Abstract
To investigate the potential for use of a well-established strain of Plasmodium falciparum as a reference strain for infected red blood cell (IRBC) surface reactivity, we monitored the binding of specific immunoglobulin G (IgG) from immune individuals to the reference Knob-positive FCR3 strain by flow cytometry. To permit interassay comparison for 162 plasma samples drawn after the rainy season, a labeling index (LI) was defined as the percentage of labeled parasites multiplied by the mean peak intensity. An LI ratio (LIR) was then calculated as the LI of the sample divided by the LI of the control. LIRs were calculated for individuals living in Dielmo and Ndiop, two Senegalese villages where P. falciparum is transmitted holoendemically and mesoendemically, respectively. The incidence (persons with an LIR of >3) observed in Dielmo was lower than that observed in Ndiop. Significantly higher LIRs were observed (i) for samples from Ndiop than for samples from Dielmo (P < 0.01) and (ii) in Ndiop, in subjects with hemoglobin AS (HbAS) than in those with hemoglobin AA (P = 0.03). No correlation with the cumulative age-associated immune status of the villagers was evidenced, contrary to antibody (Ab) responses against conserved IRBC-associated antigen (Ag) measured by enzyme-linked immunosorbent assay. These results are consistent with the notions that protection in HbAS individuals may relate to an increased IgG response to IRBC membrane Ags and that cell surface reactivity parallels IgG responses even though it is in itself a distinct indicator of the anti-P. falciparum Ab response. Measures of IgG binding to live IRBC are thus relevant for the functional screening of conserved IRBC-associated Ags that contribute to parasite destruction in vivo, as these Ags might be included in a multitarget vaccine.
Antigens (Ags) exposed on the surface of Plasmodium falciparum-infected red blood cells (IRBC) represent a first line of accessible targets for protective antibodies (Abs) involved in opsonization and immune phagocytosis of parasitized erythrocytes (7, 10, 13, 14). The large diversity of IRBC surface Ags complicates the assessment of immune responses to these Ags in humans living in areas of endemicity and the delineation of their potential role in controlling parasite density in vivo. The use of a flow cytometric technique allows accurate measures of immunoglobulin G (IgG) binding on live IRBC by handling ex vivo fresh parasites from selected patients or P. falciparum reference strains maintained in continuous culture (16). We focused attention on the use of a reference strain for the analysis of conserved cross-recognized Ags expressed on the IRBC surface. An initial investigation showed substantial levels of IgG binding to IRBC in immune individuals with the P. falciparum reference strain Palo Alto (alias FCR3) (8). However, such investigation generally requires the concentration of IRBC with mature forms of the parasite (schizonts) either by means of flotation on plasmagel or by magnet-activated cell sorting (28). Routine conditions of in vitro culture for the FCR3 strain of P. falciparum leads to 5 to 10% IRBC, which is a limiting factor for simultaneously testing a large number of plasma samples with the same cultured IRBC batch. In the present work, we aimed at further evaluating the validity of the in vivo IRBC binding properties of IgG from a substantial number of samples of immune individuals living in areas of endemic transmission, without the constraint imposed by concentration of the parasite strain. We tested for possible correlates with immunoepidemiological criteria, e.g., (i) the level of transmission intensity, by comparing individuals from two different locations (Dielmo and Ndiop) (32), (ii) the individual phenotype of hemoglobin (hemoglobin AA [HbAA] versus hemoglobin AS [HbAS]), and (iii) the content in Abs to 3 particular conserved IRBC-associated Ags.
Two Ags, PfEB200 and R23, protected Saimiri monkeys from a lethal experimental infection with the Palo Alto strain and elicited Abs mediating immune phagocytosis of IRBC in vitro (23, 24, 26). A third Ag derived from P. falciparum erythrocyte membrane 3 (PfEMP3-cl5) was identified as a target of variant-specific immune response in the Saimiri monkey (17) and induced protection in vaccinated monkeys (C. Le Scanf and O. Mercereau-Puijalon, unpublished data). Previous studies showed that these Ags were consistently recognized by naturally acquired Abs in humans exposed to P. falciparum and that their levels fluctuated depending on the period of observation, i.e., before or after the peak of parasite transmission during the rainy season (25).
We thus aimed at closely examining the relationships between the Ab responses to these conserved P. falciparum target Ags measured by enzyme-linked immunosorbent assay (ELISA) and the level of IgG binding to strain FCR3 containing the tested IRBC-associated Ags.
MATERIALS AND METHODS
Study sites and subjects.
Blood samples were collected by venipuncture after informed consent was obtained from individuals living in Dielmo and Ndiop, two neighboring villages located in southern Senegal, which had already been monitored for over 10 years. This longitudinal study was approved by an ad hoc ethical committee; the consent of villagers was renewed yearly (32, 33). Samples from 81 individuals from each village were included: the mean ages of the cohorts were 21.5 years (range, 3.1 to 63 years) and 22.3 years (range, 3.3 to 64 years) in Dielmo and Ndiop, respectively. There were no significant differences in the age distribution in individuals from Ndiop and Dielmo: 40 versus 36 children who were ≤15 years old and 41 versus 45 older individuals were enrolled in the study in Ndiop and Dielmo, respectively. Sampling was done from 24 November to 8 December 1998 simultaneously in the two villages, just after the end of the rainy season. In Dielmo (an area of holoendemic transmission), the entomological inoculation rate was estimated at 35.6 infective bites in November, with an individual cumulative risk of exposure of >100 infective bites from September to November. In Ndiop (an area of mesoendemic transmission), no transmission was recorded since the end of October and the cumulative entomological inoculation rate was estimated at 5.3 infective bites per individual from August to October.
Flow cytometry technique for the detection of IgG binding to live IRBC.
To test for the recognition of live IRBC by individual plasma, we used a double staining cytofluorimetric technique as described previously (8, 16). A knob-positive (K+) Uganda Palo Alto FUP strain of P. falciparum (alias FCR3) maintained in culture was used as a source of IRBC. K+ schizonts were continuously selected after plasmagel flotation and used when parasitemia was over 5%. Membrane-bound IgG were revealed by means of a first incubation of 30 min at 37°C with plasma diluted 1/20, which was followed by an additional 30-min incubation at 37°C with phycoerythrin-conjugated goat anti-human IgG (1/200; Cappel Organon Teknica, West Chester, Pa.). Live parasites were then labeled for 30 min at 37°C in the dark with thiazole orange (TO) (Retic-Count; Becton-Dickinson, San Jose, CA). Fluorescence was read within 1 h on a cytofluorimeter (FACScan, CELLQuest software; Becton-Dickinson). After gating on the TO-positive IRBC, 5,000 events for the overall IRBC (FL1 ≥ 101) were counted. At the end of the first set of plasma analysis, gating of IRBC was changed to select the more mature forms of parasites with subsequent nuclear material (FL1 ≥ 102), and 2,000 events were counted. In all cases, phycoerythrin-labeled membrane-bound IgG was measured in the FL2 channel. A pool of 30 plasma samples from naturally immune villagers resistant to malaria was referred to as the positive control (HIS), and pools of plasma obtained from non-P. falciparum exposed European and Senegalese (Dakar inhabitants) individuals was referred to as the negative control. For the quantification of labeled IRBC, a labeling index (LI) ratio (LIR) was defined as the LI of the sample/LI of the control; the LI was calculated as the percentage of parasites with bound IgG (FL1 ≥ 101; FL1 ≥ 102) multiplied by the geometric mean intensity of the peak. This calculation was made to allow interassay comparison.
Ags.
Two crude antigenic preparations were used. They consisted of (i) a lysate of in vitro-matured schizont-enriched P. falciparum IRBC (22) and (ii) erythrocyte membranes from IRBC. Parasitized ghosts (IRBC) and control ghosts (red blood cells) were prepared according to the method of Fairbanks et al. (11). The total protein concentration in the parasite preparations was estimated by the Bio-Rad assay. All Ags were kept frozen at −80°C in working aliquots.
Two purified recombinant proteins fused to Schistosoma japonicum glutathione S-transferase (GST) in the pGEXA vector were used. R23 contains 11 copies of a 6-amino-acid repeat derived from the central domain of Ag R45, whose consensus sequence is HKSDS N/S/H (4). PfEB200 contains 13 repeats with characteristic Glu-Glu dimers; it derives from Pf332, a conserved giant protein accessible on the IRBC surface in late schizonts (19). PfEMP3-cl5 is the recombinant product expressing the 1,450-bp EcoRI fragment recovered from clone 5, isolated from the FUP/SP Palo Alto (alias FCR3) genomic expression library. It expresses the C-proximal region of PfEMP3. PfEMP3-cl5 was identified as a target of variant immune response, and expression level of PfEMP3 was shown to be modulated during antigenic variation, with some variants expressing high levels while others expressed reduced amounts of protein (17, 18). The control protein was the GST carrier.
ELISA procedure.
As already described (22, 25), crude Ag preparation extracts were coated on MaxiSorp plates (Nunc, Roskilde, Denmark) at a concentration of 10 to 15 μg ml−1, recombinant proteins (1 μg ml−1) were coated on Immulon-4 plates (Dynatech, Springfield, Va.) and left overnight at 4°C. Reagents were from Sigma Chemicals (St. Louis, Mo.) unless stated otherwise. After three washings in phosphate-buffered saline (PBS) plus 0.5% Tween 20 and blocking with PBS-5% bovine serum albumin for 1 h at 37°C, plasma samples (diluted 1/200 in PBS-1% bovine serum albumin-0.5% Tween 20) were added and incubated for 2 h at 37°C. After three washings, peroxidase-conjugated polyclonal goat anti-human IgG [IgG(γ) chain specific] diluted 1:10,000 in the same buffer (Cappel Organon Teknika) was added for 2 h at 37°C. Following incubation and five washings, 100 μl of citrate buffer (pH 4) containing 0.16 mg of orthotoluidine ml−1 and 10% H2O2 was distributed in each well. The reaction was stopped with 4 N H2SO4, and the optical density (OD) was read with a Titertek Multiscan plate reader (Flow Laboratories, Ayshire, Scotland).
Negative controls (a pool of nonimmune European sera and a pool of naive African sera) and a positive control (a pool of sera from clinically immune adults living in Dielmo and Ndiop) were included with every assay. Results were expressed as the OD ratio (OD of the sample/OD of the negative control). The OD signals of the samples were individually corrected for the GST or red blood cell signal. In this study, responses were considered positive for individuals with an OD ratio of >3, which corresponds approximately to the signal of naive controls +3 standard deviations (after the rainy season, background signals are substantially higher than before the period of high transmission).
Statistical analysis.
Comparisons of Ab levels and/or LIRs in different assays or groups were done by means of the Wilcoxon signed-rank test and the Spearman rank correlation test for nonnormally distributed paired data. The Mann-Whitney test was used to compare unpaired data. P values of <0.05 were considered significant.
Statistical analyses were performed with Statview, version 5.0, software (SAS Institute, Cary, N.J.).
RESULTS
Incidence and characteristics of Ab responses measured by ELISA.
Levels of Ab responses of the 162 plasma samples against the different Ags tested are shown in Table 1. They are expressed as mean OD ratios ± standard deviation and the percentages of responders, i.e., proportions of individuals with an OD ratio of >3. Substantial levels of Ab responses to total P. falciparum Ags (schizont and IRBC) were evidenced. We found significantly higher Ab responses in Ndiop villagers than in Dielmo villagers (P < 0.05).
TABLE 1.
Incidence and level of Ab responses to the Ags studied
| Ag | Result (mODr ± SD [% resp.]) fora:
|
P valueb | |
|---|---|---|---|
| Dielmo | Ndiop | ||
| IgG to schizont | 7.5 ± 3.3 (98) | 10.8 ± 4.1 (96) | <0.0001 |
| IRBC membrane | 3.4 ± 1.7 (43) | 4.7 ± 2.2 (79) | 0.02 |
| PfEMP3-cl5 | 6.1 ± 7.6 (37) | 11.0 ± 7.8 (73) | 0.0002 |
| PfEB200 | 3.3 ± 3.0 (35) | 4.3 ± 3.9 (43) | NSc |
| R23 | 3.2 ± 5.7 (17) | 4.4 ± 6.9 (30) | NS |
Sampling was done simultaneously in the two villages just after the end of the rainy season (November 1998). Results are expressed as mean OD ratios (mODr) ± standard deviation and percentages of responders (% resp.), i.e., proportion of individuals with an OD ratio of >3.
Significance of comparison when lower levels of IgG were found in Dielmo versus Ndiop.
NS, not significant.
A proportion of 17 to 73% of samples from the villagers recognized the recombinant Ags under scope. The profile of every Ab response was analogous to that previously observed (2, 22, 25). Only levels of IgG against PfEMP3-cl5 were significantly higher in Ndiop villagers than in Dielmo villagers (P = 0.0002). There was a positive and significant correlation between the age of individuals and every Ab response to the Ags studied (P < 0.01; rho values ranging from 0.28 to 0.44). In this line, young individuals (<15 years old) showed significantly lower levels of Ab responses to almost all Ags, except to R23 in Ndiop and to PfEMP3-cl5 in Dielmo (P < 0.01).
There were significant relationships between Ab responses to every Ag (P < 0.05; rho values ranging from 0.38 to 0.70), underlining a substantial colinearity in the acquisition of Ab responses to different P. falciparum target Ags.
This set of data underlines the strong confounding effect of the age-associated cumulative exposure in the analysis of Ab responses against the complex pattern of P. falciparum Ags in naturally immune populations. Importantly, when comparing Ab responses with respect to the different hemoglobin phenotypes, i.e., HbAS versus HbAA (n = 12 in Dielmo; n = 20 in Ndiop), only the R23 responders from the Ndiop cohort showed a significantly different level of IgG responses (AS > AA, P < 0.01).
Recognition of live parasites as a function of location and hemoglobin phenotype.
The degree of IRBC recognition was highly variable: some individuals displayed a >3 times higher IgG binding capacity to IRBC than the positive control (Fig. 1). We measured a substantial degree of IgG binding to IRBC. The mean LIR was 5.2 ± 5.2 (49% of individuals with an LIR of >3) in Ndiop and 2.8 ± 1.6 (25% of individuals with an LIR of >3) in Dielmo, and the difference between the two measures was significant (P = 0.004).
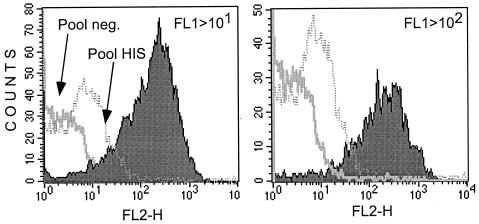
FIG. 1.
Examples of histograms of flow cytometry data acquisition. The two histograms show results from the acquisition of 5,000 events of a 3% P. falciparum culture after gating on FL1 > 101 (left panel) and on FL1 > 102 (right panel). The gating on high FL1 values (>102) selected the mature population of the cultured parasites. The binding of human IgG to IRBC was revealed by an Ig anti-IgG conjugated to TO. The fluorescence of TO-positive IRBC was measured in the FL2 channel. Shown are the reference negative control (plain curve histogram, pool neg.), the positive control (dashed curve, pool HIS), and a strong responder from Ndiop (filled curve).
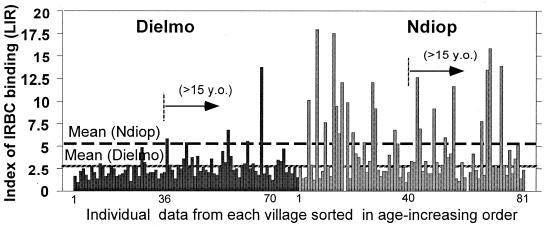
Importantly, no correlation was found between age of individuals and the indices of IRBC IgG binding. As illustrated in Fig. 2, the repartition of LIR plotted in an age-increasing order was highly variable within the two villages.
FIG. 2.
Individual results of LIR in the two villages plotted in increasing age order. Individual LIR results (LIR calculated on 5,000 events gated at >101) measured in 81 individual samples from Dielmo and Ndiop are plotted after sorting in increasing age order. Sampling was done simultaneously in the two villages just after the end of the rainy season. The arrows indicate the separation between results from young individuals (≤15 years old) and data from older individuals. The mean LIRs are plotted as dashed lines and are indicated for Dielmo and Ndiop.
By contrast, after gating on mature parasites (FL1 ≥ 102), mean LIRs were significantly lower in Ndiop than in Dielmo: 2.1 ± 2.5 versus 2.9 ± 2.5 (P < 0.01). However, for all villagers, there was a significant relationship between the individual capacities to recognize IRBC (FL1 > 101) and their mature forms (FL1 > 102) (P < 0.001; rho = 0.5).
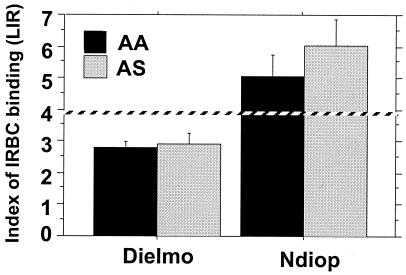
Importantly, individuals with the HbAS phenotype showed significantly higher recognition ratios than those with the HbAA phenotype for the overall cohort of 162 individuals (P = 0.006). This was true when LIRs were calculated with parasites gated for FL1 of >102. When examining the results by village, this observation was still valid in Ndiop (n = 20; P = 0.02) but not in Dielmo (Fig. 3), possibly as a consequence of the limited number of individuals with HbAS presently recruited in Dielmo (n = 12).
FIG. 3.
Mean level (± standard error) of IRBC recognition measured by flow cytometry in 81 samples from each village as a function of hemoglobin phenotype. Mean levels (± standard error) of the IRBC recognition ratio (LIR) measured in 81 individuals living in Dielmo and Ndiop are plotted. Shown are the mean LIRs from individuals with the HbAA phenotype (black) and the HbAS phenotype (grey) (n = 12 for Dielmo; n = 20 for Ndiop). The dashed line corresponds to the measure of IgG binding of the positive control (pool of immune sera).
Relation between LIRs and Ab responses.
The results of the analysis of correlation between the IgG binding indices to IRBC and the Ab responses to the Ags studied are summarized in Table 2. There was no significant correlation between the IgG binding indices to IRBC and the Ab responses to the Ag studied for Ndiop villagers, except for R23 responders and LIRs from mature forms of the parasite. In Dielmo, significant relationships were found between the LIR and the IgG response to R23 and between the LIR (FL1 > 101) and the IgG response to IRBC.
TABLE 2.
Correlation between ELISA IgG responses and LIR
| Aga | Correlation (P value [rho]) for village with gating onb:
|
|||
|---|---|---|---|---|
| FL1 > 101
|
FL1 > 102
|
|||
| Dielmo | Ndiop | Dielmo | Ndiop | |
| Schizont | NS | NS | NS | NS |
| IRBC membrane | 0.02 (0.25) | NS | NS | NS |
| PfEMP3-cl5 | NS | NS | NS | NS |
| PfEB200 | NS | NS | NS | NS |
| R23 | 0.003 (0.33) | NS | 0.001 (0.35) | 0.048 (0.22) |
Ab (IgG) responses against the different Ags measured by ELISA. Ab responses and LIRs were measured in 81 samples from each village (Table 1).
LIRs were measured after gating on IRBC (FL1 > 101) or their mature forms (late trophozoites and schizonts) (FL1 > 102). Results of the Spearman test of correlation between LIR and antibody responses are given. NS, not significant. P and rho values are given when significant.
We further examined the relationship between IgG responses with the IRBC-associated Ags by using dual-level stratified IgG responses to the different Ags, i.e., responders (OD ratio of >3) versus low responders (OD ratio of ≤3) for the IRBC, PfEMP3-cl5, PfEB200, and R23 Ags. We found a significantly increased capacity to recognize IRBC gated on mature forms of the live parasite (>102 in the FL1 channel) for individuals from Dielmo with high IgG levels against R23 (P = 0.045). No such correlate was evidenced in either village with IgG responses to IRBC measured by ELISA.
DISCUSSION
In this study, we measured the capacity of naturally acquired IgG responses from immune individuals living in two different settings of endemicity to bind live IRBC by means of a fluorescence flow cytometric technique. We deliberately chose a reference strain of P. falciparum adapted to in vitro culture to investigate possible measurement of levels of IgG binding to nonpolymorphic IRBC-related Ags. The plasma content in Abs to particular conserved Ags associated with the surface of IRBC (14, 16), previously shown to protect monkeys by either eliciting strong opsonizing Ab responses (24) or inducing a certain degree of protection (23), was taken into account in this investigation.
Several studies have focused on major IRBC surface-associated Ags such as PfEMP1 (5, 6, 12, 27, 29) by using, for example, an agglutination technique of ex vivo isolates, a method leading to comparable results with flow cytometry (27). Bull et al. demonstrated that severe malaria resulted from a substantial limitation of the Ab repertoire to variant Ags (6). In addition, recent studies showed a positive age-related IgG response to the parasite-borne variant surface Ag in healthy residents before the transmission season and a negative correlation with age in malaria patients (6, 12, 21). These data addressed the issue of partial protective immunity attributable to anti-variant surface Ag Abs. A contribution of IgG responses for clearance of parasites in humans is additionally related to particular cryptic and/or weakly immunogenic conserved IRBC-associated epitopes. Such a hypothesis is sustained by the highly efficient parasite clearance repeatedly observed in passive transfer experiments of IgG from distinct geographic settings in humans (9, 20). Similar effects were indeed demonstrated in the Saimiri model with serum from individuals living in areas of P. falciparum endemicity (15), as human IgG could bind to infected Saimiri red blood cells (13), suggesting that the parasite Ags exposed on the surface of the monkey red blood cells are naturally immunogenic in humans.
The capacity of IgG binding to live parasites (FL1 > 101) was unexpectedly higher in Ndiop than in Dielmo, but mature forms (FL1 > 102) were significantly better recognized in Dielmo than in Ndiop, a result in line with the recent cumulative history of parasite transmission and the strong individual protective immunity of Dielmo villagers (32). The non-age-related levels of LIR observed in the two villages, contrasting with Ab responses to P. falciparum Ags measured by ELISA (25), rather suggest that the LIR would rely on the functional status of circulating IgG, depending upon the individual final balance of immune responses and recent events of active parasite infection. An interesting finding was the distinct relationship between LIR and the IgG responses to R23 in Dielmo compared to Ndiop, indicating a possible in vivo contribution of IgG binding to IRBC detectable only in Dielmo, despite significantly higher levels in Ndiop (Table 1). This emphasizes the complex host-parasite imbalance of IRBC-associated Ag recognition with respect to particular conserved epitopes and variant epitopes (29, 31). The effective participation of IgG against conserved determinants of PfEMP1 to the binding of IRBC has not been demonstrated, despite an increased exposure-related magnitude of anti-PfEMP1 Ab responses (30), which is in favor of the contribution of alternate conserved IRBC-associated Ags.
An important issue concerned the significantly enhanced capacity of HbAS individuals to produce IgG responses that recognize live IRBC. The resistance of HbAS individuals to severe malaria is a well-established phenomenon recently reassessed (1); however, it is not sustained by a well-defined functional mechanism. Our findings are in line with an additional increased immune phagocytosis of the hemoglobin S-containing IRBC. Indeed, IgG binding to IRBC implies efficient phagocytosis in the presence of the relevant IgG subclass, i.e., the cytophilic IgG1 and IgG3 (2) and IgG2 in some individuals bearing a particular allele of the Fcγ RII receptor (3), despite some potential competition between the different IgG subclasses (13). Such functional capacity has to be further verified in this cohort, particularly for individuals exhibiting a high degree of IgG binding to the FCR3 strain as shown on Fig. 1. Further investigations are required to substantiate this technique for the screening of subunit Ags associated with the IRBC surface (14).
Indeed, the FCR3 strain used here has a limited relationship with natural parasites infecting the villagers of Ndiop and Dielmo. However, the adaptation of ex vivo parasites to continuous culture (when feasible) and plasmagel flotation concentration of K+ strains depict artificial in vitro procedures that result in the selection of a panel of particular clones substantially different from the original in vivo circulating clones. In this line, the FCR3 strain can be considered a reference strain, although it may probably underestimate the actual individual IgG binding capacity.
Using this flow cytometry technique, it is possible to monitor certain anti-P. falciparum function-associated parameters of IgG responses and to determine particular surrogate markers of IgG binding to IRBC before and after future vaccination trials. To circumvent the limited Ag repertoire expressed in vitro by the FCR3 strain of P. falciparum, further analysis with local strains of parasites must be conducted to demonstrate the function-associated characteristics of Ags associated with the IRBC membrane and to contribute to the screening and development of a multitarget vaccine.
Acknowledgments
We are indebted to the Dielmo and Ndiop villagers for participating in these studies for several years. We acknowledge D. Mattei and M. Guillotte for providing Ags used in this investigation and the field study team, particularly P. Nabeth, A. Spiegel, and C. Sokhna, for their participation in this work.
This work was supported in part by grants from the Institut Pasteur Fondation and from the French Ministère de la Coopération et du Développement.
REFERENCES
- 1.Aidoo, M., D. J. Terlouw, M. S. Kolczak, P. D. McElroy, F. O. ter Kuile, S. Kariuki, B. L. Nahlen, A. A. Lal, and V. Udhayakumar. 2002. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet 359:1311-1312. [DOI] [PubMed] [Google Scholar]
- 2.Aribot, G., C. Rogier, J.-L. Sarthou, A. T. Balde, J.-F. Trape, P. Druilhe, and C. Roussilhon. 1996. Pattern of immunoglobulin isotype response to Plasmodium falciparum blood-stage antigens in individuals living in a holoendemic area of Senegal (Dielmo, West Africa). Am. J. Trop. Med. Hyg. 54:449-457. [DOI] [PubMed] [Google Scholar]
- 3.Aucan, C., Y. Traore, F. Tall, B. Nacro, T. Traore-Leroux, F. Fumoux, and P. Rihet. 2000. High immunoglobulin G2 (IgG2) and low IgG4 levels are associated with human resistance to Plasmodium falciparum malaria. Infect. Immun. 68:1252-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnefoy, S., M. Guillotte, G. Langsley, and O. Mercereau Puijalon. 1992. Plasmodium falciparum: characterization of gene R45 encoding a trophozoite antigen containing a central block of six amino acid repeats. Exp. Parasitol. 74:441-451. [DOI] [PubMed] [Google Scholar]
- 5.Bull, P. C., B. S. Lowe, M. Kortok, and K. Marsh. 1999. Antibody recognition of Plasmodium falciparum erythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect. Immun. 67:733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bull, P. C., B. S. Lowe, M. Kortok, C. S. Molyneux, C. I. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med. 4:358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celada, A., A. Cruchaud, and L. H. Perrin. 1982. Opsonic activity of human immune serum on in vitro phagocytosis of Plasmodium falciparum infected red blood cells by monocytes. Clin. Exp. Immunol. 47:635-644. [PMC free article] [PubMed] [Google Scholar]
- 8.Drame, I., A. Diouf, A. Spiegel, O. Garraud, and R. Perraut. 1999. Flow cytometry analysis for the measurement of antibodies to P. falciparum infected erythrocytes membrane in immune individuals living in endemic areas of transmission. Acta Trop. 73:175-181. [DOI] [PubMed] [Google Scholar]
- 9.Druilhe, P., A. Sabchareon, H. Bouharountayoun, C. Oeuvray, and J. L. Perignon. 1997. In vivo veritas-lessons from immunoglobulin-transfer experiments in malaria patients. Ann. Trop. Med. Parasitol. 91:S37-S53.
- 10.Dubois, P., and L. Pereira da Silva. 1995. Towards a vaccine against asexual blood stage infection by Plasmodium falciparum. Res. Immunol. 146:263-275. [DOI] [PubMed] [Google Scholar]
- 11.Fairbanks, G., T. L. Steck, and D. F. Wallach. 1971. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry 17:2606-2617. [DOI] [PubMed] [Google Scholar]
- 12.Giha, H. A., T. Staalsoe, D. Dodoo, C. Roper, G. M. Satti, D. E. Arnot, L. Hviid, and T. G. Theander. 2000. Antibodies to variable Plasmodium falciparum-infected erythrocyte surface antigens are associated with protection from novel malaria infections. Immunol. Lett. 71:117-126. [DOI] [PubMed] [Google Scholar]
- 13.Groux, H., and J. Gysin. 1990. Opsonization as an effector mechanism in human protection against asexual blood stages of Plasmodium falciparum: functional role of IgG subclasses. Res. Immunol. 141:529-542. [DOI] [PubMed] [Google Scholar]
- 14.Gysin, J., S. Gavoille, D. Mattei, A. Scherf, S. Bonnefoy, O. Mercereau-Puijalon, T. Feldmann, J. Kun, B. Muller-Hill, and L. Pereira da Silva. 1993. In vitro phagocytosis inhibition assay for the screening of potential candidate antigens for sub-unit vaccines against the asexual blood stage of Plasmodium falciparum. J. Immunol. Methods 159:209-219. [DOI] [PubMed] [Google Scholar]
- 15.Gysin, J., P. Moisson, L. P. DaSilva, and P. Druilhe. 1996. Antibodies from immune African donors with a protective effect in Plasmodium falciparum human infection are also able to control asexual blood forms of the parasite in saimiri monkeys. Res. Immunol. 147:397-401. [DOI] [PubMed] [Google Scholar]
- 16.Jouin, H., Y. Goguet, C. Behr, M. Huyin Qan Dat, J.-C. Michel, J.-L. Sarthou, L. Pereira da Silva, and P. Dubois. 1995. Flow cytometry detection of surface antigens on fresh, unfixed red blood cells infected by Plasmodium falciparum. J. Immunol. Methods 179:1-12. [DOI] [PubMed] [Google Scholar]
- 17.Le Scanf, C., T. Fandeur, S. Bonnefoy, M. Guillotte, and O. Mercereau-Puijalon. 1999. Novel target antigens of the strain-specific immune response to Plasmodium falciparum identified by differential screening of an expression library. Infect. Immun. 67:64-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Scanf, C., T. Fandeur, M. E. Morales-Betoulle, and O. Mercereau-Puijalon. 1997. Plasmodium falciparum: altered expression of erythrocyte membrane-associated antigens during antigenic variation. Exp. Parasitol. 85:135-148. [DOI] [PubMed] [Google Scholar]
- 19.Mattei, D., and A. Scherf. 1992. The Pf332 gene of Plasmodium falciparum codes for a giant protein that is translocated from the parasite to the membrane of infected erythrocytes. Gene 110:71-79. [DOI] [PubMed] [Google Scholar]
- 20.McGregor, I. A., S. Carrington, and S. Cohen. 1963. Treatment of East African Plasmodium falciparum malaria with West African human gamma-globulin. Trans. R. Soc. Trop. Med. Hyg. 50:170-175. [Google Scholar]
- 21.Ofori, M. F., D. Dodoo, T. Staalsoe, J. A. Kurtzhals, K. Koram, T. G. Theander, B. D. Akanmori, and L. Hviid. 2002. Malaria-induced acquisition of antibodies to Plasmodium falciparum variant surface antigens. Infect. Immun. 70:2982-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perraut, R., M. Guillotte, I. Drame, B. Diouf, J. F. Molez, A. Tall, J. F. Trape, O. Mercereau-Puijalon, A. Spiegel, and O. Garraud. 2002. Evaluation of anti-Plasmodium falciparum antibodies in Senegalese adults using different types of crude extracts from various strains of parasite. Microbes Infect. 4:31-35. [DOI] [PubMed] [Google Scholar]
- 23.Perraut, R., O. Mercereau Puijalon, D. Mattei, E. Bourreau, S. Bonnefoy, B. Bonnemains, J. Gysin, J. C. Michel, and L. Pereira da Silva. 1997. Immunogenicity and efficacy trials with Plasmodium falciparum recombinant antigens identified as targets of opsonizing antibodies in the naive squirrel monkey Saimiri sciureus. Am. J. Trop. Med. Hyg. 56:343-350. [DOI] [PubMed] [Google Scholar]
- 24.Perraut, R., O. Mercereau Puijalon, D. Mattei, E. Bourreau, O. Garraud, B. Bonnemains, L. Pereira da Silva, and J. C. Michel. 1995. Induction of opsonizing antibodies after injection of recombinant Plasmodium falciparum vaccine candidate antigens in preimmune Saimiri sciureus monkeys. Infect. Immun. 63:554-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perraut, R., O. Mercereau-Puijalon, B. Diouf, A. Tall, C. LeScanf, A. Spiegel, J. Trape, and O. Garraud. 2000. Season-dependant fluctuation of antibody levels to P. falciparum parasitised red blood cell-associated antigens in two Senegalese villages with different transmission conditions. Am. J. Trop. Med. Hyg. 62:746-751. [DOI] [PubMed] [Google Scholar]
- 26.Perraut, R., C. Morales Betoulle, C. LeScanf, E. Bourreau, M. Guillotte, S. Bonnefoy, J. C. Michel, and O. Mercereau Puijalon. 2001. Evaluation of immunogenicity and protective efficacy of carrier-free Plasmodium falciparum R23 antigen in pre-exposed Saimiri sciureus monkeys. Vaccine 19:59-67. [DOI] [PubMed] [Google Scholar]
- 27.Piper, K. P., D. J. Roberts, and K. P. Day. 1999. Plasmodium falciparum: analysis of the antibody specificity to the surface of the trophozoite-infected erythrocyte. Exp. Parasitol. 91:161-169. [DOI] [PubMed] [Google Scholar]
- 28.Staalsoe, T., H. A. Giha, D. Dodoo, T. G. Theander, and L. Hviid. 1999. Detection of antibodies to variant antigens on Plasmodium falciparum-infected erythrocytes by flow cytometry. Cytometry 35:329-336. [DOI] [PubMed] [Google Scholar]
- 29.Staalsoe, T., and L. Hviid. 1998. The role of variant-specific immunity in asymptomatic malaria infections—maintaining a fine balance. Parasitol. Today 14:177-178. [DOI] [PubMed] [Google Scholar]
- 30.Staalsoe, T., E. Khalil, I. M. Elhassan, E. E. Zijlstra, A. M. Elhassan, H. A. Giha, T. G. Theander, and P. H. Jakobsen. 1998. Antibody reactivity to conserved linear epitopes of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1). Immunol. Lett. 60:121-126. [DOI] [PubMed] [Google Scholar]
- 31.Su, X. Z., V. M. Heatwole, S. P. Wertheimer, F. Guinet, J. A. Herrfeldt, D. S. Peterson, J. A. Ravetch, and T. E. Wellems. 1995. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82:89-100. [DOI] [PubMed] [Google Scholar]
- 32.Trape, J.-F., and C. Rogier. 1996. Combating malaria morbidity and mortality by reducing transmission. Parasitol. Today 12:236-240. [DOI] [PubMed] [Google Scholar]
- 33.Trape, J. F., C. Rogier, L. Konate, N. Diagne, H. Bouganali, B. Canque, F. Legros, A. Badji, G. NDiaye, P. NDiaye, K. Brahimi, O. Faye, P. Druilhe, and L. Pereira da Silva. 1994. The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am. J. Trop. Med. Hyg. 51:123-137. [DOI] [PubMed] [Google Scholar]