faecalis AU02 is a Gram-negative and non-endospore-forming bacillus that is motile, oxidase, catalase, and citrate positive but nitrate reduction negative, and which grows in both aerobic and anaerobic niches. Based on the results of the 16S ribosomal DNA (rDNA) partial base sequence (1,507 bp), AU02 was closest to A. faecalis SLG-02 (GenBank accession no. AY 959943.2; homology 99%, based on 16S rDNA), A. faecalis WM2012 (GenBank accession no. AY548384.1; homology 99%, based on 16S rDNA) and Alcaligenes sp. F78 (GenBank accession no. EU443097.1; homology 99%, based on 16S rDNA) (Table 1). We conclude that AU02 belongs to Alcaligenes faecalis and designated it as A. faecalis AU02 (GenBank accession no. HM145896).
Table 1.
Similarity of Alcaligenes faecalis AU02 (GenBank accession no. HM145896) with other Alcaligenes strains based on 16S rDNA sequences
| Strain | Accession number | Similarity | Nucleotide differences/compared |
|---|---|---|---|
| Alcaligenes faecalis slg-p2 | AY959943.2 | 99% | 1,499/1,507 |
| Alcaligenes sp. F78 | EU443097.1 | 99% | 1,498/1,507 |
| Alcaligenes sp. ECU0401 | EF535732.1 | 99% | 1,498/1,507 |
| Alcaligenes sp. IS-J1 | EF599759.1 | 99% | 1,498/1,507 |
| Alcaligenes faecalis WM2072 | AY548384.1 | 99% | 1,497/1,507 |
| Alcaligenes sp. IS-92 | AY346141.1 | 99% | 1,498/1,507 |
| Alcaligenes sp. IS-67 | AY346140.1 | 99% | 1,498/1,502 |
Alcaligenes faecalis AU02 was isolated from seafood industrial effluents and screened on agar plates containing 1% SCSP, 0.1% K2HPO4, 0.05% MgSO4·7H2O, and 1.5% agar powder (pH 8.0). The plates were incubated at 37°C for 2 days. The organisms obtained from screening were subcultured in liquid media containing 1% SCSP, 0.1% K2HPO4, and 0.05% MgSO4·7H2O in shake flasks at 37°C and 150 rpm. After incubation for 2 days, the culture broth was centrifuged (4°C; 8,200 g; 20 min) and the supernatants collected for measurement of chitinase activity using the standard assay. The strain Alcaligenes faecalis AU02, which showed highest chitinase activity was used for further production studies.
Among the marine wastes tested, better production was found when basal medium (0.1% K2HPO4 and 0.05% MgSO4·7H2O, pH 8.0) was supplemented with 1% SCSP at 37°C. The effect of different proportions of SCSP on growth and chitinase production was also tested in 100 ml basal medium (0.1% K2HPO4 and 0.05% MgSO4·7H2O, pH 7) containing various proportions (1:3, 1:1, 3:1) of SCSP.
The results of this experiment showed that the carbon sources with chitin (SSP, CSP and SCSP) were more suitable for chitinase production by A. faecalis AU02 than carbon sources with only chitin; 1% SCSP was found to be a more suitable substrate for chitinase production than SSP and CSP alone (Table. 2). Similar results were also found in Bacillus subtilis KU007 (Wang and Yeh 2006) and Bacillus sp. TKU004 (Wang et al. 2006).
Table 2.
Effect of various marine wastes on production of chitinase by A. faecalis AU02 and its antioxidant activity. Values represent mean ± SD. SSP Shrimp shell powder, CSP crab shell powder, SCSP mixed shrimp/crab shell powder in the proportions indicated
| Carbon source | Chitinase activity (IU/ml) | Antioxidant activity of the culture supernatant (%) |
|---|---|---|
| SSP | 226 ± 1 | 75 ± 1 |
| CSP | 194 ± 1.527 | 68 ± 1 |
| SCSP (25:75) | 233 ± 1.527 | 78 ± 1 |
| SCSP (50:50) | 258 ± 1 | 84 ± 1.527 |
| SCSP (75:25) | 229 ± 1 | 80 ± 1.527 |
To study cell growth as well as enzyme production, 100 ml medium (1% SCSP in basal medium, pH 8.0) was incubated for 72 h and chitinase activity was investigated. As shown in Fig. 1, maximum growth and chitinase production (258 U/mL) was found after 48 h of culture. Bacillus sp.TKU006 has been reported to produce protease and chitinase on SSP and CSP at 21°C for 5–10 days (Khan et al. 2003). In comparison, our experiment produced a higher amount of chitinase from A. faecalis AU02 using cheaper medium and a shorter cultivation time than Bacillus sp.TKU006. In the present study, the amount of enzyme produced was relatively higher and the fact that enzyme was produced on marine waste in water as the growth medium indicated the suitability of marine waste as a substrate for the production of chitinase. Protease and chitinase enzymes were subsequently used for the production of antioxidants from marine waste.
Fig. 1.
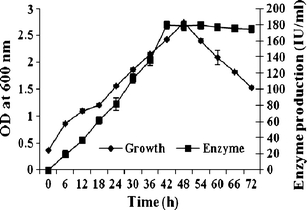
Time course of cell growth and chitinase production of Alcaligenes faecalis AU02 on 1% mixed shrimp/crab shell powder (SCSP) medium. Value represent mean ± SD
A summary of the enzyme purification steps is presented in Table 3. Purification of chitinase with ammonium sulfate followed by DEAE-cellulose and Sephadex G-50 column chromatography yielded 39.28% and 16.35% recovery, with 9.68- and 10.34-fold purification, respectively (Table 3). Purification of chitinase of only 2.4-fold was reported from Alcaligenes xylosoxydans with ultrafiltration and Sephadex G-75 gel filtration (Vaidya et al. 2003). The molecular weight of the chitinase from Alcaligenes faecalis AU02 was estimated as 36 kDa (Fig. 2). The molecular weights of chitinase from Alcaligenes xylosoxydans is 44 kDa (Vaidya et al. 2003), with chitinases of 45 kDa reported from B. circulans (Wiwat et al. 1999) and 57 kDa from Serratia marcescens (Nawani and Kapadnis 2001).
Table 3.
Summary of purification profile of chitinase from A. faecalis AU02
| Purification step | Total protein (mg) | Total activity (U) | Specific activity (U/mg) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Culture filtrate | 1,582 | 12,462 | 7.877 | 1.0 | 100 |
| (NH4)2SO4 precipitate | 106 | 7,341 | 69.25 | 8.71 | 58.90 |
| DEAE cellulose | 64 | 4,896 | 76.5 | 9.68 | 39.28 |
| Sephadex G 50 | 25 | 2,038 | 81.52 | 10.34 | 16.35 |
Fig. 2.
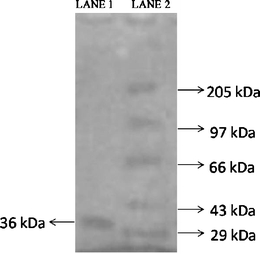
SDS-PAGE analysis of chitinase from A. faecalis AU02. Lanes: 1 Purified enzyme, 2 molecular markers (29–205 kDa)
Effect of temperature and pH on enzyme activity and stability
The enzyme was active at temperatures between 30 and 50°C, with the optimum being at 40°C. The enzyme was 100% stable even up to 50°C and also it exhibited 86% activity at 60°C. At 95°C and above it completely loses activity (Fig. 3). Chitinase from A. faecalis AU02 was active over a wide range of pH between 7 and 9, with optimum activity at pH 8.0. Regarding stability, the enzyme was 100% stable at pH 9 and retained more than 80% activity at pH 10.0 and 78% at pH 11.0 (Fig. 4).
Fig. 3.
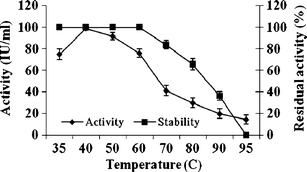
Effect of temperature on activity and stability of purified chitinase from A. faecalis AU02. Values are mean ± SD, n = 3
Fig. 4.
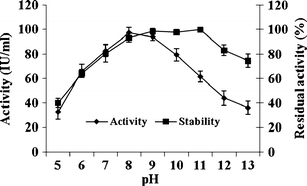
Effect of pH on activity and stability of purified chitinase from A. faecalis AU02. Values are mean ± SD, n = 3
The enzyme was highly active in the temperature range of 30–50°C but optimum at 40°C and pH 8.0. The results on temperature optimum are similar to those of chitinases previously characterized from Alcaligenes xylosoxydans, Aeromonas hydrophila (Hiraga et al. 1997), and Pseudomonas aeruginosa (Wang and Chang 1997). Most bacterial chitinases are active at acidic pH (Dahiya et al. 2005; Murao et al. 1992). Conversely, the chitinase from A. faecalis AU02 was unlike all other bacterial chitinases in remaining active at pH 8.0. Regarding stability, the enzyme was 100% stable even at 60°C and pH 11.0, whereas chitinases from Arthrobacter sp. NHBN-10 (Okazaki et al. 1999), and Vibrio alginolyticus (Ohishi et al. 1996) are stable only at temperatures between 40 and 50°C, and at pH 7.0–9.0. Hence, the chitinase characterized here is comparatively better for industrial uses.
Antioxidant activity of culture supernatant of A. faecalis AU02 by fermenting marine wastes
Many studies have reported that chitin, chitosan, and peptides have antioxidative (Lin and Chou 2004; Xing et al. 2005; He et al. 2006) and anticarcinogenic (Liang et al. 2007; Wang et al. 2008a) properties. To increase the utilization of these chitin-containing marine wastes, A. faecalis AU02 was incubated for 72 h with SCSP, and the antioxidant and enzyme activity of the culture supernatants were analyzed. Antioxidant activity was assayed by the scavenging ability on DPPH. Maximum antioxidant activity was found in the culture supernatant of Alcaligenes faecalis AU02 (1:1 SCSP) incubated for 3 days. When compared with SSP and CSP, 1:1 SCSP was the most suitable carbon source for the production of antioxidant materials by A. faecalis AU02 (Table 2). The antioxidant activity of culture supernatants of A. faecalis AU02 grown on SSP, CSP and SCSP was 75%, 68% and 84%, respectively. The results demonstrate that antioxidative oligosaccharides might be hydrolyzed by the chitinase present in the culture supernatant. Such antioxidant materials may contain oligosaccharides, which are electron donors and able to react with free radicals to terminate the radical chain reaction.
Antioxidant materials in the culture supernatants were analyzed using TLC. Culture supernatants were resolved on TLC plates, and compounds were visualized by spraying with ninhydrin reagent (Fig. 5). Although the mobility of the antioxidant material corresponded to that of chitin oligosaccharide standards, their structures need to be analyzed in future. Similarly, Wang et al. (2009) are also found antioxidant activity in the culture supernatant of Bacillus sp. TKU006 by utilizing marine waste.
Fig. 5.

Thin layer chromatography (TLC) analysis of antioxidant materials in the culture supernatant of A. faecalis AU02 grown on 1% SCSP medium. After developing, the TLC plate was visualized by spraying with ninhydrin reagent. Lanes: S N-Standard chitooligosaccharides, A sample
Compounds displaying antioxidant activity in the culture supernatant of A. faecalis AU02 were characterized by FT-IR. The FT-IR spectrum shown in Fig. 6 exhibits characteristic signals at 3421.09, 2923.81, 2843.84, 1758.90, 1647.42, 1542.67, 1459.27, 1424.66, 1117.93, 1020.55, 865.75, 669.67, 608.22, 471.23 cm−1.
Fig. 6.
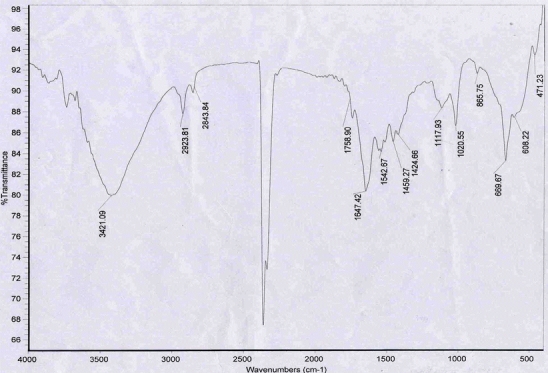
Fourier transform-infra red (FT-IR) spectrum of the antioxidant over the wave number range of 4,000–400 cm−1
The present study proposes the microbial reclamation and utilization of marine wastes. Considering production costs, the utilization of marine wastes such as shrimp and crab shell wastes for the production of chitinase seems to provide a promising approach. In addition, the culture supernatant also had antioxidant activity. Thus, marine waste can serve as a potential substrate for the recovery of medicinally useful compounds. Biological extraction of these compounds ensures good quality of the compounds and makes the process environmental friendly and economically feasible. Large scale production of these compounds from marine waste could lead to the development of low-cost technologies with great demand in the fields of medicine and neutraceuticals.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Neelamegam Annamalai, Email: annabact@gmail.com.
Mayavan Veeramuthu Rajeswari, Email: raji.shwehari@gmail.com.
Shanmugam Vijayalakshmi, Phone: +91-414-4243223, Email: vijy@msn.com.
Thangavel Balasubramanian, Phone: +91-414-4243223, Email: stbcas@nic.in.
References
- Dahiya N, Tewari R, Tiwari RP, Hoondal G. Production of an antifungal chitinase from Enterobacter sp.NRG4 and its application in protoplast production. World J Microbiol Biotechnol. 2005;21:1611–1616. doi: 10.1007/s11274-005-8343-6. [DOI] [Google Scholar]
- He H, Chen X, Sun C, Zhang Y, Gao P. Preparation and functional evaluation of oligopeptide-enriched hydrolysate from shrimp (Acetes chinensis) treated with crude protease from Bacillus sp. SM98011. Bioresour Technol. 2006;97:385–390. doi: 10.1016/j.biortech.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Hiraga K, Shou L, Kitazawa M, Takahashi S, Shimada M, Sato R, Oda K. Isolation and characterization of chitinase from a flake-chitin degrading marine bacterium, Aeromonas hydrophila H-2330. Biosci Biotechnol Biochem. 1997;61:174–176. doi: 10.1271/bbb.61.174. [DOI] [PubMed] [Google Scholar]
- Kadokura K, Rokutani A, Yamamoto M, Ikegami T, Sugita H, Itoi S, Hakamata W, Oku T, Nishio T. Purification and characterization of Vibrio parahaemolyticus extracellular chitinase and chitin oligosaccharide deacetylase involved in the production of heterodisaccharide from chitin. Appl Microbiol Biotechnol. 2007;75:357–365. doi: 10.1007/s00253-006-0831-6. [DOI] [PubMed] [Google Scholar]
- Khan A, Williams K, Molloy MP, Nevalainen H. Purification and characterization of a serine protease and chitinases from Paecilomyces lilacinus and detection of chitinase activity on 2D gels. Protein Expr Purif. 2003;32:210–220. doi: 10.1016/j.pep.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liang TW, Chen YJ, Yen YH, Wang SL. The antitumor activity of the hydrolysates of chitinous materials hydrolyzed by crude enzyme from Bacillus amyloliquefaciens V656. Process Biochem. 2007;42:527–534. doi: 10.1016/j.procbio.2006.10.005. [DOI] [Google Scholar]
- Lin HY, Chou CC. Antioxidant activities of water-soluble disaccharide chitosan derivatives. Food Res Int. 2004;37:883–889. doi: 10.1016/j.foodres.2004.04.007. [DOI] [Google Scholar]
- Liu BL, Kao PM, Tzeng YM, Feng KC. Production of chitinase from Verticillium lecanii F091 using submerged fermentation. Enzyme Microb Technol. 2003;33:410–415. doi: 10.1016/S0141-0229(03)00138-8. [DOI] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;37:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Murao S, Kawada T, Itoh H, Oyama H, Shin T. Purification and characterization of a novel type of chitinase from Vibrio alginolyticus TK-22. Biosci Biotechnol Biochem. 1992;56:368–369. doi: 10.1271/bbb.56.368. [DOI] [Google Scholar]
- Nawani NN, Kapadnis BP. One-step purification of chitinase from Serratia marcescens NK1, a soil isolate. J Appl Microbiol. 2001;90:803–808. doi: 10.1046/j.1365-2672.2001.01308.x. [DOI] [PubMed] [Google Scholar]
- Ohishi K, Yamagishi M, Ohta T, Suzuki M, Izumida H, Sano H, Nishima M, Miwa T. Purification and properties of two chitinases from Vibrio alginolyticus H-8. J Ferment Bioeng. 1996;82:598–600. doi: 10.1016/S0922-338X(97)81260-3. [DOI] [Google Scholar]
- Okazaki K, Kawabata T, Nakano M, Hayaka S. Purification and properties of chitinase from Arthrobacter sp. NHB-10. Biosci Biotechnol Biochem. 1999;63:1644–1646. doi: 10.1271/bbb.63.1644. [DOI] [PubMed] [Google Scholar]
- Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–948. doi: 10.1021/jf00018a005. [DOI] [Google Scholar]
- Suzuki K, Mikami T, Okawa Y, Tokoro A, Suzuki S, Suzuki M. Antitumor effect of hexa-N-acetylchitohexaose and chitohexaose. Carbohydr Res. 1986;151:403–408. doi: 10.1016/S0008-6215(00)90359-8. [DOI] [PubMed] [Google Scholar]
- Synowiecki J, Al-Khateeb NAAQ. The recovery of protein hydrolysate during enzymatic isolation of chitin from shrimp Crangon crangon processing discards. Food Chem. 2000;68:147–152. doi: 10.1016/S0308-8146(99)00165-X. [DOI] [Google Scholar]
- Vaidya R, Roy S, Macmil S, Gandhi S, Vyas P, Chhatpar HS. Purification and characterization of chitinase from Alcaligenes xylosoxydans. Biotechnol Lett. 2003;25:715–717. doi: 10.1023/A:1023406630791. [DOI] [PubMed] [Google Scholar]
- Wang SL, Chang WT. Purification and characterization of two bifunctional chitinase by Psudomonas aeruginos K-187 in a shrimp and crab shell powder medium. Appl Envion Microbiol. 1997;63:380–386. doi: 10.1128/aem.63.2.380-386.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SL, Yeh PY. Purification of a surfactant- and solvent stable alkaliphilic protease by bioconversion of shrimp shell wastes fermented by Bacillus subtilis TKU007. Process Biochem. 2006;41:1545–1552. doi: 10.1016/j.procbio.2006.02.018. [DOI] [Google Scholar]
- Wang SL, Lin TY, Yen YH, Liao HF, Chen YJ. Bioconversion of shellfish chitin wastes for the production of Bacillus subtilis W-118 chitinase. Carbohydr Res. 2006;341:2507–2515. doi: 10.1016/j.carres.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Wang SL, Wang CY, Huang CY. Microbial reclamation of squid pen for the production of a novel extracellular serine protease by Lactobacillus paracasei subsp. paracasei TKU012. Bioresour Technol. 2008;99:3411–3417. doi: 10.1016/j.biortech.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Wang SL, Yang CH, Liang TW, Yen YH. Optimization of conditions for protease production by Chryseobacterium taeanense TKU001. Bioresour Technol. 2008;99:3700–3707. doi: 10.1016/j.biortech.2007.07.036. [DOI] [PubMed] [Google Scholar]
- Wang SL, Chao CH, Liang TW, Chen CC. Purification and characterization of protease and chitinase from Bacillus cereus TKU006 and conversion of marine wastes by these enzymes. Mar Biotechnol. 2009;11:334–344. doi: 10.1007/s10126-008-9149-y. [DOI] [PubMed] [Google Scholar]
- Wiwat C, Siwayaprahm P, Bhumiratana A. Purification and characterization of chitinase from Bacillus circulans No. 4.1. Curr Microbiol. 1999;39:134–140. doi: 10.1007/s002849900434. [DOI] [PubMed] [Google Scholar]
- Xing R, Yu H, Liu S, Zhang W, Zhang Q, Li Z. Antioxidative activity of differently regioselective chitosan sulfates in vitro. Bioorg Med Chem. 2005;13:1387–1392. doi: 10.1016/j.bmc.2004.11.002. [DOI] [PubMed] [Google Scholar]


