Abstract
Gene expression in Anopheles gambiae shows a deficiency of testis-expressed genes on the X chromosome associated with an excessive movement of retrogene duplication. We suggest that the degeneration of sex chromosomes in this monandrous species is likely the result of pressures from X inactivation, dosage compensation, and sexual antagonism.
IN a heterogametic sex determination system, the sex chromosomes (X or Z) spend a disproportionate amount of evolutionary time in one gender over the other (Vicoso and Charlesworth 2006). For example, relative to autosome evolution, the X chromosome occurs twice as frequently in females compared to males; thus, new sex-linked mutations will be exposed to selection two-thirds of the time in females and only one-third in males. One expected consequence of this relationship is that the sex-related content of autosomes and sex chromosomes should be unequally distributed through the genome as a result of antagonism between the sexes (Rice 1984). It is predicted that dominant or partially dominant mutations beneficial to females (and detrimental to males) spread more easily if they are X-linked, whereas recessive mutations beneficial to males (and detrimental to females) should accumulate on the X chromosome because sexual antagonism will be masked in heterozygous females.
Even though the outcome of sexual antagonism is expected to be highly dependent on the dominance of mutations in question, several genomic studies have found significant differences in the gene content of autosomes and the X chromosome. First, sex-biased gene expression in somatic and germline tissue is often nonrandomly distributed among chromosomes (Parisi et al. 2003; Ranz et al. 2003; Khil et al. 2004). Second, several studies have reported an excess of duplicated genes moving from the X chromosome to the autosomes associated with testis expression (Betran et al. 2002; Emerson et al. 2004; Meisel et al. 2009; Vibranovski et al. 2009a). While sexual antagonism no doubt contributes to the observed deficiency of sex-biased genes in somatic tissues, a further consideration within the male germline is X inactivation. Evidence from Drosophila indicates that gene duplications escaping the X chromosome have a higher likelihood of testis expression and are retained on the autosomes because they are required during spermatogenesis (Betran et al. 2002; Meisel et al. 2009; Vibranovski et al. 2009a,b). Thus, X inactivation can generate selection for increased dosage and favor the fixation of autosomal gene duplications, whether or not they are in fact sexually antagonistic.
Heteromorphic sex chromosomes have evolved independently multiple times in the Diptera. In the mosquito Anopheles gambiae, males and females have fully differentiated heteromorphic sex chromosomes, whereas in Aedes aegypi the sex chromosomes are homomorphic and largely homologous (Krzywinski et al. 2004; Nene et al. 2007). By classifying the origin of retro-transposition events between these species, Toups and Hahn (2010) found evidence for an excess of X-to-A turnover, primarily in the Anopheles lineage, indicating that the Anopheles common ancestor had homomorphic sex chromosomes. Yet, in contrast to higher Diptera, Toups and Hahn (2010) found no evidence that X-to-A duplications are associated with male-biased gene expression, leading to the conclusion that sex-chromosome evolution is not driven by patterns of sex-specific gene expression in Anopheles (Hahn and Lanzaro 2005; Toups and Hahn 2010). However, an important difference between Anopheles and other species investigated to date is that females mate only once during their life time (Tripet et al. 2003), a key attribute affecting male testis size (Hosken and Ward 2001). Whereas much of the sex-biased expression displayed by Drosophila and other polygonous species results directly from expression in the gonads (Parisi et al. 2003), in Anopheles the testis are proportionately much smaller compared to body mass, and consequently, when transcription levels are assessed at a whole-body level, male-biased expression is less pronounced.
A recently published catalog of tissue-specific expression in Anopheles (MozAtlas, http://www.tissue-atlas.org) reveals that <10% of testis-enriched genes are male-biased in expression because they are largely undetected in whole-body samples (Baker et al. 2011). This contrasts with the ovaries in which >50% of genes have female-biased expression, a finding that is likely to account for elevated levels of female transcription observed in whole-body samples (Hahn and Lanzaro 2005). As we have reported previously (Baker et al. 2011), this data set reveals that there is also an under-representation of genes on the X chromosome expressed within the testis, consistent with studies in higher Diptera (Parisi et al. 2003).
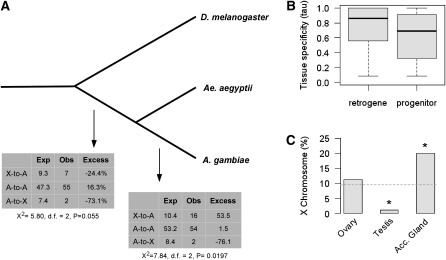
In light of these findings, we have re-examined the relationship between Anopheles retrogene duplication and male-related gene expression (supporting information, File S1 for methods). Using recent orthology assignments (Vilella et al. 2009), and in agreement with Toups and Hahn (2010), we observe an excess of retrogene relocations out of the X chromosome after the dipteran split (Figure 1A and Table S1). Importantly, while our data set is more stringent, we also find that the majority of X-to-A duplication events occur within the Anopheles lineage (n = 16/23; 53% excess). However, in contrast to earlier findings, when male-related gene expression is compared with retrogene movement, we find that testis expression is a common feature of duplications derived from the X chromosome (X to A = 17/18; 95%), whereas retrogenes originating from the autosomes are less likely to be expressed in the testis (A to A = 18/37; 48%) (χ2 = 9.08, d.f. = 1, P = 0.002). Thus, we find support for a link between the movement of gene duplications and male-related gene expression.
Figure 1—
(A) Dipteran retrogene events. We find an excess of X-to-A retrogene events occurring within the Anopheles lineage, but not on the branch prior to the Anopheles and Aedes split. X, X chromosome. A, autosomes. Excess = [(O – E)/E] × 100. (B) Tissue-specificity of retrogenes and progenitors. Retrogenes show a higher incidence of tissue-specific gene expression. Unpaired Wilcoxon test: P < 0.05. (C) X-chromosome enrichment of genes expressed in reproductive tissue. Consistent with previous studies, there is a deficiency of testis-specific genes on the Anopheles X chromosome, but an over-representation of male accessory gland genes (expected X: 9.6%; χ2 test: *P < 0.05).
One expectation for models involving X inactivation as the underlying cause of male-related genes moving off the X chromosome is that ancestral X-linked genes should have low expression levels in the testis when compared to autosomal retrogenes. Indeed, we find that derived retrogenes have a higher incidence of tissue-specific expression than ancestral genes (Figure 1B; unpaired Wilcoxon test; P < 0.05). Unfortunately, this difference in itself cannot differentiate the effects of X inactivation vs. sexual antagonism because the latter also predicts a spread of male beneficial dominant mutations to the autosomes (including testis-expressed genes). However, we find that testis enrichment, in particular, is higher for retrogenes in X-to-A relocations compared to retrogenes in A-to-A duplication events, whereas ancestral genes show the opposite effect; i.e., ancestral genes in X-to-A duplications have significantly lower testis enrichment in ancestral genes when compared to A-to-A duplication events (Table 1). This finding was repeated specifically for genes within the Anopheles lineage on the basis of testis log-fold enrichment vs. the carcass (Figure S1). Since sexual antagonism does not predict a difference in gene expression between autosomes on the basis of where the ancestral gene was located, deviation in testis expression of X-to-A vs. A-to-A retro-duplicated genes suggests the involvement of X inactivation (Vibranovski et al. 2009a).
Table 1. Testis gene expression enrichment.
| Progenitors (Culicidae/Anopheles) | Retrogenes (Culicidae/Anopheles) | |||
|---|---|---|---|---|
| Direction | Enriched | Not enriched | Enriched | Not enriched |
| X to A | 0/0 | 3/12 | 3/6 | 0/6 |
| A to A | 2/6 | 12/5 | 2/2 | 12/9 |
Testis enrichment calls for the Culicidae and Anopheles branches. Testis enrichment is significantly higher for retrogenes in X-to-A relocations when compared to A-to-A duplications across branches, whereas testis enrichment of ancestral genes (progenitors) in X-to-A relocations is significantly lower than in A-to-A duplications. Significant differences within progenitor and retrogene sets were determined by pooling branch samples and comparing the observed χ2 statistic with 100,000 permuted χ2 values via Monte Carlo simulation. See File S1 and File S2 for expression values and significance tests. For progenitors, P(χ2) < 0.032; for retrogenes, P(χ2) < 0.004.
However, a third mechanism that we must consider that could deplete male-related genes on the X chromosome is the action of dosage compensation systems. Dosage compensation acts to balance gene expression between the X chromosome and autosomes of the heterogametic sex and may contribute to the paucity of male-biased genes in a number of ways. For instance, when the X chromosome is hyper-activated in males, increased testis expression may be harder to achieve for single X-linked genes if transcription rates are limited, thus reducing the accumulation of male-biased genes on the X chromosome (Vicoso and Charlesworth 2009; Bachtrog et al. 2010). Alternatively, if the X chromosome lacks dosage compensation in males, selection for increased dosage may favor the fixation of male-related genes on the autosomes in a fashion similar to X inactivation during spermatogenesis. We find that while male and female somatic tissues are expressed at the same level on autosomes and on the X chromosome in Anopheles (soma: Xfemale/Afemale: 1.07–1.29; Xmale/Amale: 1.12–1.26; see Table S2), male germline expression on the X chromosome is half of the autosomal levels, as would be expected in the absence of dosage compensation (germline: Xfemale/Afemale—1.05; Xmale/Amale—0.48; unpaired Wilcoxon test—P < 2.38 × 10−12). Subsequently, unlike Drosophila (Gupta et al. 2006), we detect X chromosome hyper-activation only in the male soma, indicating that an absence of male germline dosage compensation may contribute to a deficiency of male-related genes on the X chromosome in Anopheles.
Taken together, these observations suggest that retrogene movement, as a result of expression in the male testis, may be a general feature of sex-chromosome evolution in the Diptera. However, lower expression of the X chromosome in the male germline could correspond to an absence of dosage compensation or meiotic X inactivation in Anopheles. In the future, it will be of considerable interest to measure X chromosome and autosomal expression in mitotic and meiotic cells of this species to help resolve which mechanisms contribute to this trend. Since Y chromosome degeneration appears to have evolved rapidly in Anopheles after its divergence from Aedes, further study is likely to yield important insight into how these processes and modes of sexual antagonism contribute to sex-chromosome evolution in monandrous species like the mosquito. Given that Anopheles appears to have evolved sex chromosomes independently of Drosophila, much is to be gained from understanding the generality of phenomena that we have observed.
For example, if we extend our original analysis of germline and somatic expression to consider the chromosomal distribution of genes expressed only in single tissues, unlike Drosophila melanogaster (Mueller et al. 2005), we find an excess of genes detected solely in the male accessory glands of Anopheles (Figure 1C and Table S3). Male accessory gland (MAG) proteins are an essential component of seminal fluid deposited into the female reproductive tract during copulation, often associated with male reproductive success (Klowden and Russell 2004; Rogers et al. 2008). While the relative importance of such proteins to sexual conflict in the mosquito is still debated, an over-representation of MAG proteins on the X chromosome suggests that at least some components of male reproductive biology are potentially antagonistic in this species. Indeed, it is predicted that recessive genes conferring a male advantage at the expense of females should be located on the X chromosome (Rice 1984). However, if sexually antagonistic mutations are often dominant, this could explain a deficiency of male-biased genes on the X chromosome, including somatically expressed genes.
Acknowledgments
This study was funded by a Grant from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health initiative.
Literature Cited
- Bachtrog D., Toda N. R., Lockton S., 2010. Dosage compensation and demasculinization of X chromosomes in Drosophila. Curr. Biol. 20: 1476–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. A., Nolan T., Fischer B., Pinder A., Crisanti A., et al. , 2011. A comprehensive gene expression atlas of sex- and tissue-specificity in the malaria vector, Anopheles gambiae. BMC Genomics 12: 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betran E., Thornton K., Long M., 2002. Retroposed new genes out of the X in Drosophila. Genome Res. 12: 1854–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson J. J., Kaessmann H., Betran E., Long M. Y., 2004. Extensive gene traffic on the mammalian X chromosome. Science 303: 537–540 [DOI] [PubMed] [Google Scholar]
- Gupta V., Parisi M., Sturgill D., Nuttall R., Doctolero M., et al. , 2006. Global analysis of X-chromosome dosage compensation. J. Biol. 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M. W., Lanzaro G. C., 2005. Female-biased gene expression in the malaria mosquito Anopheles gambiae. Curr. Biol. 15: R192–R193 [DOI] [PubMed] [Google Scholar]
- Hosken D. J., Ward P. I., 2001. Experimental evidence for testis size evolution via sperm competition. Ecol. Lett. 4: 10–13 [Google Scholar]
- Khil P. P., Smirnova N. A., Romanienko P. J., Camerini-Otero R. D., 2004. The mouse X chromosome is enriched for sex-biased genes not subject to selection by meiotic sex chromosome inactivation. Nat. Genet. 36: 642–646 [DOI] [PubMed] [Google Scholar]
- Klowden M. J., Russell R. C., 2004. Mating affects egg maturation in Anopheles gambiae Giles (Diptera: Culicidae). J. Vector Ecol. 29: 135–139 [PubMed] [Google Scholar]
- Krzywinski J., Nusskern D. R., Kern M. K., Besansky N. J., 2004. Isolation and characterization of Y chromosome sequences from the African malaria mosquito Anopheles gambiae. Genetics 166: 1291–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel R. P., Han M. V., Hahn M. W., 2009. A complex suite of forces drive gene traffic from Drosophila X chromosomes. Genome Biol. Evol. 1: 176–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller J. L., Ravi Ram K., McGraw A., Bloch Gazi M. C., Siggia E. D., et al. , 2005. Cross-species comparison of Drosophila male accessory gland protein genes. Genetics 171: 131–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nene V., Wortman J. R., Lawson D., Haas B., Kodira C., et al. , 2007. Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316: 1718–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi M., Nuttall R., Naiman D., Bouffard G., Malley J., et al. , 2003. Paucity of genes on the Drosophila X chromosome showing male-biased expression. Science 299: 697–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranz J. M., Castillo-Davis C. I., Meiklejohn C. D., Hartl D. L., 2003. Sex-dependent gene expression and evolution of the Drosophila transcriptome. Science 300: 1742–1745 [DOI] [PubMed] [Google Scholar]
- Rice W. R., 1984. Sex chromosomes and the evolution of sexual dimorphism. Evolution 38: 735–742 [DOI] [PubMed] [Google Scholar]
- Rogers D. W., Whitten M. M., Thailayil J., Soichot J., Levashina E. A., et al. , 2008. Molecular and cellular components of the mating machinery in Anopheles gambiae females. Proc. Natl. Acad. Sci. USA 105: 19390–19395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toups M. A., Hahn M. W., 2010. Retrogenes reveal the direction of sex-chromosome evolution in mosquitoes. Genetics 186: 763–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripet F., Toure Y. T., Dolo G., Lanzaro G. C., 2003. Frequency of multiple inseminations in field-collected Anopheles gambiae females revealed by DNA analysis of transferred sperm. Am. J. Trop. Med. Hyg. 68: 1–5 [PubMed] [Google Scholar]
- Vibranovski M. D., Zhang Y., Long M. Y., 2009a General gene movement off the X chromosome in the Drosophila genus. Genome Res. 19: 897–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibranovski M. D., Lopes H. F., Karr T. L., Long M. Y., 2009b Stage-specific expression profiling of Drosophila spermatogenesis suggests that meiotic sex chromosome inactivation drives genomic relocation of testis-expressed genes. PLoS Genet. 5: e1000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicoso B., Charlesworth B., 2006. Evolution on the X chromosome: unusual patterns and processes. Nat. Rev. Genet. 7: 645–653 [DOI] [PubMed] [Google Scholar]
- Vicoso B., Charlesworth B., 2009. The deficit of male-biased genes on the D. melanogaster X chromosome is expression-dependent: A consequence of dosage compensation? J. Mol. Evol. 68: 576–583 [DOI] [PubMed] [Google Scholar]
- Vilella A. J., Severin J., Ureta-Vidal A., Heng L., Durbin R., et al. , 2009. EnsemblCompara GeneTrees: complete, duplication-aware phylogenetic trees in vertebrates. Genome Res. 19: 327–335 [DOI] [PMC free article] [PubMed] [Google Scholar]