Abstract
Meningococcal serogroup-specific immunoglobulin G (IgG), IgG1, and IgG2 concentrations were assigned to three reference sera, CDC 1992, 89-SF, and 96/562, for meningococcal serogroups A, C, Y, and W135 via the method of cross standardization. The sum of the serogroup-specific IgG1 and IgG2 concentrations determined for the four meningococcal serogroups showed good agreement with the serogroup-specific IgG either determined here or as previously represented. Following the assignment of meningococcal serogroup-specific IgG1 and IgG2 concentration to these reference sera, a meningococcal serogroup-specific IgG1 and IgG2 enzyme-linked immunosorbent assay protocol was developed. The serogroup A and C specific subclass distribution of a panel of adult sera collected following vaccination with any combination of meningococcal serogroup C conjugate, bivalent, or tetravalent polysaccharide vaccines was determined. For the determination of serogroup W135 and Y specific subclass distribution, an adolescent panel 28 days following a single dose of either tetravalent polysaccharide or conjugate vaccine was used. The sum of the serogroup-specific IgG1 and IgG2 showed strong correlation with the serogroup-specific total IgG determined. The assignment here of IgG1 and IgG2 subclasses to these reference sera will allow more detailed evaluation of meningococcal conjugate and polysaccharide vaccines.
Meningococcal disease, which occurs mainly as either septicemia or meningitis, represents a major health problem worldwide. Outbreaks and epidemics of meningococcal disease occur throughout the world, caused by serogroups B, C, Y, and W135 causing endemic disease, whereas epidemics are mainly caused by serogroup A. Meningococcal serogroup C conjugate (MCC) vaccines have been introduced into several countries and have had a significant impact on the reduction of meningococcal serogroup C disease in the targeted age groups within the United Kingdom (2, 17). Recently, an increase in cases due to serogroup W135, especially in association with the hajj pilgrimage to Saudi Arabia, and serogroup Y within the United States has occurred (20). Currently, only tetravalent A, C, Y, and W135 polysaccharide vaccines are available against all four serogroups, with conjugate vaccines under development (3, 17). Therefore, the need for international reference sera with assigned serogroup-specific IgG and IgG subclass values is valuable for vaccine development and evaluation.
There is no standard method for assigning antibody concentrations to a standard reference serum. Arbitrary or weight-based units can be assigned, with the latter being of preference. However, achieving a consensus antibody level can prove difficult, as in the case of Haemophilus influenzae reference serum FDA 1983, in which a variety of techniques were used (8, 9, 12). Various methods can be used to assign reference sera antibody concentrations, including quantitative precipitation assays (14, 19, 21), homologous or heterologous enzyme-linked immunosorbent assay (ELISA) formats (10) and the use of myeloma protein standards (11), all methodologies used in the past. The method of cross standardization between different antigens has been reported by Concepcion and Frasch (5), who evaluated previously assigned IgG values in the pneumococcal reference serum 89SF by this method. Available reference sera would facilitate the comparison of ELISA results and interlaboratory standardization of subclass assays.
IgG1 and IgG2 comprise about 90% of the total human serum IgG. Little information is available concerning the IgG subclass response following meningococcal disease or vaccination with meningococcal polysaccharides or conjugates and this is in part due to the absence of an international reference serum that has been quantified for IgG subclasses. The determination of IgG subclass response following vaccination or infection will give an insight into the type of immune response induced by the immunological challenge present.
This study had two main aims, the assignment of serogroup-specific IgG and IgG subclass values to three international reference sera and the development of a subclass ELISA protocol, enabling the use of the assigned values to quantify sera from individuals following vaccination or disease.
MATERIALS AND METHODS
Reference sera.
CDC 1992 (NIBSC code 99/706) is a pool of serum from 20 adults following vaccination with a tetravalent meningococcal polysaccharide (A, C, W135, and Y) vaccine, which is distributed by the National Institute for Biological Standards and Control (NIBSC, Potters Bar, United Kingdom) and the Centers for Disease Control and Prevention (CDC, Atlanta, Ga.).
Meningococcal reference serum (NIBSC code 96/562) is a pool of serum from adults following bivalent meningococcal polysaccharide (A and C) which is also distributed by NIBSC and will be referred to as 96/562 throughout this article.
U.S. reference serum lot 89-SF is a pool of postvaccination adult sera following immunization with a 23-valent pneumococcal vaccine, a meningococcal tetravalent (A, C, Y, W135) polysaccharide vaccine, and an H. influenzae type b conjugate vaccine which is distributed by the Food and Drug Administration (FDA), Bethesda, Md.
All reference sera were reconstituted as directed.
Monoclonal antibodies against human IgG subclasses.
The mouse anti-human monoclonal antibodies used were anti-IgG1 (clone HP6069) and anti-IgG2 (clone HP6002), both purchased from Zymed Laboratories (San Francisco, Calif.).
Human serum panel.
A panel of 10 adult sera was used to determine serogroup A and C specific IgG and IgG subclass distribution. These samples were obtained from laboratory staff at Manchester Health Protection Agency Laboratory, subjects had received any combination of MCC, bivalent (A and C), or tetravalent (A, C, Y, and W135) polysaccharide vaccines, and blood samples were obtained at various time points following vaccination. A different panel was used to determine serogroup W135 and Y specific IgG and IgG subclass distribution. This panel consisted of 10 adolescent sera, 28 days following a single dose of either a tetravalent polysaccharide or conjugate vaccine.
Meningococcal polysaccharides.
Meningococcal serogroup A (MenA) and C (MenC) polysaccharide and methylated human serum albumin are available upon request from NIBSC and were reconstituted as directed. Meningococcal serogroup W135 (MenW135) and Y (MenY) polysaccharides were provided by Aventis Pasteur, Swiftwater, Pa.
Polysaccharide plate coating took place in a mixture of methylated human serum albumin in 10 mM phosphate-buffered saline dissolved in pyrogen-free sterile water, overnight at 4°C. The concentrations of MenA and MenC polysaccharides and methylated human serum albumin were as described by Holder et al. (10) and MenW135 and MenY were at a final concentration of 1 μg/ml with methylated human serum albumin at 2 μg/ml.
All buffers and plate washings were as described previously (4).
ELISA for determination of anticapsular meningococcal serogroup A, C, W135, and Y IgG concentrations in reference sera.
Immulon 2 microtiter plates (ThermoLabsystems, Franklin, Mass.) were coated with meningococcal polysaccharides and always contained MenA and MenC and either MenW135 or MenY, with four columns being coated with each serogroup polysaccharide. Following blocking, seven twofold dilutions of reference sera were made directly in the microtiter plate by well to well transfer, leaving row H as a buffer blank. Each reference serum was tested in duplicate on each plate and incubation was overnight at 4°C.
Microtiter plates were developed with monoclonal-PAN anti-human Fcγ peroxidase antibody (diluted in serum/conjugate [S/C] buffer) for 2.5 h at room temperature, followed by the chromogenic substrate tetramethylbenzidine dihydrochloride monohydrate, and the reaction was stopped after 30 min with 2 M H2SO4. The optical density of each well was then read at 450 nm.
Data analysis.
Data analysis was performed as previously described by Soininen et al. (22). Differences between values assigned with MenA and MenC as reference standards were analyzed with a paired student's t test.
ELISA for determination of anticapsular meningococcal serogroup A, C, W135, and Y IgG1 and IgG2 concentrations in reference sera.
The ELISA for the determination of IgG1 and IgG2 concentrations in reference sera was a modification of the ELISA described above for IgG. Costar, enzyme immunoassay enzyme immunoassay/radioimmunoassay medium binding microtiter plates (Corning Life Sciences, Netherlands) were coated with meningococcal polysaccharides as above. Following overnight incubation, plates were incubated sequentially with mouse monoclonal antibodies to human IgG subclasses for 3 h at room temperature, with alkaline phosphatase-conjugated rabbit anti-mouse antibody for 2.5 h at room temperature, and with p-nitrophenyl phosphate (1 mg/ml) in 1 M diethanolamine buffer containing 0.5 mM MgCl2 (pH 9.8) for 2 h at room temperature. Both the monoclonal antibodies and conjugated anti-mouse antibody were diluted in S/C buffer at predetermined dilutions. The reaction was stopped by the addition of 50 μl of 3 M sodium hydroxide. The plates were read on an Anthos 2001 plate reader at 405 nm with a reference filter at 690 nm.
Data analysis.
Data analysis was carried out as above except only MenC CDC 1992 was used as a standard with values previously assigned by Hutchins et al. (11).
ELISA for antimeningococcal serogroup-specific IgG antibodies in unknown samples.
A standardized ELISA was performed as described previously by Carlone et al. (4) except reference serum CDC 1992 and a different conjugate, monoclonal-PAN anti-human Fcγ peroxidase antibody, were used.
ELISA for antimeningococcal serogroup-specific IgG1 and IgG2 antibodies in unknown samples.
The ELISA specific for IgG1 and IgG2 antibodies in unknown samples was a modification of the ELISA described earlier by Carlone et al. (4). Following nonspecific protein binding blocking, reference sera, a quality control serum (either 89-SF or 96/562) and unknown samples were added in duplicate to a Costar enzyme immunoassay/radioimmunoassay medium binding plate coated with the required polysaccharide and eight twofold dilutions made directly in the plate leaving two wells at the base of the quality control as buffer blanks. Following overnight serum incubation plates were processed as in the IgG subclass reference serum concentration determination above.
Data analysis.
Serogroup-specific IgG and IgG subclass concentrations were calculated with a four-parameter, logistic curve model in the SOFTmax PRO (Molecular Devices, Wokingham, UK) data analysis software program.
RESULTS
Assignment of serogroup-specific IgG concentrations to reference sera.
The method of cross-standardization was used to assign meningococcal serogroup-specific IgG, IgG1, and IgG2 antibody concentrations to CDC 1992, 89-SF, and 96/562. Assignment of meningococcal serogroup-specific IgG used the reference serum CDC 1992 on the MenA and MenC portions of the plate as the standard reference. There was no significant difference between the values assigned when CDC 1992 on either the MenA or MenC portion of the plate was used as the reference standard (P < 0.05 for all comparisons), and therefore the values shown in Table 1 are the means of the values determined with both. Means were determined from at least five independent assays, with the coefficient of variation for values assigned on different days remaining below 23% (n = 5 to 27) in all cases. Standard reference curves and unknown sample curves obtained by the cross standardization assay were parallel as determined by the guidelines of Plikaytis et al. (15).
TABLE 1.
IgG1 and IgG2 values assigned to CDC 1992, 89-SF, and NIBSC reference sera
| Sero- group | Mean % ± SD (no. of determinations)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CDC 1992
|
89-SF
|
96/562
|
||||||||||
| IgG1 | IgG2 | IgG1 + IgG2a | IgG | IgG1 | IgG2 | IgG1 + IgG2 | IgG | IgG1 | IgG2 | IgG1 + IgG2 | IgG | |
| A | 10.17 ± 1.17 (9) | 72.39 ± 8.88 (9) | 82.56 (100.5) | 82.15 ± 17.33 (16) | 3.03 ± 0.63 (5) | 20.19 ± 3.45 (5) | 23.22 (99.7) | 23.29 ± 3.24 (12) | 3.34 ± 0.82 (5) | 25.76 ± 2.16 (6) | 29.1 (114) | 25.38 ± 5.72 (17) |
| C | 8.9b | 14b | 22.9 (95) | 27.52 ± 5.74 (21) | 3.48 ± 0.5 (9) | 11.33 ± 1.16 (12) | 14.81 (108) | 13.62 ± 2.54 (20) | 7.11 ± 1.16 (9) | 9.75 ± 1.71 (15) | 16.86 (98) | 17.11 ± 3.54 (23) |
| W135 | 3.84 ± 0.72 (13) | 17.48 ± 3.54 (15) | 21.33 (105.4) | 20.24 ± 2.76 (27) | 3.27 ± 0.57 (9) | 3.51 ± 0.37 (6) | 6.78 (118) | 5.72 ± 0.94 (10) | Too low to be detected | 3.08 ± 0.52 (10) | 3.08 (106) | 2.9 ± 0.52 (21) |
| Y | 5.09 ± 1.18 (9) | 30.28 ± 4.88 (9) | 35.37 (121.3) | 29.15 ± 2.90 (16) | 5.65 ± 1.04 (10) | 22.88 ± 4.31 (6) | 28.53 (90) | 31.65 ± 4.24 (13) | 2.83 ± 0.65 (6) | 10.63 ± 1.21 (5) | 13.46 (113.0) | 11.91 ± 2.34 (12) |
Values are expressed as the mean ± standard deviation. Values in parentheses in the IgG1 + IgG2 column are the sum of IgG1 plus IgG2 of the total IgG.
Previously assigned values (11).
Assignment of serogroup-specific IgG1 and IgG2 concentrations to reference sera.
Meningococcal serogroup C-specific IgG subclass antibody concentrations have previously been assigned to CDC 1992 (11), and this was used as the reference standard to assign IgG and IgG subclass antibody concentrations to the three reference sera for all four serogroups. Again, each test was run at least five times and the means were assigned as the IgG subclass specific concentrations (Table 1). Standard reference and unknown curves were parallel in the IgG subclass ELISA and the coefficient of variation for values assigned on different days remained below 25% (n = 5 to 14). The sum of IgG1 and IgG2 was 98.8 to 121.3% of the total IgG determined by independent assays for all four serogroups. The ratio of IgG subclass serogroup C CDC 1992 concentration to the IgG subclass concentration of the unknown were similar to the dilution ratio required to produce equivalent optical densities on standard dilution curves for all serogroups and reference sera. For example, the ratio (1.14) of serogroup A IgG1 CDC 1992 concentration (10.17 μg/ml) to serogroup C IgG1 CDC 1992 concentration (8.9 μg/ml) is similar to the dilution ratio required to produce equivalent optical densities on dilution curves for both serogroups (100/100 = 1).
Comparison of the IgG subclass-specific assay to the IgG-specific assay.
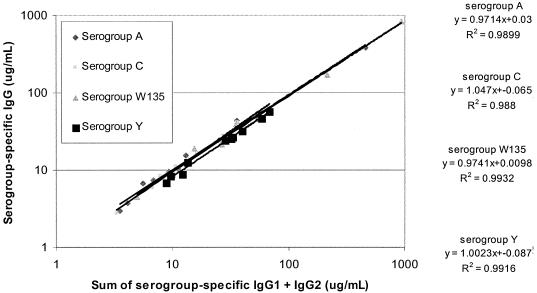
Adult sera were quantified for antimeningococcal serogroup A, C, W135 and Y specific IgG, IgG1 and IgG2 with the reference serum CDC 1992 as a reference standard, with values determined in this study (Table 1) or earlier (10, 11). The correlation between independently determined IgG concentrations and the sum of IgG1 and IgG2 concentrations measured by the IgG subclass-specific ELISA was determined, with correlation coefficients of 0.99 for all four serogroups (Fig. 1).
FIG. 1.
Comparison of the sum of IgG1 and IgG2 concentrations determined by the IgG subclass ELISA with IgG concentrations of antibodies to meningococcal serogroups A, C, Y, and W135 in adult sera.
DISCUSSION
This study has determined the IgG, IgG1, and IgG2 concentrations of antibodies to meningococcal serogroups A, C, W135, and Y in three reference sera and describes an IgG subclass protocol for determining IgG subclass distribution in unknown sera. The method of cross-standardization was used to assign IgG and IgG subclass values to the three reference sera. Cross-standardization allows the assigned antibody concentration in a reference serum against one meningococcal serogroup to assign antibody values to additional meningococcal serogroups. This assumes that if equal amounts of antibody are bound to the two different solid-phase antigens, the optical density of the bound labeled secondary antibody will be the same regardless of the specificities of the antibodies being measured. This format allows a previously calibrated standard reference serum to be used to assign antibody concentrations to any designated reference serum.
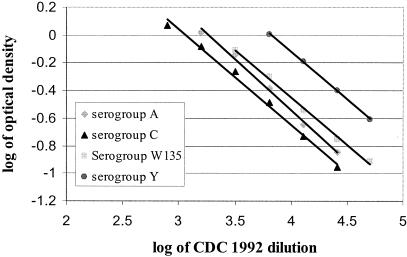
For the method of cross-standardization to measure meningococcal serogroup-specific IgG and IgG subclasses, several requirements must be met (13). First, the antigen must not be limiting. Optimal antibody binding was obtained when MenA and MenC were used at 5 μg/ml and when MenW135 and MenY were used at 1 μg/ml. The dilution curves of the two samples (reference and unknown) must be parallel to support the assumption that the antibody-binding characteristics are similar enough to allow the determination of antibody levels in the diluted serum sample. Standard reference curves and unknown samples in the cross-standardization ELISAs were judged to be parallel by the guidelines of Plikaytis et al. (15). Figure 2 shows an example of typical curves obtained in the assays described here in which parallelism was present. Finally, the standard and unknown serum samples must be assayed under the same experimental conditions. Three meningococcal polysaccharides were coated per plate and only these data were used in the cross standardization, and therefore all assay parameters were identical.
FIG. 2.
Typical parallel curves obtained in the determination of meningococcal serogroup-specific IgG and IgG subclass in the reference sera CDC 1992, 89-SF, and 96/562.
Meningococcal serogroup A and C capsular polysaccharide and meningococcal reference serum, CDC 1992 with previously assigned IgG antibody concentrations (10) were used to generate standard curves used to measure IgG-specific antibody concentrations in the three reference sera for meningococcal serogroups A, C, W135, and Y. The serogroup A and C IgG-specific concentration in CDC 1992 assigned by Holder et al. (10) were confirmed by the use of human IgG myeloma (data not shown). Recently serogroup Y and serogroup W135 IgG values have been assigned to CDC 1992 (7) with cross standardization in which a polysaccharide coating concentration of 5 μg/ml was used. It has been noted that subjects immunized with polysaccharide vaccine recognize specific epitopes of serogroup W135 and Y polysaccharide antigens that varied with the individual. The availability of these epitopes in the ELISA also varied with the coating concentration (P. Giardina, R. E. Evans, D. J. Sikkema, and S. W. Hildreth, abstracts 13th International Pathogenic Neisseria Conference, Oslo, Norway).
Titration of serogroup W135 and Y polysaccharide and methylated human serum albumin was performed and a polysaccharide coating concentration of 1 μg/ml with 2 μg/ml of methylated human serum albumin was found to be optimal for both serogroups. With this coating concentration, serogroup-specific IgG antibody concentrations were assigned for MenW135 and MenY by cross-standardization from CDC 1992 on serogroup A and C as reference standards. For MenW135, the value assigned via this method was different to that assigned previously by Elie et al. (7). This difference is probably accountable to the different coating conditions used. Giardiana et al. (P. Giardina, R. E. Evans, D. J. Sikkema, and S. W. Hildreth, abstracts 13th International Pathogenic Neisseria Conference, Oslo, Norway) reported that some serum samples display different IgG values based on the coating concentrations.
IgG subclass values have previously been assigned to CDC 1992 for MenC, with human myeloma proteins as standards (11). These values were used in this study to assign serogroup A, Y, and W135 IgG1 and IgG2 specific antibody concentrations in reference sera CDC 1992 via the method of cross standardization. The assignment of IgG and IgG subclass serogroup-specific concentrations to additional reference sera would enable their use as either reference standards or quality control sera.
Pneumococcal anti-serotype IgG and IgG subclass values have been previously assigned to 89-SF (16, 22) but no such values have been assigned for meningococcal serogroups for either 89-SF or 96/562. The same method of cross-standardization was used assign IgG, IgG1, and IgG2 serogroup-specific antibody concentrations to 89-SF and 96/562. Those values obtained with either standard were not significantly different (P < 0.05). IgG subclass values were assigned with CDC 1992 as a reference standard on serogroup C. The sum of IgG1 and IgG2 was in good agreement with the IgG assigned here.
These assays used adhered to the criteria mentioned previously, but several other factors confirm the validity of IgG1 and IgG2 assignments to the three reference sera.
The sum of IgG1- and IgG2-specific antibody levels for all three reference sera for the meningococcal serogroups was consistent with the IgG values either assigned in this study or previously (7, 10) with the sum of IgG1 and IgG2 being within 20% agreement of the IgG-specific antibody as measured in a separate assay. Also, the ratio of the unknown IgG subclass antibody concentrations to the serogroup C CDC 1992 IgG subclass antibody concentration was similar to the dilution ratio required to produce equivalent optical densities on dilution curves.
IgG3 and IgG4 values were assigned to CDC 1992 for MenC (10), but values were too low for this to be used as a standard.
The values assigned in this study were used to quantify IgG1 and IgG2 in ten serum samples from individuals who had received none or any combination of serogroup A/C polysaccharide vaccine, serogroup C conjugate vaccine, or serogroup A,C, Y, W135 polysaccharide vaccine. IgG subclass concentrations were determined with reference serum, CDC 1992 as a reference standard with values assigned either in this study or previously and either 89-SF or 96/562 as a quality control serum. Serogroup-specific IgG concentrations were determined in independent assays with standardized ELISA methodology (4). There was good agreement between the sum of IgG1 and IgG2 with the IgG concentrations determined in independent assays with correlation coefficients of 0.99 for all four serogroups, indicating that the concentrations for reference serum CDC 1992 have been reliably determined.
Little information is available concerning the IgG subclass response following vaccination with meningococcal polysaccharides. The determination of IgG subclass response to vaccination or infection will give an insight into the type of immune response induced by the immunological challenge present. Bactericidal polysaccharides are thought to stimulate the production of IgG2 in adults and IgG1 in infants, whereas proteins elicit predominantly an IgG1 response. The production of IgG1 is thought to involve antigenic activation of T helper cells that promote class switching in B cells from IgM to IgG1. An IgG2 response is thought to involve limited T cell help for B cell antibody production (1). This is reflected in the response to meningococcal polysaccharide and conjugate vaccines, where the polysaccharide response is T cell independent and short term (6), but the conjugate vaccine stimulates a T-cell-dependent response, priming immunological memory. Therefore, it would be of interest to analyze the IgG subclass response to both types of vaccine. The assignment of meningococcal serogroup-specific IgG and IgG subclass values to three reference sera will allow further evaluation of the immune response to both vaccination and meningococcal disease and enable interlaboratory comparison.
Acknowledgments
We thank D. Sikkema, G. Carlone, and C. Frasch for helpful discussions about cross-standardization assays.
REFERENCES
- 1.Ambrosino, D. M., M. Wang, A. Ciamarra, M. Chan, D. L. Bolon, J. Minn, D. A. Jacobsohn, and R. W. Finberg. 1994. T cells and natural killer cells regulate human IgG subclass concentrations in SCID mice. Cell. Immunol 155:134-143. [DOI] [PubMed] [Google Scholar]
- 2.Balmer, P., R. Borrow, and E. Miller. 2002. Impact of meningococcal C conjugate vaccine in the United Kingdom. J. Med. Microbiol. 51:717-722. [DOI] [PubMed] [Google Scholar]
- 3.Cambell, J. D., R. Edelman, J. C. King, Jr., T. Papa, R. Ryall, and M. B. Rennels. 2002. Safety, reactogenicity, of a tetravalent meningococcal polysaccharide-diphtheria toxoid conjugate vaccine given to healthy adults. J Infect. Dis. 186:1848-1851. [DOI] [PubMed] [Google Scholar]
- 4.Carlone, G., C. E. Frasch, G. R. Siber, et al. 1992. Multicenter comparisons of levels of antibody to the Neisseria meningitidis group A capsular polysaccharide measured by an enzyme linked immunosorbent assay. J. Clin. Microbiol. 30:154-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Concepcion, N., and C. E. Frasch. 1998. Evaluation of previously assigned antibody concentrations in pneumococcal polysaccharide reference serum 89-SF by the method of cross-standardisation. Clin Diagn Lab Immunol. 5:199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diakun, K. R., D. C. Martin, T. Mininni, J. Skuse, N. Ziembiec, and S. Quataert. 1997. Immunoassay of human Neisseria meningitidis serogroup A antibody. Immunol. Investig. 26:661-679. [DOI] [PubMed] [Google Scholar]
- 7.Elie, C. M., P. K. Holder, S. Romero-Steiner, and G. M. Carlone. 2002. Assignment of additional anticapsular antibody concentrations to the Neisseria meningitidis group A, C, Y, and W-135 meningococcal standard reference serum CDC 1992. Clin. Diagn. Lab. Immunol. 9:725-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton, R. G. 1991. Application of engineered chimeric antibodies to the calibration of human antibody standards. Ann, Biol, Clin. 49:242-248. [PubMed] [Google Scholar]
- 9.Herrmann, D. J., R. G. Hamilton, T. Barington, et al. 1992. Quantitation of human IgG subclass antibodies to Haemophilus influenzae type b capsular polysaccharide. J. Immunol. Methods 148:101-114. [DOI] [PubMed] [Google Scholar]
- 10.Holder, P. K., S. E. Maslanka, L. B. Pais, J. Dykes, B. D. Plikaytis, and G. M. Carlone. 1995. Assignement of Neisseria meningitidis serogroup A and C class-specific anticapsular antibody concentrations to the new standard reference serum CDC 1992. Clin. Diagn. Lab. Immunol. 2:132-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchins, W. A., G. M. Carlone, and M. A. Westerink. 1999. Elderly immune responses to a TI-2 antigen: heavy and light chain use and bactericidal activity to Neisseria meningitidis serogroup C polysaccharide. J Infect. Dis. 179:1433-1440. [DOI] [PubMed] [Google Scholar]
- 12.Madore, D. V., C. L. Johnson, D. C. Phipps, Pennridge Pediatric Associates, M. G. Myers, R. Eby, and D. H. Smith. 1990. Safety and immunogenicity of Haemophilus influenzae type b oligosaccharide-CRM197 conjugate vaccine in infants aged 15 to 23 months. Pediatrics 86:527-534. [PubMed] [Google Scholar]
- 13.Makela, O., and F. Peterfy. 1983. Standard sera in solid-phase immunoassays. Eur. J. Immunol. 13:815-819. [DOI] [PubMed] [Google Scholar]
- 14.Makela, O., P. Mattila, N. Rautonen, I. Seppala, J. Eskola, and H. Kayhty. 1987. Isotype concentrations of human antibodies to Haemophilus influenzae type b polysaccharide (Hib) in young adults immunized with the polysaccharide as such or conjugated to a protein (diphtheria toxoid). Eur. J. Immunol. 139:1999-2004. [PubMed] [Google Scholar]
- 15.Plikaytis, B. D., P. F. Holder, L. B. Pais, S. E. Maslanka, L. L. Gheesling, and G. M. Carlone. 1994. Determination of parallelism and nonparallelism in bioassay dilution curves. J. Clin. Microbiol. 32:2441-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quataert, S. A., C. S. Kirch, L. J. Quackenbush Wiedl, D. C. Phipps, S. Stromeyer, C. O. Cimino, J. Skuse, and D. Madore. 1995. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89s. Clin. Diagn. Lab. Immunol. 2:590-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramsay, M.E., N. J. Andrews, C. L. Trotter, E. B. Kaczmarski, and E. Miller. 2003. Herd Immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. Br. Med. J. 326:365-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rennels, M., J. King, Jr., R. Ryall, S. Manoff, T. Papa, A. Weddle, and J. Froeschle. 2002. Dose escalation, safety and immunogenicity study of a tetravalent meninogococcal polysaccharide diphtheria conjugate vaccine in toddlers. Pediatr. Infect. Dis. J. 21:978-979. [DOI] [PubMed] [Google Scholar]
- 19.Robbins, J. B., J. C. Parke, R. Schneerson, and J. K. Whisnant. 1973. Quantitative me.asurement of “natural” and immunization-induced Haemophilus influenzae type b capsular polysaccharide antibodies. Pediatr Res. 7:103-110. [DOI] [PubMed] [Google Scholar]
- 20.Rosenstein, N. E,. B. A. Perkins, D. S. Stephens, L. Lefkowitz, M. L. Cartter, R. Danila, P. Cieslak, K. A. Shutt, T. Popovic, A. Schuchat, L. H. Harrison, and A. L. Reingold. 1999. The changing epidemiology of meningococcal disease in the United States, 1992-1996. J Infect. Dis. 180:1894-1901. [DOI] [PubMed] [Google Scholar]
- 21.Seppala, I., H. Sarvas, O. Makela, J. Mattila, J. Eskola, and H. Kayhty. 1988. Human antibody responses to two conjugate vaccines of Haemophilus influenzae type b saccharides and diphtheria toxin. Scand. J. Immunol. 28:471-479. [DOI] [PubMed] [Google Scholar]
- 22.Soininen, A., I. Seppala, T. Wuorimaa, and H. Kayhty. 1998. Assignment of immunoglobulin G1 and G2 concentrations to pneumococcal capsular polysaccharides 3, 6B, 14, 19F, and 23F in pneumococcal reference serum 89-SF. Clin. Diagn. Lab. Immunol. 5:561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]