Abstract
Cross-sectional analysis of human immunodeficiency virus-exposed, uninfected infants revealed high proportions of CXCR4-expressing cells in their cord blood, which declined at 4.5 months and increased between 9 and 15 months to levels approaching those of uninfected adults. Proportions of CCR5-expressing cells, however, were very low in cord blood and subsequently increased with age.
The immune system of the infant is functionally less mature at birth and undergoes a process of sequential maturation and development that is reflected in qualitative and quantitative changes in a number of leukocyte subsets. Infants have increased numbers of lymphocytes compared with adults, as well as different distributions of the major lymphocyte subsets (8, 15, 24, 25).
Chemokines and their receptors are involved in controlling lymphocyte trafficking, which is a critical component of systemic immunity. Human immunodeficiency virus type 1 (HIV-1) has evolved the means to utilize chemokine receptors to facilitate its entry into CD4+ cells, the major ones being CXCR4 and CCR5, which mediate infection with T-cell-tropic and macrophage-tropic viral strains, respectively (1, 7, 10, 12).
The purpose of this study was to assess whether age-related changes exist in the expression of CXCR4 and CCR5 in infants, as this could have relevance for infant immune responses to microbes and for differences in disease susceptibility and progression found between HIV-1-infected adults and children. Ethical clearance for this study was obtained from the University of the Witwatersrand Ethical Committee.
We monitored coreceptor expression in cord blood (n = 27) and in infants 4.5 months (n = 9), 9 months (n = 16), and 15 months (n = 9) of age who were born to HIV-1-seropositive mothers, none of whom breastfed, recruited as part of the PETRA trial (18). All 61 of the infants included had been determined to be HIV-1 seronegative by enzyme-linked immunosorbent assay (ELISA; Abbott HIV-1 IMX) at 18 months of age, and hence defined as HIV-exposed, uninfected infants. T-lymphocyte maturation abnormalities have been detected in uninfected, HIV-exposed infants (4), and so the results reported here must not be seen as necessarily representative of uninfected infants who are not exposed to HIV-1. However, a direct comparison of cord blood subsets of infants born to non-HIV-infected mothers showed no significant differences from those of uninfected infants born to HIV-infected mothers. This suggests that at least in utero exposure is not sufficient to result in substantial alterations in the cell subsets studied (data not shown). Healthy, non-HIV-infected adults (n = 18; mean age, 37.8 years; range, 26 to 57 years) were included for comparison. Mononuclear cell subsets outlined in Table 1 and Fig. 1 and 2 were analyzed by flow cytometry as described by Shalekoff et al. (26).
TABLE 1.
Percentages of lymphocyte subsets for study subjects
| Cell type or ratio | Median % total lymphocytes (range) for group
|
||||
|---|---|---|---|---|---|
| Cord Blood (n = 27) | 4.5 mo (n = 9) | 9 mo (n = 16) | 15 mo (n = 9) | Adult (n = 18) | |
| CD3+ T cells | 62 (48-73) | 59 (49-66) | 57 (39-71) | 61 (59-68) | 71a (57-77) |
| CD4+ cells | 41 (24-59) | 41 (28-51) | 35 (20-54) | 41 (30-48) | 40 (33-54) |
| CD8+ cells | 27 (11-37) | 20a (11-29) | 20 (9-38) | 18 (9-35) | 28a (18-39) |
| CD4/CD8 ratio | 1.6 (0.7-5.7) | 2.1 (1.1-4.4) | 1.9 (0.8-5.6) | 2.2 (1.0-5.0) | 1.5a (0.9-3.0) |
| CD3− CD16+/CD56+ NK cells | 14 (5-36) | 8a (3-13) | 8 (2-25) | 7 (6-15) | 15a (4-22) |
| CD19+ B cells | 15 (6-24) | 28a (20-39) | 30 (14-48) | 25 (17-84) | 11a (5-17) |
| CD45RA+ lymphocytes | 89 (80-99) | 90 (84-92) | 86a (65-93) | 84 (83-87) | 65a (43-94) |
| CD45RA+ CD4 cells | 89 (55-98) | 85 (80-89) | 80a (57-89) | 79 (72-87) | 51a (27-93) |
| CD45RA+ CD8 cells | 98 (92-100) | 93a (70-96) | 85 (62-98) | 83 (66-94) | 75 (44-99) |
| CD45RO+ lymphocytes | 8 (4-25) | 12a (7-31) | 16a (6-32) | 16 (11-19) | 38a (27-62) |
| CD45RO+ CD4 cells | 9 (4-23) | 15a (9-34) | 23a (13-45) | 21 (12-25) | 55a (37-78) |
| CD45RO+ CD8 cells | 4 (2-9) | 8a (3-45) | 20a (5-55) | 21 (11-34) | 34a (10-61) |
| CD38+ lymphocytes | 95 (85-97) | 91a (82-95) | 91 (73-95) | 86a (77-92) | 59a (42-77) |
| CD38+ CD8 cells | 97 (87-100) | 95 (86-98) | 95 (83-98) | 93 (73-97) | 48a (22-75) |
Differences compared to the previous age group were determined by the Mann-Whitney U test (P < 0.05).
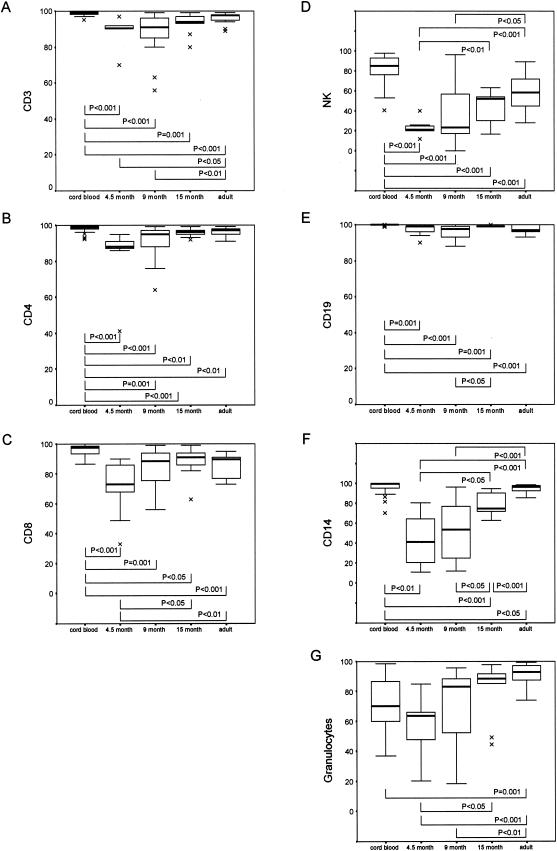
FIG.1.
Expression of CXCR4 on whole-blood leukocytes from subjects in five different age groups. Lymphocytes were identified by using a forward scatter-side scatter gating strategy, and then the percentages of CXCR4-expressing CD3, CD4, CD8, NK, and CD19 cells were determined. CXCR4-expressing monocytes were analyzed by gating on CD14-positive cells, and CXCR4-expressing granulocytes were analyzed on the basis of their forward scatter and side scatter properties and absence of CD14 staining. Results are shown as percentages of positive cells. Data are presented as medians (horizontal bar), 25th and 75th percentiles (boxes), and 10th and 90th percentiles (bars). Significant differences between groups are indicated. Comparison of CXCR4 expression on different leukocyte subsets between the different study groups was done by use of the Mann-Whitney U test with SPSS software (SPSS, Chicago, Ill.).
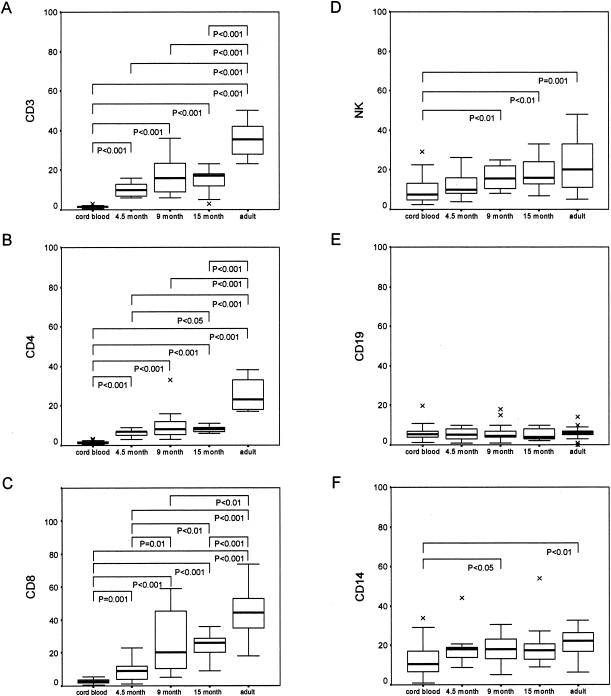
FIG. 2.
Expression of CCR5 on whole-blood leukocytes from subjects in five different age groups. Lymphocytes were identified by using a forward scatter-side scatter gating strategy, and then the percentages of CCR5-expressing CD3, CD4, CD8, NK, and CD19 cells were determined. CCR5-expressing monocytes were analyzed by gating on CD14-positive cells. Results are shown as percentages of positive cells. Data are presented as medians (horizontal bar), 25th and 75th percentiles (boxes), and 10th and 90th percentiles (bars). Significant differences between groups are indicated. Comparison of CCR5 expression on different leukocyte subsets between the different study groups was done by use of the Mann-Whitney U test.
Percentages of lymphocyte subsets among different age groups are shown in Table 1. Other studies have assessed cell subsets in healthy, non-HIV-exposed infants but looked at different age groups (5, 6, 11, 25). However, there appear to be no major differences in any subsets compared to the present study of HIV-exposed, uninfected children.
The relative expression of CXCR4 and CCR5 on various leukocyte subsets of infants of various ages is shown in Fig. 1 and 2, respectively. The proportion of cells expressing CXCR4 was highest in cord blood (Fig. 1). Thereafter, proportions of CXCR4-expressing cells declined at 4.5 months and then increased to adult levels, a pattern similar among the different cell types. CXCR4 expression was significantly higher in cord blood than in adult blood, although differences were very modest. The most dramatic modulation of CXCR4 with respect to age occurred on NK cells, CD14+ monocytes, and granulocytes (Fig. 1D, F, and G, respectively). In contrast to that found for CXCR4, the proportions of cells expressing CCR5 were initially very low and gradually increased with age (Fig. 2). Similar patterns were observed for all cell types except CD19+ lymphocytes, where there was no significant age-related increase in CCR5 expression (Fig. 2E), and CD14+ monocytes, where there was only a significant increase in expression in the 9-month (P < 0.05) and adult (P < 0.01) groups compared with the cord blood samples (Fig. 2F). Correlations between CXCR4 (cord blood samples excluded) and CCR5 expression and age are shown in Table 2.
TABLE 2.
Correlations between CXCR4 and CCR5 expression and ageb
| Cell type | CXCR4 expression
|
CCR5 expression
|
||
|---|---|---|---|---|
| r value | P valuea | r value | P valuea | |
| CD3+ | 0.457 | 0.001 | 0.896 | <0.001 |
| CD4+ | 0.519 | <0.001 | 0.908 | <0.001 |
| CD8+ | 0.242 | 0.084 | 0.862 | <0.001 |
| NK | 0.570 | <0.001 | 0.433 | <0.001 |
| CD19+ | 0.074 | 0.601 | −0.46 | 0.689 |
| CD14+ | 0.760 | <0.001 | 0.402 | <0.001 |
| Granulocytes | 0.621 | <0.001 | ||
Significant P values are indicated in bold.
The Spearman correlation coefficient was calculated to analyze correlations between age and CXCR4 and CCR5 expression.
Results presented here confirm those from other studies (2, 16) in that the percentage of CD4 cells that expressed CCR5 was lower in cord blood than in adult blood (1.4 versus 23%, respectively). However, Mo et al. (16) found that cord blood monocytes expressed only slightly less CCR5 than did adult cells (62 versus 78%), and although in our study a higher proportion of cord blood monocytes (11%) expressed CCR5 than did cord blood CD4 cells, this was still significantly less than that of adult monocytes (22%). The reason for these differences may be that isolated peripheral blood monocytes were used for their experiments (16), whereas whole blood was used in our study. Possible effects of HIV exposure on our study also cannot be excluded.
Virus populations in infected infants have been shown to be more homogeneous than those of their mothers (14, 21, 29), which suggests that a single genotype is either transmitted to or initially replicating in the child. Whether such a restriction in the virus transmitted is in any way related to the coreceptor expression on permissive cells remains largely unknown. However, HIV-1 has been shown to primarily infect CXCR4-expressing cells in placentae from nontransmitting, HIV-1-infected mothers, whereas infection of predominantly CCR5-expressing cells was demonstrated in placentae from transmitting women (3). Macrophage tropism of a mother's HIV-1 isolates has been shown to be associated with an increased risk of transmission of HIV-1 to the infant (9). Taken together, these data are consistent with transmission of a population of non-syncytium-inducing (NSI) isolates that use CCR5 as a coreceptor.
Persistent use of the CCR5 receptor has been associated with slow progression of HIV-1 disease (30), while use of the CXCR4 receptor is associated with rapid progression (22). Perinatally infected children experience more rapid progression to disease than do HIV-1-infected adults (20, 23), and thus, the question of an association between CXCR4 and CCR5 receptor expression and clinical disease is important. However, infants are more likely to be infected with NSI strains of HIV-1 and progress to symptomatic disease rapidly with no evidence of syncytium-inducing (SI) isolates (27). SI isolates are, however, found in older HIV-1-infected children (27).
It is unlikely that peripheral blood receptor expression is a restricting factor for susceptibility to infection by CCR5-utilizing strains of HIV-1, as these strains are very readily transmitted from mother to child (9). An explanation for this may be that CCR5 can be upregulated by cellular activation, thereby allowing increased viral entry, or that entry of even small amounts of virus can lead to active virus turnover through some postentry enhancement of replication promoted through cellular activation. In addition, in vitro experiments have shown that the threshold of CCR5 expression required for HIV-1 replication in thymocytes is very low (17). Further support favoring the lack of restriction afforded by low CCR5 expression in cord blood cells comes from a study showing that unstimulated cord blood mononuclear cells are preferentially infected by macrophage-tropic NSI HIV-1 isolates, whereas adult peripheral blood mononuclear cells are more susceptible to T-cell-tropic SI viral strains (19). Two other studies show an increased susceptibility of neonatal monocyte-derived macrophages to infection with NSI HIV-1 strains (13, 28).
It is clear that differences in cell maturity are reflected in the differential expression of CXCR4 and CCR5 on infant and adult peripheral leukocytes that are also mirrored by alterations in the naive and memory T-cell phenotype with age (Table 1). These findings have implications for cellular trafficking in response to the specific ligands of CXCR4 and CCR5, in that these capabilities would be expected to improve as the infant ages. Such differences may in part underlie differences in HIV disease progression between adults and children and have implications for age-dependent susceptibility to other microbial infections in early infancy. In the latter regard, it is particularly noteworthy that CXCR4-expressing NK, CD14, and granulocyte populations increase remarkably with age, whereas CCR5-expressing NK and CD14 cells show a more gradual increase. These findings suggest that CXCR4 may play a more prominent role in the innate arm of the immune system, as opposed to CCR5, which appears to be more strongly associated with cells of the acquired arm of the immune system. How persistent exposure to HIV (intrapartum and postpartum exposure through breastfeeding) might alter these immune parameters and so alter predisposition to infection, or alter the disease profiles of other infecting organisms, remains an important future question for study.
Taken together, the data show that studies of children need to take into account the differences in expression of these two important receptors in relation to age. These data add to our knowledge of the developing immune system of the infant that will provide valuable understanding with respect to the pathogenesis of neonatal disease, especially with respect to perinatal HIV-1 infection.
(This study formed part of a Ph.D. thesis, by S. Shalekoff, approved for that degree by the University of the Witwatersrand, Johannesburg, South Africa.)
Acknowledgments
This work was supported by a grant from the Poliomyelitis Research Foundation of South Africa.
REFERENCES
- 1.Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. [DOI] [PubMed] [Google Scholar]
- 2.Auewarakul, P., K. Sangsiriwut, K. Pattanapanyasat, C. Wasi, and T. H. Lee. 2000. Age-dependent expression of the HIV-1 coreceptor CCR5 on CD4+ lymphocytes in children. J. Acquir. Immune Defic. Syndr. 24:285-287. [DOI] [PubMed] [Google Scholar]
- 3.Behbahani, H., E. Popek, P. Garcia, J. Andersson, A. L. Spetz, A. Landay, Z. Flener, and B. K. Patterson. 2000. Up-regulation of CCR5 expression in the placenta is associated with human immunodeficiency virus-1 vertical transmission. Am. J. Pathol. 157:1811-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clerici, M., M. Saresella, F. Colombo, S. Fossati, N. Sala, D. Bricalli, M. L. Villa, P. Ferrante, L. Dally, and A. Vigano. 2000. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood 96:3866-3871. [PubMed] [Google Scholar]
- 5.Comans-Bitter, W. M., R. de Groot, R. van den Beemd, H. J. Neijens, W. C. Hop, K. Groeneveld, H. Hooijkaas, and J. J. van Dongen. 1997. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J. Pediatr. 130:388-393. [DOI] [PubMed] [Google Scholar]
- 6.D'Arena, G., P. Musto, N. Cascavilla, G. Di Giorgio, S. Fusilli, F. Zendoli, and M. Carotenuto. 1998. Flow cytometric characterization of human umbilical cord blood lymphocytes: immunophenotypic features. Haematologica 83:197-203. [PubMed] [Google Scholar]
- 7.Deng, H., R. Liu, W. Ellmeier, S. Choe, D. Unutmaz, M. Burkhart, P. Di Marzio, S. Marmon, R. E. Sutton, C. M. Hill, C. B. Davis, S. C. Peiper, T. J. Schall, D. R. Littman, and N. R. Landau. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661-666. [DOI] [PubMed] [Google Scholar]
- 8.Denny, T., R. Yogev, R. Gelman, C. Skuza, J. Oleske, E. Chadwick, S. C. Cheng, and E. Connor. 1992. Lymphocyte subsets in healthy children during the first 5 years of life. JAMA 267:1484-1488. [PubMed] [Google Scholar]
- 9.De Rossi, A., L. Ometto, S. Masiero, M. Zanchetta, and L. Chieco-Bianchi. 1997. Viral phenotype in mother-to-child HIV-1 transmission and disease progression of vertically acquired HIV-1 infection. Acta Paediatr. Suppl. 421:22-28. [DOI] [PubMed] [Google Scholar]
- 10.Dragic, T., V. Litwin, G. P. Allaway, S. R. Martin, Y. Huang, K. A. Nagashima, C. Cayanan, P. J. Maddon, R. A. Koup, J. P. Moore, and W. A. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667-673. [DOI] [PubMed] [Google Scholar]
- 11.Erkeller-Yuksel, F. M., V. Deneys, B. Yuksel, I. Hannet, F. Hulstaert, C. Hamilton, H. Mackinnon, L. T. Stokes, V. Munhyeshuli, F. Vanlangendonck, et al. 1992. Age-related changes in human blood lymphocyte subpopulations. J. Pediatr. 120:216-222. [DOI] [PubMed] [Google Scholar]
- 12.Feng, Y., C. C. Broder, P. E. Kennedy, and E. A. Berger. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272:872-877. [DOI] [PubMed] [Google Scholar]
- 13.Ho, W. Z., J. Lioy, L. Song, J. R. Cutilli, R. A. Polin, and S. D. Douglas. 1992. Infection of cord blood monocyte-derived macrophages with human immunodeficiency virus type 1. J. Virol. 66:573-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jansson, M., P. Orlandi, G. Scarlatti, V. Moschese, M. L. Romiti, C. Cancrini, L. Mancia, S. Livadiotti, G. Castelli-Gattinara, P. Rossi, and E. Halapi. 1997. Role of immunity in maternal-infant HIV-1 transmission. Acta Paediatr. Suppl. 421:39-45. [DOI] [PubMed] [Google Scholar]
- 15.McKinney, R. E., Jr., and C. M. Wilfert. 1992. Lymphocyte subsets in children younger than 2 years old: normal values in a population at risk for human immunodeficiency virus infection and diagnostic and prognostic application to infected children. Pediatr. Infect. Dis. J. 11:639-644. [PubMed] [Google Scholar]
- 16.Mo, H., S. Monard, H. Pollack, J. Ip, G. Rochford, L. Wu, J. Hoxie, W. Borkowsky, D. D. Ho, and J. P. Moore. 1998. Expression patterns of the HIV type 1 coreceptors CCR5 and CXCR4 on CD4+ T cells and monocytes from cord and adult blood. AIDS Res. Hum. Retrovir. 14:607-617. [DOI] [PubMed] [Google Scholar]
- 17.Pedroza-Martins, L., K. B. Gurney, B. E. Torbett, and C. H. Uittenbogaart. 1998. Differential tropism and replication kinetics of human immunodeficiency virus type 1 isolates in thymocytes: coreceptor expression allows viral entry, but productive infection of distinct subsets is determined at the postentry level. J. Virol. 72:9441-9452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.PETRA Study Team. 2002. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (PETRA study): a randomised, double-blind, placebo-controlled trial. Lancet 359:1178-1186. [DOI] [PubMed] [Google Scholar]
- 19.Reinhardt, P. P., B. Reinhardt, J. L. Lathey, and S. A. Spector. 1995. Human cord blood mononuclear cells are preferentially infected by non-syncytium-inducing, macrophage-tropic human immunodeficiency virus type 1 isolates. J. Clin. Microbiol. 33:292-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers, M. F., P. A. Thomas, E. T. Starcher, M. C. Noa, T. J. Bush, and H. W. Jaffe. 1987. Acquired immunodeficiency syndrome in children: report of the Centers for Disease Control National Surveillance, 1982 to 1985. Pediatrics 79:1008-1014. [PubMed] [Google Scholar]
- 21.Scarlatti, G., T. Leitner, E. Halapi, J. Wahlberg, P. Marchisio, M. A. Clerici-Schoeller, H. Wigzell, E. M. Fenyo, J. Albert, M. Uhlen, et al. 1993. Comparison of variable region 3 sequences of human immunodeficiency virus type 1 from infected children with the RNA and DNA sequences of the virus populations of their mothers. Proc. Natl. Acad. Sci. USA 90:1721-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuitemaker, H., M. Koot, N. A. Kootstra, M. W. Dercksen, R. E. de Goede, R. P. van Steenwijk, J. M. Lange, J. K. Schattenkerk, F. Miedema, and M. Tersmette. 1992. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J. Virol. 66:1354-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott, G. B., C. Hutto, R. W. Makuch, M. T. Mastrucci, T. O'Connor, C. D. Mitchell, E. J. Trapido, and W. P. Parks. 1989. Survival in children with perinatally acquired human immunodeficiency virus type 1 infection. N. Engl. J. Med. 321:1791-1796. [DOI] [PubMed] [Google Scholar]
- 24.Shahabuddin, S., I. H. al Ayed, M. O. el-Rad, and M. I. Qureshi. 1998. Lymphocyte subset reference ranges in healthy Saudi Arabian children. Pediatr. Allergy Immunol. 9:44-48. [DOI] [PubMed] [Google Scholar]
- 25.Shahabuddin, S., I. Al-Ayed, M. O. Gad El-Rab, and M. I. Qureshi. 1998. Age-related changes in blood lymphocyte subsets of Saudi Arabian healthy children. Clin. Diagn. Lab. Immunol. 5:632-635. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Shalekoff, S., and C. T. Tiemessen. 2001. Duration of sample storage dramatically alters expression of the human immunodeficiency virus coreceptors CXCR4 and CCR5. Clin. Diagn. Lab. Immunol. 8:432-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spencer, L. T., M. T. Ogino, W. M. Dankner, and S. A. Spector. 1994. Clinical significance of human immunodeficiency virus type 1 phenotypes in infected children. J Infect. Dis. 169:491-495. [DOI] [PubMed] [Google Scholar]
- 28.Sperduto, A. R., Y. J. Bryson, and I. S. Chen. 1993. Increased susceptibility of neonatal monocyte/macrophages to HIV-1 infection. AIDS Res. Hum. Retrovir. 9:1277-1285. [DOI] [PubMed] [Google Scholar]
- 29.Wolinsky, S. M., C. M. Wike, B. T. Korber, C. Hutto, W. P. Parks, L. L. Rosenblum, K. J. Kunstman, M. R. Furtado, and J. L. Munoz. 1992. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science 255:1134-1137. [DOI] [PubMed] [Google Scholar]
- 30.Xiao, L., D. L. Rudolph, S. M. Owen, T. J. Spira, and R. B. Lal. 1998. Adaptation to promiscuous usage of CC and CXC-chemokine coreceptors in vivo correlates with HIV-1 disease progression. AIDS 12:F137-F143. [DOI] [PubMed] [Google Scholar]