Abstract
The steroid hormone 20-hydroxyecdysone (20E) regulates gene transcription through the heterodimeric nuclear receptor composed of ecdysone receptor (EcR) and Ultraspiracle (USP). The EcR gene encodes three protein isoforms—A, B1, and B2—with variant N-terminal domains that mediate tissue and developmental stage-specific responses to 20E. Ariadne-1a is a conserved member of the RING finger family of ubiquitin ligases first identified in Drosophila melanogaster. Loss-of-function mutations at key cysteines in either of the two RING finger motifs, as well as general overexpression of this enzyme, cause lethality in pupae, which suggests a requirement in metamorphosis. Here, we show that Ariadne-1a binds specifically the isoform A of EcR and ubiquitylates it. Co-immunoprecipitation experiments indicate that the full sequence of EcRA is required for this binding. Protein levels of EcRA and USP change in opposite directions when those of ARI-1a are genetically altered. This is an isoform-specific, E3-dependent regulatory mechanism for a steroid nuclear receptor. Further, qRT-PCR experiments show that the ARI-1a levels lead to the transcriptional regulation of Eip78C, Eip74EF, Eip75B, and Br-C, as well as that of EcR and usp genes. Thus, the activity of this enzyme results in the regulation of dimerizing receptors at the protein and gene transcription levels. This fine-tuned orchestration by a conserved ubiquitin ligase is required during insect metamorphosis and, likely, in other steroid hormone-controlled processes across species.
STEROID hormones regulate multiple processes during development and adult life. In Drosophila, the steroid hormone 20-hydroxyecdysone (20E) triggers the transcriptional changes required for gonad development, larval molting, and the onset and completion of metamorphosis (Sliter and Gilbert 1992; Henrich et al. 1993; Carney and Bender 2000; Riddiford et al. 2000; Beckstead et al. 2005). Differentiation of imaginal structures and neuronal remodeling, two major features of metamorphosis, have been used as cellular systems to analyze the functional role of proteins that elicit morphogenetic changes at this phase of the life cycle (Schubiger et al. 2005; Brown et al. 2006; Santos et al. 2006). 20E regulates gene expression by binding to its nuclear receptor, EcR (King-Jones and Thummel 2005). To bind 20E and stimulate transcription, however, EcR must heterodimerize with Ultraspiracle (USP) to reconstitute specific activation domains (Yao et al. 1992). Different 20E levels activate transcription of different sets of genes (Champlin and Truman 1998; Li and White 2003; Schubiger et al. 2003). Like their vertebrate cognates (Chen and Evans 1995), unliganded EcR and USP act as repressors of transcription, whereas the liganded receptor stimulates expression of target genes (Tsai et al. 1999; Ghbeish et al. 2001; Schubiger et al. 2003).
On the basis of sequence identities, it is considered that the mammalian orthologs of EcR are the group H of nuclear receptor subfamily 1 that include LXR and FXR, while USP is represented by the retinoic X receptor (Robyr et al. 2000; Fitzgerald et al. 2002). The regulated activity of these receptors has a widespread effect on multiple aspects of development. For example, in mammals, they regulate cholesterol, osteoclast differentiation and triglyceride metabolism, and their impaired function leads to cardiovascular, bone, metabolic, and, possibly, Alzheimer’s diseases (Patel and Forman 2004; Beaven and Tontonoz 2006; Mark et al. 2006; Robertson et al. 2006; Xiong et al. 2008; Spyridon et al. 2011).
The EcR gene of Drosophila produces three protein isoforms (EcRA, EcRB1, and EcRB2) by using two promoters and alternative splicing (Talbot et al. 1993). The three isoforms are able to heterodimerize USP and share the same carboxy terminus, which includes the hormone-binding and DNA-binding domains, while the amino termini are unique to each isoform. The three EcR isoforms are hypothesized to play specific functions on the basis of their distinct temporal and spatial expression patterns and the distinct biochemical properties of their specific amino terminal domains (Kim et al. 1999; Sung and Robinow 2000; Davis et al. 2005). Mutational analyses of the EcR gene support the proposed EcR isoform functional specificity. Mutants that fully inactivate EcR are lethal in embryogenesis while, in isoform specific alleles, lethality occurs at characteristic stages of development. For instance, isoform B1 mutants fail to pupate while the majority of EcRA mutants die later during metamorphosis (Carney et al. 2004; Davis et al. 2005). Development is also halted by an excess of EcR function, with virtually the same phenotypes as the loss-of-function alleles (Schubiger et al. 2003), suggesting that EcR levels must be kept tightly regulated for normal development. The A and B1 isoforms are expressed in complementary patterns, with one notable exception: the prothoracic gland, which exhibits expression of both isoforms (Talbot et al. 1993). The other nuclear receptor, USP, is expressed ubiquitously and exhibits a DNA-binding motif that is required for repression, but dispensable for activation, of metamorphosis (Schubiger and Truman 2000; Ghbeish et al. 2001; Erezyilmaz et al. 2006). The mechanisms that lead to the activation of a specific receptor or, more often, a receptor isoform are still poorly known for ecdysone and most other steroid hormones across species. Thus, identifying a mechanism for the specific activation of a receptor isoform would be of general interest in the field of steroid hormone signaling due to the conserved nature of the proteins involved in this study.
Ubiquitin–proteasome degradation is one of the major processes to regulate protein levels and function (Bedford et al. 2010). Increasing evidence supports a key role of ubiquitylation and proteasome-dependent proteolysis in gene transcription (Collins and Tansey 2006). Activity of the 26S proteasome is required for proper transcription of genes encoding the glucocorticoid and many other steroid hormone receptors (Dennis et al. 2005; Kinyamu and Archer 2007). The ubiquitin pathway includes the activity of at least three different enzymes: a ubiquitin-activating enzyme or E1, a ubiquitin-conjugating enzyme or E2, and a ligase enzyme or E3. The specificity of the pathway is determined mainly by the E3 ligase, and, consequently, in mammals there is a large number of E3 (>100) compared to the 25 E2 or the single E1 (Glickman and Ciechanover 2002). Two families of E3 ligases are known so far: the HECT (homologous to E6-AP C terminus) and the RING finger. Drosophila Ariadne-1a (ARI-1a) belongs to the latter and is the founder member of a subfamily containing the RBR (RING finger, in between RING fingers) string motif (Aguilera et al. 2000). Indeed, most RBR proteins function as E3 enzymes (Lorick et al. 1999; Joazeiro and Weissman 2000; Kamura et al. 2002; Marin and Ferrus 2002; Capili et al. 2004; Marin et al. 2004). The gene ari-1a is expressed throughout development, but its lack of function (Aguilera et al. 2000) or its generalized overexpression results in lethality at the end of the third larval instar, indicating a quantitatively regulated requirement for this ubiquitin ligase activity in metamorphosis. Here we investigated the in vivo targets of ARI-1a, a conserved E3 enzyme, and the corresponding cellular effects to explain its role in metamorphosis. We have previously described UbcD10 as a novel ubiquitin-conjugating enzyme (E2) that interacts with ARI-1a (Aguilera et al. 2000). Now we identify EcRA as a bona fide substrate of ARI-1a, since it is regulated in response to ARI-1a levels and is ubiquitylated in vitro by ARI-1a with the concourse of UbcD10. In addition, ARI-1a interacts with USP and contributes to the transcriptional activity of the EcRA/USP complex.
Materials and Methods
Fly strains, genetic interaction, and target gene expression studies
The mutant allele ari-1a2 was described previously (Aguilera et al. 2000). The following fly stocks were obtained from the Drosophila Stock Center (FlyBase http://flybase.bio.indiana.edu): EcRM554fs/SM6b (EcRA, B1, B2 defective); EcR31/CyO (EcRB1 defective); EcR225/CyO (EcRB1, B2 defective); Df(2)EcRA112/CyO (EcRA defective) (Carney et al. 2004); usp4/FM7a (allele mutated at the DNA-binding domain); bon21B/TM6 (Bonus defective); P(ry+)ftz-f103649/TM3 (βFtz-f1 defective); Df(3R)Spf,P(ry+)l(3)ArBEip78C/MKRS (Eip78C defective); P(y+)Eip55EKG02526/CyO (Eip55E defective); P(y+)Eip75BKG03025 (Eip75B defective); P(y+)CG15523KG07288 (Eif2b defective); Df(2)Drlrv7(Spn1 defective); P(w+)UAS-EcRB13b; P(w+)UAS-EcRB23a; P(w+)UAS-EcRA3a; P(w+)UAS-CD8GFP; Beadex-Gal4-MS1096; how-Gal4-24B/TM2; tubulin-Gal4-LL7/TM3; Dpp-Gal4-PS6A/CyO; Distal-lesGal4-MD23/CyO; Gal4-C649; EcR.GET-BD-Gal4 (expressed in an EcRA domain); ari-1a-Gal4-NP1063 (expressed in an ari-1a domain); and phm-Gal4UAS-CD8-GFP (expressed in the phantom domain, the prothoracic cells of the ring gland). The expression domains of Gal4 drivers were tested using the reporters UAS-LacZ or UAS-GFPS65T. For gene interaction studies, ari-1a2/FM7i females were crossed with spn1 (serine protease inhibitor 1); eIF2b (translation initiation factor); Bonus (nuclear receptor cofactor); and EcRA, EcRA-B1-B2, EcRB1-B2 and transcription factors βFtz-f1, Eip78C, Eip75B, and Eip55E mutant males. The percentage of viability was obtained as the number of ari-1a2; */+ males rescued from lethality divided by the number of sibling adults of the most viable genotype in each cross. Lethal phases were determined as previously described (Aguilera et al. 2000).
For the generation of the pUAS-ari-1a line, the ari-1a open reading frame (ORF) was cloned into the pUASt vector. To generate the myc-tagged ARI-1a construct (pUAS-myc-ari-1a), the ari-1a ORF was first cloned into the plasmid pCMA vector (Hu et al. 2003) containing 6× myc in the N terminus. The myc-ari-1a chimera was subcloned into pUASt. Germline transformations were performed in y w embryos following standard procedures (Rubin and Spradling 1982; Spradling and Rubin 1982). The expression of selected genes was severely attenuated by using the corresponding interference RNA (RNAi) (Dietzl et al. 2007). The lines used for that purpose were P(w+)UAS-ari-1a-RNAi35029; P(w+)UAS-usp-RNAi16893 (https://stockcenter.vdrc.at) and P(w+)UAS-EcR-RNAi104; P(w+)UAS-EcRA-dsRNA91 and P(w+)UAS-EcRB1-dsRNA168 (http://flybase.bio.indiana.edu). Their expression was driven into the desired group of cells by means of the Gal4/UAS system (Brand and Perrimon 1993).
Yeast two-hybrid assay
The ari-1a ORF lacking 47 amino acids of the N-terminal region was cloned into plasmid pAS21 (Gal4 DNA-binding domain). Full-length cDNAs of EcRA, EcRB1, USP, eIf2b, and spn1 were obtained from the Berkeley Drosophila Genome Project EST collection (Drosophila Genomics Resource Center) and cloned in plasmid pACT2 (Gal4-activating domain). For each assay, normal or mutated ARI-1a bound to the Gal4 DNA-binding domain was co-expressed with each of the proteins bound to the Gal4-activating domain into the yeast strain Y190. Thereafter, each cotransformation was tested for β-galactosidase activity.
Generation of anti-ARI-1a antibody
The ARI-1a-specific polyclonal antibody was generated in mice immunized with the peptide EVDLPSSADRQMDQDDYQ, which corresponds to amino acids 30–47. Peptide synthesis, immunization, and affinity purification of serum were performed by GenScript. Specificity of the antibody was tested by Western blot of BL21 bacteria expressing ARI-1a.
Immunoprecipitation and Western blot
For co-immunoprecipitations (CoIPs), white prepupae or transfected cells were lysed in immunoprecipitation (IP) buffer [20 mm Tris, pH 7.4, 150 mm NaCl, 2 mm MgCl, and 0.5% NP-40 with protease inhibitor cocktail (Roche)]. A 500-μg protein extract was incubated overnight at 4° with antibody anti-c-myc (Sigma) or mouse anti-goat IgG (Pierce) as negative control. Following incubation for 2 hr with protein G-coupled magnetic beads (Invitrogen), beads were extensively washed with IP buffer and boiled in Laemli buffer for 10 min, and eluted proteins were subjected to SDS-PAGE electrophoresis. Immunoblotting was carried out with anti-EcRA (15G1a) antibody 1:100 (Hybridoma Bank), anti-c-myc 1:2000 (Sigma), anti EcR antibody 1:100 (Hybridoma bank), or anti-flag antibody 1:2000 (Sigma). To minimize unspecific bands from the antibodies used for the IP, the blots were then incubated with HRP-conjugated anti-mouse IgG TrueBlot (eBioscience), which preferentially detects the native disulfide form of mouse IgG. For Western blotting analysis of protein levels, at least 10 white prepupae from each genotype were homogenized in lysis buffer [150 mm NaCl, 1% Triton X100, 50 mm Tris–HCl, pH 7.4, supplemented with complete protease inhibitor cocktail (Roche)]. For the S2 fractioning, nuclear and cytoplasmic fractions were obtained from S2 cells according to Kawasaki et al. (2005). In all cases, protein extracts were resolved by SDS-PAGE, and blots were incubated with antibodies against ARI-1a; EcRA (15G1a) (Hybridoma Bank) at 1:100 dilution; EcRB1 (AD4.4) (Hybridoma Bank) at 1:500 dilution; USP (kindly provided by F. Kafatos, European Molecular Biology Laboratory) at 1:200; actin (JLA20) (Hybridoma Bank) at 1:2000 dilution; HA (Sigma) at 1:2000 dilution; myc antibody at 1:2000 (Sigma); or anti-nucleoporin p62 antibody at 1:1000 (BD Bioscience) overnight at 4° followed by the secondary HRP-conjugated antibodies (Sigma) at 1:5000. The signal was detected by autoradiography using Supersignal West Pico substrate (Pierce). When required, blots were quantified by densitometry using the Quantity-1 software (BioRad), normalizing values to actin content.
In vitro ubiquitylation assay
cDNAs for the E2-conjugating enzyme UbcD10, ari1a, and EcRA were cloned in frame with polyhistidine tag in the vector pRSET (Invitrogen). Proteins were purified using His Gravitrap columns (GE Healthcare) following the manufacturer’s instructions. Previously, KRX bacteria (Promega) containing the plasmids were induced with IPTG and rhamnose for 16 hr at 30° and then lysated and homogenized by sonication. For the ubiquitylation assays, the E1 enzyme (Boston Biochem) and affinity-purified UbcD10, ARI-1a, and EcRA were incubated with or without ubiquitin (Boston Biochem), or, in other type of approach, E1, UbCd10, and EcRA were incubated in the presence of HA–ubiquitin (Boston Biochem) with or without affinity-purified ARI-1a. In both cases, the reaction was incubated at 30° for 2 hr in ubiquitylation buffer (50 mm Tris–HCl, pH 7.5, 2.5 mm MgCl2, 1 mm ATP, and 0.5 mm dithiothreitol) and then stopped by adding Laemli buffer. The samples were then resolved by SDS-PAGE.
Cell culture and cell transfection assays
Schneider line 2 and L57-3-11 (EcR-deficient line) (Cherbas and Cherbas 1997) cells were grown as described (Cherbas et al. 1994). All transfections were carried out with Lipofectamine 2000 (Invitrogen) according to manufacturer instructions. For the CoIP assays, the USP, the N-terminal domain of EcRA (AF1), and the C-terminal domain, common to all EcR isoforms (AF2), were cloned by recombination in PAWF (Drosophila Gateway Vector collection) to introduce the Flag tag in the C terminus of each protein. The EcR-deficient L57-3-11 cell line was transfected with EcRA or EcRB1 cloned in PCMA (Hu et al. 2003) or with those constructs cloned in PAWF, and all of them cotransfected with myc-ari1a in PCMA.
Immunocytochemistry and histology
Untransfected S2 cells or cells transfected with PCMA myc-ari1a or PCMA GFP-ari1a were fixed with 4% paraformaldehyde in PBS for 20 min and then washed with PBS. GFP was directly visualized, and myc-tagged ARI-1a or endogenous ARI-1a were detected using anti-c-myc (Sigma) or anti-ARI-1a antibodies, incubated overnight in PBS containing 0.1% Triton-X100 5% normal goat serum and 5% bovine serum albumin. After washing with PBS, they were incubated with Alexa 488-conjugated secondary antibodies (Invitrogen). For mounting, Vectashield (Vector Laboratories) containing DAPI was used. Images were acquired using a Leica TSC SP5 confocal microscope. Malpighian tubuli were dissected from mutant and control larvae of the third instar expressing the GFP-tagged membrane protein CD8 under the Gal4 driver C649 whose domain of expression includes the stellate cells of the Malpighian tubuli and placed in Vectashield (Vector Laboratories). Images were taken under a Leica TSC SP5 confocal microscope. To quantify the amount of membrane material, the GFP intensity for each confocal image series was measured using ImageJ software.
Gene expression microarray analysis and quantitative RT-PCR
Offspring from (♀ y w ari-1a1/FM7i,GFP × ♂ FM7i,GFP) for ari-1a lack-of-function transcriptome and from (♀ y w; tubulin-Gal-LL7/TM6,TbGFP × ♂ y w UAS-ari1a) for ari-1a overexpression transcriptomes were used as source of experimental and control larvae. For each cross, egg-laying periods of 2 hr were obtained and kept under constant rearing conditions until larval extraction. Male mutant or overexpressing late third instar larvae and their corresponding controls were collected from the container’s wall and staged under the criterion of everted spiracles. RNA extraction and microarray analysis was performed by Progenika Biopharma, using the Affimetrix Genechip platform. The resulting control transcriptomes were used to determine whole-genome expression differences due to ari-1a mutant or ari-1a overexpressing condition, respectively. For quantitative RT-PCR (qRT-PCR) assays, RNA from at least eight larvae per genotype was extracted using Trizol (Invitrogen). A total of 5 μg RNA was used for reverse transcription (RT) performed with Supercript III Kit (Invitrogen) according to the manufacturer’s instructions. Quantitative PCR was carried out using Taqman MGB probes (Applied Biosystems) for the different genes analyzed. RNA polymerase II (RNApolII) was used as a housekeeping gene. Data were captured on a 7500 Real Time PCR System (Applied Biosystems) and analyzed using relative expression to RNApolII and plotted as a percentage of the respective control larva. For the Malpighian tubuli RT-PCR, 15 pairs of tubuli were collected from wild-type LIII larvae, and RNA extraction and RT were carried out as described previously; for the PCR, Taqman MGB probes specific for ECRA or ECRB1/B2 or Ari 1a were used, but instead of running the reaction in the real-time PCR system, reactions were carried out in a standard PCR (MJ Research) and run in an agarose gel.
Results
Changes in ARI-1a levels lead to phenotypes similar to ecdysone pathway gene deregulation
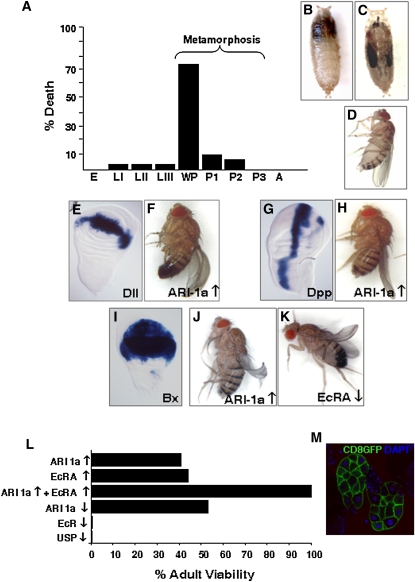
Since we had previously described the ari-1a loss-of-function mutant phenotypes (Aguilera et al. 2000), we addressed here the overexpression counterpart to explore possible trait similarities. Phenotypes caused by the excess of ARI-1a function were investigated using the UAS/Gal4 system (Brand and Perrimon 1993). Generalized overexpression by the tubulin-Gal4-LL7 driver causes lethality mostly at the white prepupae stage (Figure 1A). These mutants degenerate in their pupal cases and never emerge as adults. Using Gal4 drivers with more restricted expression domains (how-Gal4-24B) (see Supporting Information, Table S1), individuals develop further but arrest before head eversion and leg elongation, resulting in a cryptocephalic-like phenotype (Figure 1, B and C). In general, these traits are quite similar to those of genes involved in the ecdysone-signaling pathway (Bender et al. 1997; D’Avino and Thummel 2000; Fortier et al. 2003; Schubiger et al. 2003; Beckstead et al. 2005; Davis et al. 2005; King-Jones and Thummel 2005). Interestingly, the Gal4 driver NP1063 that corresponds to some regulatory sequences of the endogenous ari-1a gene does not cause lethality (not shown). This driver, however, is expressed in the fat body and salivary glands at the late LIII and prepupae stages, becoming general (in Malpighian tubuli, nervous system, muscles, etc.) at the late pupae stage and throughout adulthood. This observation indicates that the ARI-1a overexpression becomes deleterious at a critical time window around the onset of metamorphosis. This indication prompted our in-depth study of the putative relationship between ARI-1a and ecdysone pathway genes.
Figure 1 .
ARI-1a overexpression phenotypes. (A) Generalized overexpression of Ari-1a (tubulin-Gal4-LL7 > UAS-ari-1a) is lethal, mostly at the white prepupae stage. (B) Overexpression in the muscles (how-Gal4-24B > UAS-ari-1a) yields cryptocephalic pupae with defective head eversion and compressed thorax at the anterior end of the pupal case (anterior is up). (C) Wild-type pupae at the same age to serve as a reference. (D–J) Wing morphology is affected compared to a wild type (D) when ari-1a is overexpressed using the Gal4 drivers Distal-lessGal4-MD23 (Dll) (E and F), Decapentaplegic-PS6A (Dpp) (G and H), or Beadex-Gal4-MS1096 (Bx) (I and J). (K) Similar phenotype results from the underexpression of EcRA. Gal4 expression domains in the wing disc are revealed by the LacZ reporter (see also Figure S1 for additional Gal4 domains). (L) Viability of adults expressing the various constructs in the prothoracic gland, using the phm-Gal4 driver. (M) Domain of expression of phm-Gal4 in the prothoracic gland visualized by the CD8-GFP reporter. The excess-of-function for each gene (↑) was obtained using the corresponding UAS construct. The loss-of-function (↓) results from the expression of the RNAi constructs against ari-1a, EcRA, or usp. Note that the co-expression of EcRA and ARI-1a compensate each other and that there is no reduction in viability (L). E, embryo; LI–III, first through third larval instars; WP, white pupae; P1–3, pupae stages 1–3; A, adult.
EcR isoforms are differentially expressed in larval vs. imaginal tissues, with isoform A being predominant in imaginal structures, and in the wing disc in particular (Talbot et al. 1993; Cherbas et al. 2003; Davis et al. 2005). We found that wing discs are sensitive to the overexpression of ARI-1a. All tested Gal4 drivers for wing disc regions lead to abnormal morphology of the adult wing when ARI-1a is in excess, although each domain of expression yielded characteristic morphological abnormalities (Figure 1, D–J, and Fig. S1). Also, we explored the effect of overexpressing ARI-1a or down-expressing EcRA using Beadex-Gal4-MS1096, and in both cases abnormal wing morphology of the same type was observed (Figure 1, I–K). The joint effect of overexpressing ARI-1a and down-expressing EcRA resulted in an enhanced wing phenotype (Fig. S1). In agreement with the lack of expression of the EcRB1 isoform in the imaginal discs, silencing EcRB1 by means of an RNAi construct in the Dpp-Gal4 domain yielded normal wings (not shown).
In most insects, ecdysone biosynthesis occurs in the cells of the prothoracic gland during larval and pupal stages (Gilbert and Warren 2005). These cells are particularly interesting in the context of this study because they express all EcR isoforms (Talbot et al. 1993). We manipulated the expression of ARI-1a, EcR, or USP in the prothoracic gland using phm-Gal4, which drives expression specifically in the gland. The expression of RNAi against usp or EcR genes results in lethality, demonstrating the requirement of the EcR/USP complex in this gland (Figure 1L). The over- or down-expression of ARI-1a causes a reduction of adult viability of ∼50%, and the same effect is observed with the overexpression of EcRA. Interestingly, the joint overexpression of ARI-1a and EcRA rescued the lethality that the overexpression of each protein separately causes (Figure 1L). Taken together, the data so far suggest a possible functional relationship between ARI-1a and ecdysone-related proteins, particularly EcRA, justifying further experiments to identify the actual molecular mechanisms.
ARI-1a translocates to the nucleus, binds EcRA, and functionally interacts with ecdysone-signaling genes
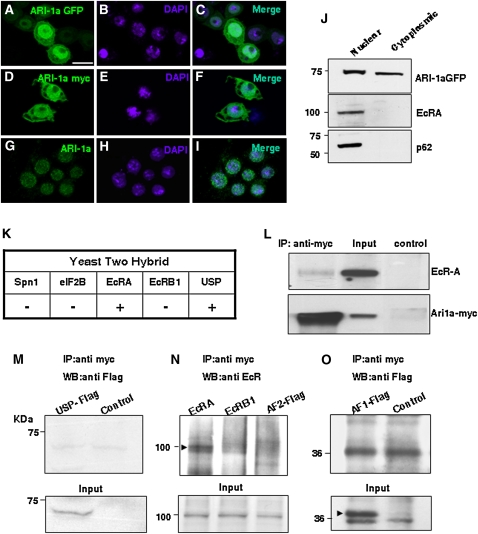
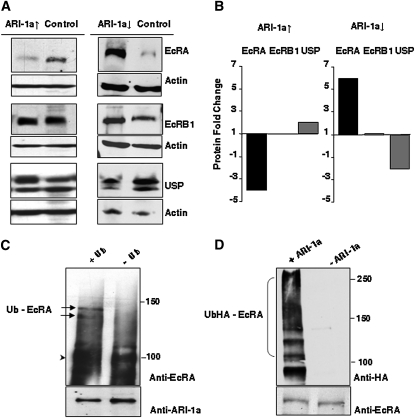
Since EcR and its partner, USP, are nuclear receptors and the previous set of data suggested a possible functional interaction between EcR and ARI-1a, we analyzed the cellular localization of ARI-1a. Its sequence exhibits a nuclear localization motif, KKWIKK, although cell fractionation experiments from whole-body adults had indicated a cytoplasmic, rather than nuclear, localization (Aguilera et al. 2000). To determine if ARI-1a could translocate to the nucleus, S2 cells were transfected with GFP-tagged or myc-tagged ARI-1a constructs. Colocalization of tagged ARI-1a and DAPI can be observed after immunostaining (Figure 2, A–F). For a further assessment, we checked the cellular localization of endogenous ARI-1a in S2 cells using an ARI-1a-specific antibody and DAPI for nuclear identification (Figure 2, G–I). ARI-1a appears colocalized to a large extent with the DAPI nuclear signal. In most cells, the tag signal can be detected in the cytoplasm as well as in the nucleus. Finally, a direct cell fractionation assay on S2 cells transfected with myc-tagged ARI-1a clearly shows that the signal can be present in the nucleus as well as in the cytoplasm (Figure 2J). These observations indicate that ARI-1a, either endogenous or transfected, can locate to the nucleus, as reported for EcR and USP (Gwozdz et al. 2007) (see Discussion).
Figure 2 .
ARI-1a localizes to the nucleus and binds EcRA. (A–C) Tagged expression of ARI-1a in transfected S2 cells revealed by an anti-GFP antibody. (D–F) Equivalent experiments using a myc tag. (G–I) Localization of endogenous ARI-1a in nontransfected cells using an anti-ARI serum. Note that tagged or untagged ARI-1a can localize in both the nucleus and the cytoplasm. Nuclei are labeled by DAPI. (J) Western blot of nuclear and cytoplasmic fractions from S2 cells cotransfected with GFP-tagged ARI-1a and EcRA. Detection of endogenous nucleoporin p62 is the control for the nuclear fraction. Note ARI-1a is present in both cell compartments while EcRA is nuclear. (K) Direct yeast two-hybrid assays show that ARI-1a interacts with EcRA and USP, but not with EcRB1, eIF2B (translation initiation factor), or Spn1 (serin protease inhibitor 1). (L) Immunoprecipitation (IP) of myc-ARI-1a from overexpressing white prepupae using an anti-myc antibody. ARI-1a is revealed using an anti-myc antibody and EcRA by anti-EcRA antibody. The negative control consisted of immunoprecipitation with a goat anti-mouse IgG. Input lane was loaded with 10% of the whole-cell extract used for IP. (M–O) IP experiments in L57-3-11 cells transfected with USP-flag, EcRA, EcRB1, AF2-flag (C-terminal of EcR, flag tagged), or AF1-flag (N-terminal of EcRA flag tagged), in all cases cotransfected with Ari1a-myc. Western blots were stained with the indicated antibodies. Control lanes contained extracts from cells transfected with Ari1a only. Input lanes were 10% of the cell extracts used for IPs (arrowhead in O indicates the correct band for AF1-flag). Note that only full-length EcRA is co-immunoprecipitated with Ari1a-myc (arrowhead in N). Bar, 10 µm.
Yeast two-hybrid assays were used to check if the putative interactions were direct. Of the five candidate proteins tested, only EcRA and USP gave a positive result (Figure 2K). The choice of the five candidates was based on their reported role in ecdysone-dependent responses during metamorphosis and the results of the genetic interaction assay (see below and Figure 3A). It is important to note that EcR isoform B1 gave a negative result in this assay, suggesting that the interaction between ARI-1a and EcR could be isoform specific. As a validation of the yeast two-hybrid interaction, CoIP assays were carried out between EcRA and ARI-1a. To that end, myc-tagged ari-1a UAS construct transformant fly lines were established. The functionality of the myc-ARI-1a chimera was tested by reproducing the prepupal lethal phenotypes described above under ARI-1a overexpression using the tub-Gal4-LL7 driver. These white prepupae were used for protein extractions, CoIP, and Western blots. The data demonstrate that EcRA and ARI-1a interact in a cellular context (Figure 2L). The equivalent attempt with USP failed because of the available USP antibody was ineffective in Western blots. As an alternative, we generated a Flag-tagged USP construct with which we cotransfected L57-3-11 cells. Here, the anti-Flag antibody did not recognize any product of CoIP with ARI-1a-myc. Thus, the YTH interaction detected between ARI-1a and USP could not be validated in this assay. This negative result could indicate a false positive in the YTH assay or the existence of an inhibitory factor in that particular cell line. Since equivalent assays in a HEK cell line also failed to yield a positive result (not shown), we concluded that the YTH result between ARI-1a and USP is likely a false positive. Nevertheless, as shown below, ARI-1a does regulate the protein levels of USP, and hence there must be a functional interaction between these two proteins.
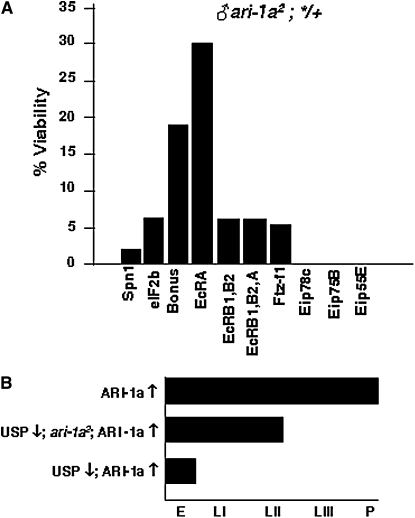
Figure 3 .
Genetic interactions of ARI-1a. (A) Rescue of lethal ari-1a2 adult males in heterozygous condition with ecdysone pathway mutants. Viability is calculated as a percentage with respect to the most viable sibling genotype. The allele ari-1a2 is a fully penetrant lethal. Thus, all rescue values are significant. Note that the mutant in EcRA is the most effective rescuer at 30%. The following ecdysone pathway mutant alleles were used: Df(2)Drlrv7(Spn1 defective); P(y+)CG15523KG07288 (Eif2b defective); bon21B (Bonus defective); Df(2)EcRA112 (EcRA defective) EcR225 (EcRB1, B2 defective) (Carney et al. 2004); EcRM554fs (EcRA, B1, B2 defective); P(ry+)ftz-f103649 (βFtz-f1 defective); Df(3R)Spf,P(ry+)l(3)ArBEip78C (Eip78C defective); P(y+)Eip75BKG03025 (Eip75B defective); and P(y+)Eip55EKG02526 (Eip55E defective). Data are from crosses in which a minimum of 100 individuals of the most viable genotype were screened. (B) Developmental phase reached by genotypes in which ari-1a and/or usp expression is altered. The various genotypes exhibit a progressively earlier lethality phase as the levels of ARI-1a increase and those of USP decrease. The excess of function for each gene (↑) was obtained using the tubulin-Gal4-LL7 driver and the corresponding UAS construct. The loss of function (↓) corresponds to mutant alleles. Genotypes used were the following: ♀ tub-Gal4-LL7/UAS-ari-1a (top), ♀ usp4ari-1a2/+; tub-Gal4-LL7/UAS-ari-1a (middle), and ♀ usp4/+; tub-Gal4-LL7/UAS-ari-1a (bottom). The allele usp4 yields a protein unable to bind DNA and to repress transcription (Ghbeish et al. 2001), and the allele ari-1a2 is defective in the binding of the E2 enzyme UbcD10 required for substrate ubiquitylation (Aguilera et al. 2000).
Furthermore, we aimed to determine the specificity and structural bases that sustain the interaction between ARI-1a and EcRA. To that end, we used L57-3-11 line cells cotransfected with the corresponding constructs. The data show no evidence of interaction with USP (Figure 2M). By contrast, EcRA, but not EcRB1, co-immunoprecipitates with ARI-1a (Figure 2N). Since all EcR isoforms share the same C terminus, the AF2 fragment, we included in the same CoIP experiment a Flag-tagged AF2 fragment. The result, however, was negative, pointing to the differential N- terminus, the AF1 fragment as a potential site for the interaction. However, a further set of CoIP experiments with a Flag-tagged AF1 fragment also yielded negative results (Figure 2O). Taken together the structural data, we concluded that the ARI–1a/EcRA interaction is a bona fide feature of the biology of the cell, but the fully normal sequences of both proteins are required. Also, the interaction specificity among the EcR isoforms is not dependent on the differential N termini (AF1 fragment), probably because it requires a 3D feature that only the entire protein can exhibit. Indeed, E3 enzymes frequently use these high-order structural motifs to recognize their substratum (Qian et al. 2009).
Beyond these structural considerations, we searched for genetic evidence of the ARI–1a/EcRA interaction in an attempt to validate it at the organism level. Ten different genes of the ecdysone-signaling pathway were tested. We crossed ♀ y w ari-1a2/FM7i,GFP and ♂ */Balancer and screened for the possible rescue from lethality of ♂ y w ari-1a2; */+. Each ecdysone-related gene (*) was represented by the most extreme allele available (http://flybase.bio.indiana.edu). We used the allele ari-1a2 because its Cys150 > Tyr change in the N terminus RING finger motif causes fully penetrant lethality (Aguilera et al. 2000). Seven of 10 genes tested showed evidence of functional interaction by producing a number of ari-1a2 survivors (Figure 3A). The most effective suppression was detected with EcR mutants, in particular with those of isoform A. In general, rescued males did not appear healthy and survived for only ∼5 days in which they seldom moved or fed. This result provides in vivo evidence for the interaction between ARI-1a and EcRA. Furthermore, since the rescued genotypes still contain one normal copy of the tested gene, it suggests that the functional relationship between ARI-1a and these ecdysone-related genes relies on their relative stoichiometry rather than on a switch-type process. Additional genetic interaction tests were carried out with USP, the known partner of EcR. We found that their corresponding lethal phases changed as a function of the relative contents of these two proteins (Figure 3B). In a background of excess of ARI-1a, lethality occurs at the pupal stage. However, if the endogenous ari-1a and usp genes are inactivated by means of null mutant alleles, lethality occurs at progressively earlier stages. In the most extreme case studied—the null condition for USP and the generalized overexpression of ARI-1a—development is stopped at midembryogenesis (for specific genotypes, see legend in Figure 3).
Transcription of ecdysone-signaling genes is modified by ARI-1a
We performed microarray analyses from mutant and overexpressing ARI-1a genotypes to investigate if the interaction of ARI-1a with ecdysone-signaling genes translates into significant changes in transcription. The transcriptome data (Table S2; Table S3; Figure S2) were filtered by two criteria: (1) genes that change their expression in the experimental genotypes but not between the two corresponding sibling control genotypes and (2) genes that change their expression in one direction in the ari-1a lack-of-function genotypes and in the opposite direction in the ari-1a excess-of-function genotypes.
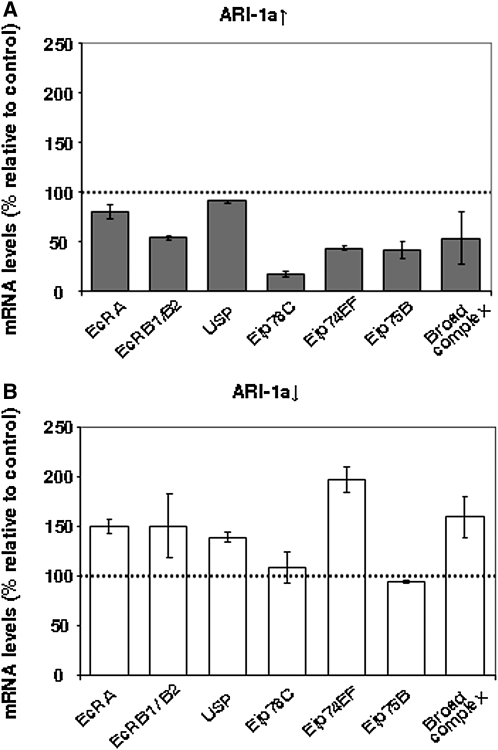
Gene ontology of the genes that are upregulated in the mutant and, at the same time, downregulated in the overexpressing larvae and vice versa was analyzed using GOEAST (Zheng and Wang 2008) (Table S2; Table S3; Figure S2). The analysis showed that, in the group of 184 upregulated genes in the ari-1a mutant and downregulated in the ari-1a overexpressing larva, there are several 20E-dependent genes [e.g., Eip78C, one of the genes induced through the EcR/USP dimer (Beckstead et al. 2005)]. We further analyzed by qRT-PCR the expression of a sample of 20E-activated early genes, including Eip78C, Eip74EF, Eip75B, Broad complex, and the receptors EcRA, EcRB(B1/B2 isoforms), and USP. In general, they showed either up- or downregulation, depending on the status of ari-1a expression levels (Figure 4). Thus, we concluded that ARI-1a elicits transcriptional changes in a dose-dependent manner over a set of genes that include several of the 20E-signaling pathway.
Figure 4 .
Transcriptional effects of up- and downregulation of ari-1a on ecdysone-signaling genes. (A) The mRNA levels of the ecdysone receptors EcRA, EcRB1/B2, and USP and the transcription factors Eip78C, Eip74EF, Eip75B, and Broad complex were analyzed by qRT-PCR assays in whole larvae, overexpressing ARI-1A (tub-Gal4-LL7 > UAS-ari-1a). The mRNA levels of RNApol II were used as internal control. Values are shown as the percentage relative to the mRNA levels for the non-overexpressing siblings that are considered 100% (dotted line). (B) Equivalent assays on loss-of-function ari-1a2 mutant larvae. Note that most genes are upregulated in ARI-1a (B, ↓) and downregulated in ARI-1a (A, ↑) conditions, suggesting that the activity of ARI-1a results in the transcriptional repression of these genes.
In vivo effects of ARI-1a at the single-cell level
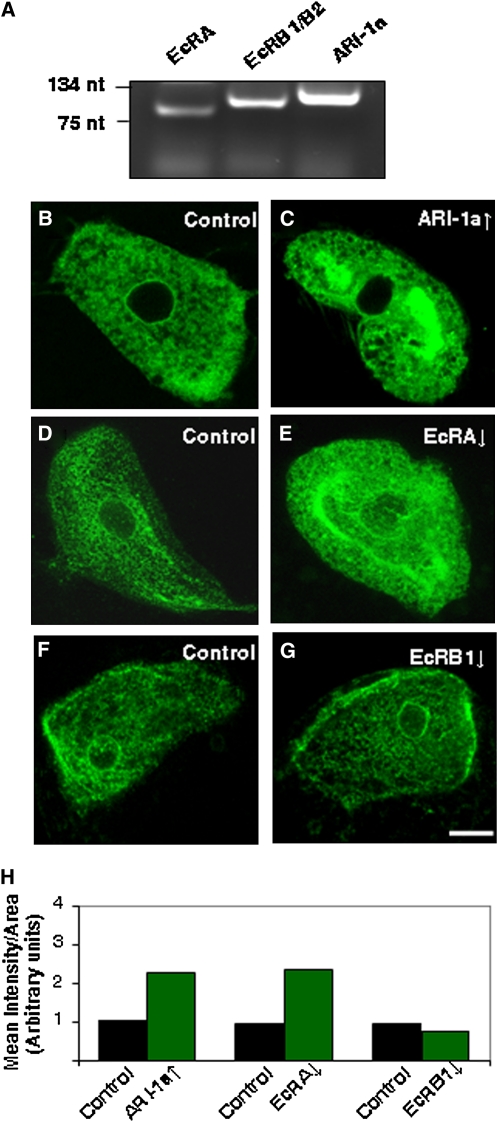
To visualize in single cells the phenotypes caused by modifying ARI-1a levels, we choose the Malpighian tubuli cells because of their relatively large size and accessibility. The C649 Gal4 driver is expressed in the stellate cells of this tissue (Sozen et al. 1997). First, we carried out RT-PCR assays to check if the partners under study were present in this tissue. The data show that ari-1a, EcRA, and EcRB1/2 genes are expressed (Figure 5A). In the driven expression experiments, we found that the overexpression of ARI-1a always leads to an increment in the fluorescent signal of the GFP-tagged membrane protein CD8 used as a reporter (Figure 5, B, C, and H). This effect correlates with the loss of endoplasmic reticulum in the ari-1a lack-of-function mutant that we reported previously in photoreceptor cells (Aguilera et al. 2000). Thus, both phenotypes are consistent with the level of ARI-1a expression. To test if this phenomenon could be due to the regulation of EcRA specifically, we used an UAS-RNAi to downregulate EcRA. The resulting phenotype on cell membranes is very similar to that produced by the excess of ARI-1a (Figure 5, D, E, and H). As in the previous experiments, this effect is also EcR isoform-specific, as the reduction on EcRB1 levels had no effect on the CD8-GFP signal (Figure 5, F, G, and H). The same phenotypes were reproduced using two other membrane reporters that are specific for the endoplasmic reticulum, the proteins Rtnl1 and Pdi (data not shown). These phenotypes are consistent with the high requirement of membranogenesis during metamorphosis, the developmental stage where the loss or the excess of ari-1a function causes lethality.
Figure 5 .
ARI-1a phenotypes at the single-cell level. (A) RT-PCR from Malpighian tubuli showing expression of EcRA, EcRB1/B2, and ari-1a genes. (B and C) Effect of overexpressing (↑) ARI-1a in larval Malpighian tubuli (stellate cells) co-expressing the GFP-tagged membrane protein CD8 under the driver Gal4-C649. (D and E) Effect of underexpressing (↓) EcRA. Note that in both cases the amount of membranous signal is increased with respect to controls. (F and G) By contrast, the underexpression of EcRB1 yields no effect, demonstrating that the phenomenon is specific of EcRA. The underexpression of EcRA and EcRB1 was performed by driving the expression of the corresponding RNAi constructs with Gal4-C649. (H) Relative quantification of GFP intensity signals in each genotype. Controls always correspond to sibling genotypes. Laser settings were kept constant in all images. Bar, 10 µm.
Taken together, the data so far indicate that ARI-1a regulates metamorphosis, most likely as a result of its direct interaction with the nuclear transcriptional receptor EcRA and, perhaps indirectly, with its heterodimeric partner USP. The mechanism of this regulation, however, could be executed at the protein or the gene transcription levels. Thus, we set out to analyze these two alternatives.
ARI-1a regulates the protein levels of EcRA and USP in vivo and ubiquitylates EcRA in vitro
Since ARI-1a is an E3 enzyme, we addressed the effects on the putative substrates. We measured protein levels of EcRA and USP under lack- and excess-of-function conditions for ARI-1a. Similar experiments were carried out for EcRB1 to define the specificity of ARI-1a activity. Prepupae of ari-1a2 and tub-Gal4-LL7 > UAS-ari-1a lethal mutants were used for protein extracts and Western blotting with EcRA, EcRB1, and USP-specific antibodies. Quantitative differences for EcRA, and to a lesser extent for USP, were clearly detected (Figure 6, A and B). While EcRB1 showed no apparent change, EcRA levels decreased fourfold when ARI-1a was overexpressed and increased sixfold in ari-1a lack-of-function mutants. By contrast, USP levels changed in the opposite direction to those of EcRA: they doubled in the first case and decreased twofold in the second.
Figure 6 .
ARI-1a regulates the protein levels of EcRA and Utraspiracle (USP) but not those of EcRB1. (A, left column) Western blots of EcRA, EcRB1, and USP from white pupae overexpressing ARI-1a (genotype: tub-Gal4-LL7; UAS-ari-1a/+). (A, right column) Equivalent blot from lack-of-function ari-1a2 mutant white pupae. Sibling normal males were used as controls. Densitometry values were normalized for actin content. (B) Fold change of EcRA, EcRB1, and USP under ARI-1a overexpression (left) or underexpression (right). (C and D) In vitro ubiquitylation assays. Purified recombinant proteins were incubated with ubiquitylation reaction components with or without Ubiquitin (Ub) (C) and with or without ARI-1a (D). Reactions were analyzed by EcRA antibody immunoblot. Arrowhead shows the unmodified EcRA, and arrows indicate two of the polyubiquitylated EcRA bands. As a loading control, we monitored ARI-1a by immunoblot. In the second set of experiments (D), recombinant E1, UbcD10, EcRA, and Ubiquitin tagged with HA were incubated in the presence or absence of purified recombinant ARI-1a. Ubiquitylated EcRA was detected by anti-HA antibody, and the EcRA loading control was monitored by anti-EcRA antibody. Note that the levels of ARI-1a determine those of EcRA and USP, which change in opposite directions. In addition, the ubiquitylation of EcRA is ARI-1a dependent.
Furthermore, since the EcRA levels changed in concert with those of ARI-1a and the two proteins directly bind to each other, we tested the putative ubiquitylation of EcRA by ARI-1a. In the in vitro assay used to that end, we included the E2 enzyme previously identified as a direct interactor with ARI-1a, UbcD10. In the presence of ubiquitin and the enzymes E1, UbcD10, and ARI-1a, at least two high-molecular-weight bands were detected that corresponded to ubiquitylated EcRA as shown by the anti-EcRA-specific antibody. Under the same conditions, but in the absence of ubiquitin, EcRA remained unmodified (Figure 6C). To test the dependence of this ubiquitylation on the presence of the E3 enzyme, we carried out an additional test under the presence or absence of ARI-1a (Figure 6D). In this latter experiment, we used a HA-tagged ubiquitin to improve ubiquitin detection.
Interpreting the full set of data, we conclude that ARI-1a regulates the EcRA/USP protein levels via ubiquitylation of EcRA and, indirectly, the RNA levels of both genes, perhaps through a feedback loop of the proteins over the transcription of their encoding genes. This interpretation is akin to the case of the vertebrate estrogen receptor α, ERα, where the two processes, ubiquitin/proteasome degradation of the protein and transcriptional activation of the gene, are independent and mediated through distinct structural motifs (Tateishi et al. 2004; Valley et al. 2005).
Discussion
We show here that the conserved E3 ligase Ariadne-1a specifically binds the isoform A of EcR. This interaction results in the quantitative regulation of protein levels of EcRA and its heterodimeric partner, USP, that change in opposite directions. In addition, this E3 enzyme is able to ubiquitylate EcRA in vitro in the presence of its native E2, UbcD10. The levels of ARI-1a also lead to transcriptional changes in several 20E-dependent genes, including EcR and usp.
ARI-1a in metamorphosis
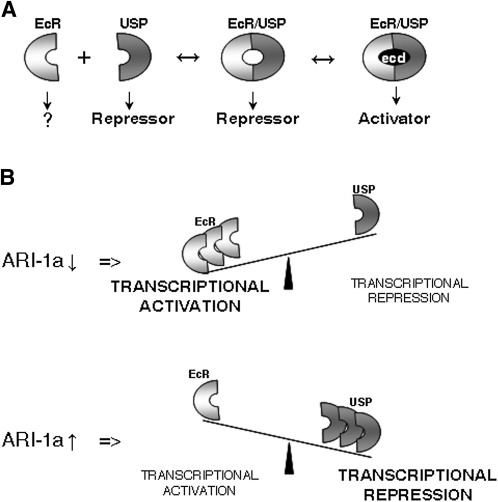
The excess as well as the lack-of-function conditions for ARI-1a cause lethality at the same developmental phase, metamorphosis. Since isoform A of EcR is particularly abundant in imaginal tissues, the lethality phase is coherent with the molecular interaction. The fact that metamorphosis is affected by deviations, in either direction, of EcRA and USP protein levels underlies the significance of the proper stoichiometry of these two transcription factors at this stage of development. Previous studies have led to a functional model in which the heterodimer EcR/USP acts as a transcriptional repressor until binding 20E, when additional cofactors are recruited and the complex becomes an activator (Schubiger et al. 2005; Beck et al. 2009). In an attempt to summarize the data reported here and merge them with those previously published, we assume that EcRA and USP are in equilibrium between the bound and dissociated states (Figure 7A). The ubiquitylation activity of ARI-1a will determine, via the proteasome, the levels of EcRA. If EcRA increases, the equilibrium will be displaced to favor the dimerized complexes that will act as transcriptional repressors since ecdysone is not yet present. In addition, to explain the opposite direction of the changes in the protein levels of EcRA and USP, we speculate that the unliganded dimer EcRA/USP could repress each other’s gene transcription. As ecdysone titers peak at the onset of metamorphosis, the equilibrium would further displace toward the formation of liganded dimmers, which should then act as transcriptional activators. In the ari-1a2 mutant background, the ratio of activator–repressor complexes will be altered, and thus lethality will ensue (Figure 7B). Further and focused experiments will determine if this tentative mechanism is indeed correct. This proposal is a generic model of titration between EcRA and USP, and cellular heterogeneities throughout the body could represent significant functional differences. Nevertheless, the fact that metamorphosis is the lethal phase for both the lack and the excess of ARI-1a function compels us to give a key role to 20E.
Figure 7 .
Summary of results and functional proposal. (A) It is known that EcRA and USP need to dimerize to reconstitute a nuclear transcriptional activator. The isolated monomers are located in the cytoplasm (EcRA) or in the nucleus (USP) where the latter behaves as a repressor (Gwozdz et al. 2007). As previously suggested, the two monomers could bind generating unliganded complex units, which would act as a transcriptional repressor (Schubiger et al. 2005). At metamorphosis, ecdysone (ecd) titers increase, and the complex can become a transcriptional activator upon ecd binding. (B) On the basis of the data reported here, we propose that the equilibrium between EcRA and USP proteins can be displaced as a function of ARI-1a activity. If ARI-1a is diminished (RNAi) or absent (ari-1a2), the balance would displace in favor of EcRA, and the predominant transcriptional effect upon the tested genes, including EcR, would be activation. By contrast, if ARI-1a increases (tub-Gal4LL7 > UAS-ari-1a), the equilibrium would move in the opposite direction, and transcriptional repression would dominate most of the tested genes. This functional proposal is a generalization that does not take into account the possible tissue specificities in terms of stoichiometry of the proteins involved.
Mechanisms of ARI-1a activity
ARI-1a exhibits two types of regulatory effects on EcRA, at the protein and at the transcription levels. The effects at the protein level can be interpreted as a result of the E3 ubiquitylation activity and degradation through the proteasome pathway. The effects on EcRA transcription may be indirect and result from a feedback transcriptional regulation of EcRA on its own gene, EcR. This putative feedback mechanism could explain the transcriptional effects of ARI-1a on the other EcR isoforms, B1 and B2 (Figure 4). However, although EcR transcription is known to depend on 20E (Karim and Thummel 1992; Varghese and Cohen 2007), the direct implication of EcRA in a feedback mechanism remains to be demonstrated. The isoform-specific regulation of EcR by an E3 enzyme invites us to explore if a similar mechanism could regulate other isoforms. Different E3s could exert this regulation on EcRB1 and EcRB2. This hypothetical regulation would make biological sense in those cells where several EcR isoforms are co-expressed. This is known to occur only in the prothoracic cells of the ring gland, where ecdysone is synthesized. Most other tissues appear to express mainly one isoform (Talbot et al. 1993). However, we have shown here that the Malpighian tubules express at least the RNAs for all isoforms (Figure 5). Thus, the tissue expression data for this gene and its three isoforms may require revision. Alternatively, ARI-1a could be inhibited in a tissue- or cell-specific manner. In any case, the changes in the relative stoichiometry of EcR isoforms are likely to have widespread transcriptional consequences since they are nuclear receptors and act differently as monomers vs. dimerized with USP and 20E bound.
At present, we cannot resolve if ARI-1a binds EcRA as a complex with USP or as a monomere. Although speculative at this time, it is conceivable that ARI-1a could elicit cytoplasm-nuclear translocation by means of monoubiquitylation of EcRA where the third element, USP, would join in and titrate EcRA concentration via proteasome by means of polyubiquitylation. As a precedent, the protein Parkin, a RING finger protein related to ARI-1a and a cause of Parkinson’s disease, is capable of mono- and polyubiquitylation of its substrates (Hampe et al. 2006). On the other hand, while USP seems to localize within the nucleus only, the presence of EcRA (Gwozdz et al. 2007) and ARI-1a (Aguilera et al. 2000) in the cytoplasm makes plausible that their interaction could elicit fast, nontranscriptional events such as G-protein-coupled cellular responses to 20E (Srivastava et al. 2005). In this report, however, we have dealt with the functional bases of EcRA/ARI-1a interactions only.
These features from Drosophila are likely to be relevant to other species as well, given the conservation of the ARI type of E3 enzymes (Marin and Ferrus 2002; Mladek et al. 2003) and the EcR type of nuclear receptors (King-Jones and Thummel 2005). Thus, it will be worth investigating if the human liver X receptor, an ortholog of EcR, undergoes a similar regulatory process by the human ARI-1a. Considered in a wider context, the specificity of hormone receptor signaling seems to depend not only on the particular activated isoform, but also on the isoform-specific mechanism of modulation. The data reported here illustrate one such mechanism that yields specific phenotypes and has gene transcriptional effects. In addition, the described process represents a mechanism to coregulate other functionally linked nuclear receptors—in this case, USP.
Acknowledgments
We are indebted to the Iowa Hybridoma Bank for antibodies, to Bloomington Stock Center for most fly strains, and to M. Bender (University of Georgia) for EcR mutants. The S2 cell line deficient for EcR and the pCMA vector were produced by Lucy Cherbas. The anti-USP antibody was a gift from F. Kafatos (European Molecular Biology Laboratory). The contributions from our lab members are most appreciated. R. Willson corrected the English text. The work was supported by grant BFU2009-12410 from the Spanish Ministry of Research and Innovation. A.-C.G. was employed by the Universidad de Guadalajara (Jalisco, México). A.M. holds a Junta para la AmpliaciÓn de Estudios research contract.
Literature Cited
- Aguilera M., Oliveros M., Martinez-Padron M., Barbas J. A., Ferrus A., 2000. Ariadne-1: a vital Drosophila gene is required in development and defines a new conserved family of ring-finger proteins. Genetics 155: 1231–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaven S. W., Tontonoz P., 2006. Nuclear receptors in lipid metabolism: targeting the heart of dyslipidemia. Annu. Rev. Med. 57: 313–329 [DOI] [PubMed] [Google Scholar]
- Beck Y., Delaporte C., Moras D., Richards G., Billas I. M., 2009. The ligand-binding domains of the three RXR-USP nuclear receptor types support distinct tissue and ligand specific hormonal responses in transgenic Drosophila. Dev. Biol. 330: 1–11 [DOI] [PubMed] [Google Scholar]
- Beckstead R. B., Lam G., Thummel C. S., 2005. The genomic response to 20-hydroxyecdysone at the onset of Drosophila metamorphosis. Genome Biol. 6: R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford L., Paine S., Sheppard P. W., Mayer R. J., Roelofs J., 2010. Assembly, structure, and function of the 26S proteasome. Trends Cell Biol. 20: 391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender M., Imam F. B., Talbot W. S., Ganetzky B., Hogness D. S., 1997. Drosophila ecdysone receptor mutations reveal functional differences among receptor isoforms. Cell 91: 777–788 [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415 [DOI] [PubMed] [Google Scholar]
- Brown H. L., Cherbas L., Cherbas P., Truman J. W., 2006. Use of time-lapse imaging and dominant negative receptors to dissect the steroid receptor control of neuronal remodeling in Drosophila. Development 133: 275–285 [DOI] [PubMed] [Google Scholar]
- Capili A. D., Edghill E. L., Wu K., Borden K. L., 2004. Structure of the C-terminal RING finger from a RING-IBR-RING/TRIAD motif reveals a novel zinc-binding domain distinct from a RING. J. Mol. Biol. 340: 1117–1129 [DOI] [PubMed] [Google Scholar]
- Carney G. E., Bender M., 2000. The Drosophila ecdysone receptor (EcR) gene is required maternally for normal oogenesis. Genetics 154: 1203–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney G. E., Robertson A., Davis M. B., Bender M., 2004. Creation of EcR isoform-specific mutations in Drosophila melanogaster via local P element transposition, imprecise P element excision, and male recombination. Mol. Genet. Genomics 271: 282–290 [DOI] [PubMed] [Google Scholar]
- Champlin D. T., Truman J. W., 1998. Ecdysteroid control of cell proliferation during optic lobe neurogenesis in the moth Manduca sexta. Development 125: 269–277 [DOI] [PubMed] [Google Scholar]
- Chen J. D., Evans R. M., 1995. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377: 454–457 [DOI] [PubMed] [Google Scholar]
- Cherbas L., Cherbas P., 1997. “Parahomologous” gene targeting in Drosophila cells: an efficient, homology-dependent pathway of illegitimate recombination near a target site. Genetics 145: 349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbas L., Moss R., Cherbas P., 1994. Transformation techniques for Drosophila cell lines. Methods Cell Biol. 44: 161–179 [DOI] [PubMed] [Google Scholar]
- Cherbas L., Hu X., Zhimulev I., Belyaeva E., Cherbas P., 2003. EcR isoforms in Drosophila: testing tissue-specific requirements by targeted blockade and rescue. Development 130: 271–284 [DOI] [PubMed] [Google Scholar]
- Collins G. A., Tansey W. P., 2006. The proteasome: A utility tool for transcription? Curr. Opin. Genet. Dev. 16: 197–202 [DOI] [PubMed] [Google Scholar]
- D’Avino P. P., Thummel C. S., 2000. The ecdysone regulatory pathway controls wing morphogenesis and integrin expression during Drosophila metamorphosis. Dev. Biol. 220: 211–224 [DOI] [PubMed] [Google Scholar]
- Davis M. B., Carney G. E., Robertson A. E., Bender M., 2005. Phenotypic analysis of EcR-A mutants suggests that EcR isoforms have unique functions during Drosophila development. Dev. Biol. 282: 385–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis A. P., Lonard D. M., Nawaz Z., O’Malley B. W., 2005. Inhibition of the 26S proteasome blocks progesterone receptor-dependent transcription through failed recruitment of RNA polymerase II. J. Steroid Biochem. Mol. Biol. 94: 337–346 [DOI] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K. C., Barinova Y., et al. , 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156 [DOI] [PubMed] [Google Scholar]
- Erezyilmaz D. F., Riddiford L. M., Truman J. W., 2006. The pupal specifier broad directs progressive morphogenesis in a direct-developing insect. Proc. Natl. Acad. Sci. USA 103: 6925–6930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. L., Moore K. J., Freeman M. W., 2002. Nuclear hormone receptors and cholesterol trafficking: the orphans find a new home. J. Mol. Med. 80: 271–281 [DOI] [PubMed] [Google Scholar]
- Fortier T. M., Vasa P. P., Woodard C. T., 2003. Orphan nuclear receptor betaFTZ-F1 is required for muscle-driven morphogenetic events at the prepupal-pupal transition in Drosophila melanogaster. Dev. Biol. 257: 153–165 [DOI] [PubMed] [Google Scholar]
- Ghbeish N., Tsai C. C., Schubiger M., Zhou J. Y., Evans R. M., et al. , 2001. The dual role of ultraspiracle, the Drosophila retinoid X receptor, in the ecdysone response. Proc. Natl. Acad. Sci. USA 98: 3867–3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert L. I., Warren J. T., 2005. A molecular genetic approach to the biosynthesis of the insect steroid molting hormone. Vitam. Horm. 73: 31–57 [DOI] [PubMed] [Google Scholar]
- Glickman M. H., Ciechanover A., 2002. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 82: 373–428 [DOI] [PubMed] [Google Scholar]
- Gwozdz T., Dutko-Gwozdz J., Nieva C., Betanska K., Orlowski M., et al. , 2007. EcR and Usp, components of the ecdysteroid nuclear receptor complex, exhibit differential distribution of molecular determinants directing subcellular trafficking. Cell. Signal. 19: 490–503 [DOI] [PubMed] [Google Scholar]
- Hampe C., H. Ardila-Osorio, M. Fournier, A. Brice, and O. Corti, 2006. Biochemical analysis of Parkinson’s disease-causing variants of Parkin, an E3 ubiquitin-protein ligase with monoubiquitylation capacity. Hum. Mol. Genet. 15: 2059–2075 [DOI] [PubMed] [Google Scholar]
- Henrich V. C., Livingston L., Gilbert L. I., 1993. Developmental requirements for the ecdysoneless (ecd) locus in Drosophila melanogaster. Dev. Genet. 14: 369–377 [DOI] [PubMed] [Google Scholar]
- Hu X., Cherbas L., Cherbas P., 2003. Transcription activation by the ecdysone receptor (EcR/USP): identification of activation functions. Mol. Endocrinol. 17: 716–731 [DOI] [PubMed] [Google Scholar]
- Joazeiro C. A., Weissman A. M., 2000. RING finger proteins: mediators of ubiquitin ligase activity. Cell 102: 549–552 [DOI] [PubMed] [Google Scholar]
- Kamura T., Conaway J. W., Conaway R. C., 2002. Roles of SCF and VHL ubiquitin ligases in regulation of cell growth. Prog. Mol. Subcell. Biol. 29: 1–15 [DOI] [PubMed] [Google Scholar]
- Karim F. D., Thummel C. S., 1992. Temporal coordination of regulatory gene expression by the steroid hormone ecdysone. EMBO J. 11: 4083–4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H., Hirose S., Ueda H., 2005. A simple and quick method to isolate nuclear extracts from pupae of Drosophila melanogaster. Cytotechnology 49: 67–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J., Park J. G., Lee C. C., 1999. Transcript titers of ecdysteroid receptor components vary between tissues and stages during Drosophila development. Mol. Cells 9: 61–66 [PubMed] [Google Scholar]
- King-Jones K., Thummel C. S., 2005. Nuclear receptors: a perspective from Drosophila. Nat. Rev. Genet. 6: 311–323 [DOI] [PubMed] [Google Scholar]
- Kinyamu H. K., Archer T. K., 2007. Proteasome activity modulates chromatin modifications and RNA polymerase II phosphorylation to enhance glucocorticoid receptor-mediated transcription. Mol. Cell. Biol. 27: 4891–4904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T. R., White K. P., 2003. Tissue-specific gene expression and ecdysone-regulated genomic networks in Drosophila. Dev. Cell 5: 59–72 [DOI] [PubMed] [Google Scholar]
- Lorick K. L., Jensen J. P., Fang S., Ong A. M., Hatakeyama S., et al. , 1999. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc. Natl. Acad. Sci. USA 96: 11364–11369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin I., Ferrus A., 2002. Comparative genomics of the RBR family, including the Parkinson’s disease-related gene parkin and the genes of the ariadne subfamily. Mol. Biol. Evol. 19: 2039–2050 [DOI] [PubMed] [Google Scholar]
- Marin I., Lucas J. I., Gradilla A. C., Ferrus A., 2004. Parkin and relatives: the RBR family of ubiquitin ligases. Physiol. Genomics 17: 253–263 [DOI] [PubMed] [Google Scholar]
- Mark M., Ghyselinck N. B., Chambon P., 2006. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu. Rev. Pharmacol. Toxicol. 46: 451–480 [DOI] [PubMed] [Google Scholar]
- Mladek C., Guger K., Hauser M. T., 2003. Identification and characterization of the ARIADNE gene family in Arabidopsis: a group of putative E3 ligases. Plant Physiol. 131: 27–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, N. V., and B. M. Forman, 2004 Linking lipids, Alzheimer’s and LXRs? Nucl. Recept. Signal. 2: e001. [DOI] [PMC free article] [PubMed]
- Qian S. B., Waldron L., Choudhary N., Klevit R. E., Chazin W. J., et al. , 2009. Engineering a ubiquitin ligase reveals conformational flexibility required for ubiquitin transfer. J. Biol. Chem. 284: 26797–26802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddiford L. M., Cherbas P., Truman J. W., 2000. Ecdysone receptors and their biological actions. Vitam. Horm. 60: 1–73 [DOI] [PubMed] [Google Scholar]
- Robertson K. M., Norgard M., Windahl S. H., Hultenby K., Ohlsson C., et al. , 2006. Cholesterol-sensing receptors, liver X receptor alpha and beta, have novel and distinct roles in osteoclast differentiation and activation. J. Bone Miner. Res. 21: 1276–1287 [DOI] [PubMed] [Google Scholar]
- Robyr D., Wolffe A. P., Wahli W., 2000. Nuclear hormone receptor coregulators in action: diversity for shared tasks. Mol. Endocrinol. 14: 329–347 [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C., 1982. Genetic transformation of Drosophila with transposable element vectors. Science 218: 348–353 [DOI] [PubMed] [Google Scholar]
- Santos J. G., Pollak E., Rexer K. H., Molnar L., Wegener C., 2006. Morphology and metamorphosis of the peptidergic Va neurons and the median nerve system of the fruit fly, Drosophila melanogaster. Cell Tissue Res. 326: 187–199 [DOI] [PubMed] [Google Scholar]
- Schubiger M., Truman J. W., 2000. The RXR ortholog USP suppresses early metamorphic processes in Drosophila in the absence of ecdysteroids. Development 127: 1151–1159 [DOI] [PubMed] [Google Scholar]
- Schubiger M., Tomita S., Sung C., Robinow S., Truman J. W., 2003. Isoform specific control of gene activity in vivo by the Drosophila ecdysone receptor. Mech. Dev. 120: 909–918 [DOI] [PubMed] [Google Scholar]
- Schubiger M., Carre C., Antoniewski C., Truman J. W., 2005. Ligand-dependent de-repression via EcR/USP acts as a gate to coordinate the differentiation of sensory neurons in the Drosophila wing. Development 132: 5239–5248 [DOI] [PubMed] [Google Scholar]
- Sliter T. J., Gilbert L. I., 1992. Developmental arrest and ecdysteroid deficiency resulting from mutations at the dre4 locus of Drosophila. Genetics 130: 555–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozen M. A., Armstrong J. D., Yang M., Kaiser K., Dow J. A., 1997. Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proc. Natl. Acad. Sci. USA 94: 5207–5212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M., 1982. Transposition of cloned P elements into Drosophila germ line chromosomes. Science 218: 341–347 [DOI] [PubMed] [Google Scholar]
- Spyridon M., Moraes L. A., Jones C. I., Sage T., Sasikumar P., et al. , 2011. LXR as a novel antithrombotic target. Blood 117: 5751–5761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava D. P., Yu E. J., Kennedy K., Chatwin H., Reale V., et al. , 2005. Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J. Neurosci. 25: 6145–6155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung C., Robinow S., 2000. Characterization of the regulatory elements controlling neuronal expression of the A-isoform of the ecdysone receptor gene of Drosophila melanogaster. Mech. Dev. 91: 237–248 [DOI] [PubMed] [Google Scholar]
- Talbot W. S., Swyryd E. A., Hogness D. S., 1993. Drosophila tissues with different metamorphic responses to ecdysone express different ecdysone receptor isoforms. Cell 73: 1323–1337 [DOI] [PubMed] [Google Scholar]
- Tateishi Y., Kawabe Y., Chiba T., Murata S., Ichikawa K., et al. , 2004. Ligand-dependent switching of ubiquitin-proteasome pathways for estrogen receptor. EMBO J. 23: 4813–4823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. C., Kao H. Y., Yao T. P., McKeown M., Evans R. M., 1999. SMRTER, a Drosophila nuclear receptor coregulator, reveals that EcR-mediated repression is critical for development. Mol. Cell 4: 175–186 [DOI] [PubMed] [Google Scholar]
- Valley C. C., Metivier R., Solodin N. M., Fowler A. M., Mashek M. T., et al. , 2005. Differential regulation of estrogen-inducible proteolysis and transcription by the estrogen receptor alpha N terminus. Mol. Cell. Biol. 25: 5417–5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese J., Cohen S. M., 2007. microRNA miR-14 acts to modulate a positive autoregulatory loop controlling steroid hormone signaling in Drosophila. Genes Dev. 21: 2277–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H., Callaghan D., Jones A., Walker D. G., Lue L. F., et al. , 2008. Cholesterol retention in Alzheimer’s brain is responsible for high beta- and gamma-secretase activities and Abeta production. Neurobiol. Dis. 29: 422–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao T. P., Segraves W. A., Oro A. E., McKeown M., Evans R. M., 1992. Drosophila ultraspiracle modulates ecdysone receptor function via heterodimer formation. Cell 71: 63–72 [DOI] [PubMed] [Google Scholar]
- Zheng Q., Wang X. J., 2008. GOEAST: a web-based software toolkit for Gene Ontology enrichment analysis. Nucleic Acids Res. 36: W358–W363 [DOI] [PMC free article] [PubMed] [Google Scholar]