Abstract
Mycoplasma genitalium is associated with acute and chronic urethritis in men. Existing data on infection in women are limited and inconsistent but suggest that M. genitalium is associated with urethritis, cervicitis, pelvic inflammatory disease, and possibly female infertility. Data are inconclusive regarding the role of M. genitalium in adverse pregnancy outcomes and ectopic pregnancy. Available data suggest that azithromycin is superior to doxycycline in treating M. genitalium infection. However, azithromycin-resistant infections have been reported in 3 continents, and the proportion of azithromycin-resistant M. genitalium infection is unknown. Moxifloxacin is the only drug that currently seems to uniformly eradicate M. genitalium. Detection of M. genitalium is hampered by the absence of a commercially available diagnostic test. Persons with persistent pelvic inflammatory disease or clinically significant persistent urethritis or cervicitis should be tested for M. genitalium, if possible. Infected persons who have not previously received azithromycin should receive that drug. Persons in whom azithromycin therapy fails should be treated with moxifloxicin.
Identified in 1980 [1], Mycoplasma genitalium is a bacterium of the Mollicutes class that colonizes the male and female reproductive tract. It is well-known as the smallest of any free-living cell and, given the small size of its genome (580 kb), was one of the first bacteria to be fully sequenced [2] and the first genome to be chemically synthesized [3]. Epidemiologic studies of this somewhat novel bacterium’s role in disease processes have been conducted since the early 1990s, subsequent to the development of nucleic acid amplification tests [4, 5], and a number of studies have evaluated associations with male and female reproductive tract disease syndromes. Increasing evidence regarding the role of M. genitalium as a sexually transmitted disease (STD) raises questions about the clinical management of STD syndromes in general and of M. genitalium infection in particular. Primary among these questions is under what circumstances should clinicians treat for M. genitalium, and what pharmacologic therapy is most effective? Because there is currently no commercially available assay for M. genitalium, we were particularly interested in how data on the role of M. genitalium infection in STD syndromes should inform empirical treatment recommendations.
Against this background, we examined 6 specific questions, with the goal of informing and potentially revising the United States Centers for Disease Control and Prevention (CDC) STD Treatment Guidelines. These questions are as follows: (1) Does M. genitalium cause significant morbidity in adult men and women? (2) Is 1 of the 2 recommended treatment regimens for male urethritis, azithromycin (1 gram) and doxycycline (100 milligrams twice daily for 7 days), superior to the other in the treatment of M. genitalium infection? (3) Is a longer course of azithromycin superior to a single 1-gram dose of azithromycin in the treatment of M. genitalium infection? (4) Which, if any, quinolones are effective in the treatment of M. genitalium infection? (5) What is the preferred therapy for M. genitalium infection? and (6) Should the therapy for STD syndromes, such as nongonococcal urethritis (NGU), persistent NGU, cervicitis, or pelvic inflammatory disease (PID), be altered in recognition of the potential role played by M. genitalium? To address these questions, we evaluated the evidence in the published literature and sought expert opinion.
METHODS
We searched the English-language literature using PubMed and the MeSH search term Mycoplasma genitalium for articles published through 15 August 2011. After excluding reports on the development of laboratory assays, studies on genomics, and editorials, a total of 112 studies evaluating disease associations, treatment outcomes, and antimicrobial susceptibility were reviewed. Data in tables summarizing these studies were directly abstracted from the article when available and were calculated from data presented when not directly available. Significance testing and calculation of unadjusted odds ratios was done using EpiInfo, version 6 (CDC).
RESULTS
Significant Morbidity in Men and Women Caused by M. genitalium
To address the question of whether M. genitalium causes significant morbidity among adults, we reviewed studies evaluating its association with urethritis in men, lower and upper genital tract disease syndromes in women, infertility, and adverse pregnancy outcomes. Special attention was paid to the amount and consistency of the evidence.
Male Urethritis
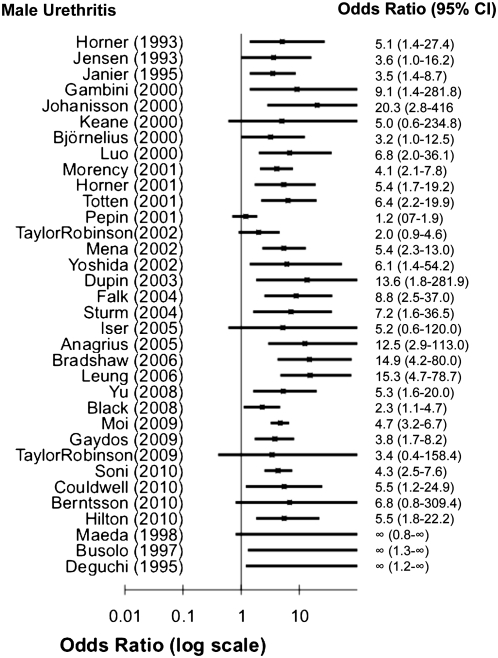
We identified 34 studies published during 1993–2011 that enrolled >10 men with NGU and used polymerase chain reaction (PCR) analysis to evaluate the role of M. genitalium as a cause of acute urethritis in men (Supplementary Table 1; online only). Twenty-eight of the 34 studies enrolled populations from high-income countries (United States, Europe, Japan, Australia, New Zealand, Hong Kong, or Russia) [6–33]; 4 additional studies enrolled men from lower-income countries (sub-Saharan Africa [34–37], Central Africa [38], and China [39]). The 28 studies in high-income countries included a total of 5650 men with acute NGU (range, 36–2406 men). Across all studies, 955 (13%) of the 7123 men with NGU tested positive for M. genitalium (median, 15%; range, 5%–42%). The median prevalence of M. genitalium infection among men with nonchlamydial NGU in 11 studies for which data were available was 25% (range, 10%–38%) [11, 15, 18, 19, 21, 23, 25, 29, 30, 34, 36].
Pooling study results, the proportion of NGU cases associated with M. genitalium was similar in studies that defined NGU only by the presence of signs or symptoms (16%) and in studies that defined NGU by both signs or symptoms and ≥5 polymorphonuclear (PMN) leukocytes on Gram stain (17%), although Gram stain criteria were not uniform across studies. However, all 4 studies that used >1 criterion to define NGU found that the prevalence of M. genitalium infection was higher when the syndrome was defined both by the presence of symptoms or signs and by the finding of ≥5 PMN leukocytes per high-power field than when only symptoms or Gram stain findings were used to define the syndrome [12, 20, 33, 40].
Twenty-two of 28 studies compared the presence of M. genitalium in men with urethritis with the prevalence of infection in an asymptomatic control group. All found that M. genitalium was more common in men with NGU than in men without urethritis, and this difference was significant in 16 (73%) of 22 studies, with odds ratios ranging from 2.2 to 20.3 (Figure 1). Additional evidence supporting a causal role for M. genitalium in male urethritis includes studies demonstrating that the organism can cause urethritis in primate models [41–43], evidence of a dose-response relationship between M. genitalium bacterial load as measured by quantitative PCR and signs and symptoms of urethritis [23, 44, 45], and studies associating successful eradication of M. genitalium with the clinical resolution of urethritis and microbiologic persistence with clinical treatment failure [23, 46–50]. On the basis of the consistent epidemiologic evidence associating the presence of the organism with the clinical syndrome of NGU, experimental animal model data, and both observational and experimental clinical data associating microbiologic and clinical treatment failures in humans, we conclude that M. genitalium can cause acute urethritis.
Figure 1.
Odds ratios and 95% confidence intervals for studies of the association between Mycoplasma genitalium assessed by polymerase chain reaction (PCR) and nongonococcal urethritis (NGU).
Persistent or Chronic Urethritis in Men
Eight studies have shown either a significant association or a trend toward such an association between microbiologic treatment failure for acute M. genitalium urethritis and clinically persistent or recurrent urethritis [12, 20, 40–45] (Table 1). Four additional studies have assessed the prevalence of M. genitalium infection among men presenting with persistent or recurrent urethritis, reporting that 19%–41% of men with the syndrome are infected with M. genitalium [16, 51–53]. Three studies included comparison groups of men without chronic urethritis; in 1, M. genitalium was not detected in any control subjects (resulting in an undefined odds ratio) [16], 1 found a statistically significant association [51], and 1 found no association [52]. On the basis of treatment studies associating the microbiologic persistence of M. genitalium with persistent symptoms of urethritis and evidence of urethral inflammation and studies associating M. genitalium with the clinical syndrome of persistent or recurrent urethritis, we conclude that M. genitalium can cause persistent or recurrent urethritis.
Table 1.
Summary of Studies Presenting Data on the Proportion of Men With or Without Microbiologic Eradication of Mycoplasma genitalium Who Experienced Clinical Cure
| Proportion, % |
|||
| Study | Clinical cure but microbiologic failure | Both clinical and microbiologic cure | P |
| Dupin et al [20] | 25 | 100 | .03 |
| Maeda et al [40] | |||
| 7 days | 86 | 100 | .43 |
| 8–28 days | 29 | — | — |
| Gambini et al [12] | 0 | 100 | <.0001 |
| Bradshaw et al [41] | 0 | 100 | <.0001 |
| Stamm et al [42] | 0 | 100 | — |
| Bradshaw et al [43] | 17a | 91a | <.0001 |
| Björnelius et al [44] | 29 | 77 | <.0001 |
| Mena et al [45] | |||
| <21 days | 11 | 20 | .39 |
| >21 daysb | 14 | 77 | .001 |
Numbers are not presented separately for men and women, because differences were the same in both groups.
Only a minority of persons had follow-up testing at >21 days.
Cervicitis
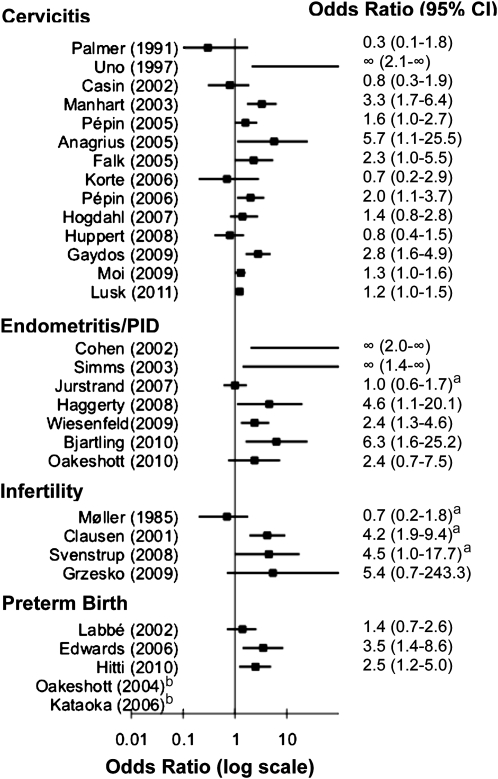
Fourteen studies conducted in the United States, Europe, Japan, West Africa, and Australia evaluated the relationship between M. genitalium and cervicitis [23, 46–50, 54–61]. Study populations ranged in size from 57 to 7676 women, and all but 1 [50] used nucleic acid amplification tests (primarily PCR) to detect M. genitalium. Nearly all studies used different definitions of cervicitis, but most incorporated some objective measure of inflammation, such as number of cervical PMN leukocytes or clinical assessment of signs of cervicitis (eg, cervical mucopus, discharge, edema, and erythema). Taken together, the results of these studies are somewhat conflicting, with 8 (57%) of 14 reporting a significant association [23, 47, 54–58, 61] and 6 (43%) of 14 reporting no association [46, 48–50, 59, 60]. In general, the studies that used only clinical diagnoses of cervicitis were less likely to show an association with M. genitalium [49, 50], whereas all studies that defined cervicitis as ≥30 PMN leukocytes per high-power field reported higher prevalence of M. genitalium infection among women with cervicitis than among control subjects [23, 48, 54, 55, 57, 60, 61]; however, in 1 case, this was not statistically significant [48]. Statistically significant odds ratios for the association between M. genitalium and cervicitis ranged from 1.2 to 5.7 but were rarely adjusted for other factors. We conclude that existing evidence provides some support for the hypothesis that M. genitalium may cause cervicitis but is conflicting. Supporting evidence comes from the observation that the vast majority of studies using an objective assessment of cervical inflammation reported a significant association between M. genitalium and cervicitis. Nevertheless, the magnitude of this association is highly variable in studies conducted to date, and nearly half of the studies report no association.
Female Urethritis
M. genitalium has been detected in 4%–9% of women with urethritis, and 2 of the 3 studies that assessed this reported a significant association, with odds ratios ranging from 2.1 to 2.5 [23, 60] (Figure 2 and Supplementary Table 2; online only). In contrast, one-third of the studies found no significant association [47], although M. genitalium was more common among women with than without urethritis. Current evidence suggests that M. genitalium may cause female urethritis in addition to male urethritis, but data are too limited to draw definitive conclusions.
Figure 2.
Odds ratios and 95% confidence intervals for studies of the association between Mycoplasma genitalium (assessed by polymerase chain reaction [PCR], unless otherwise specified) and female reproductive tract disease syndromes. aM. genitalium assessed by serology bRR could not be calculated for Oakeshott (2004 [70]) and Kataoka (2006 [82]); no M. genitalium was detected among women with preterm birth.
Pelvic Inflammatory Disease
Nine studies assessed the association between female upper reproductive tract disease and M. genitalium. Three studies used serologic testing [62–64], whereas 6 applied PCR to cervical and endometrial [65–69] or urine specimens [70]. All but 3 [64, 66, 70] were hampered by the absence of a comparison group of women without PID, and the evidence overall is somewhat conflicting. Two of the 3 serologic studies found no association with either M. genitalium antibody positivity or titer level [63, 64], although 1 showed that 38.7% of women with acute PID and no antibodies to Chlamydia trachomatis or Mycoplasma hominis experienced a ≥4-fold increase in M. genitalium antibody titer between acute and convalescent phase serum samples [62]. However, in the only seroepidemiologic study to include women without clinically diagnosed PID, antibodies to M. genitalium were detected in similar proportions of women with PID and healthy pregnant women (17% vs 15%; P = .48), although there was a trend toward an association among younger women (age, 15–30 years) [64].
Of the 6 studies using PCR and comparison groups, M. genitalium was detected more often in women with endometritis [65, 68, 71] or clinically diagnosed PID [66, 69] than in women without disease, with odds ratios ranging from 4.6 to 6.3. In the only cohort study, the risk of incident clinically diagnosed PID in the Prevention of Pelvic Infection (POPI) trial was higher among young British women with M. genitalium at baseline, but this was not statistically significant (relative risk, 2.4; 95% CI 0.74–7.46; P = .12), and the overall incidence of M. genitalium infection over the course of 1 year was low (0.9%) [70]. M. genitalium has been detected in the fallopian tube of a Kenyan woman with mild salpingitis, indicating that it can ascend high into the upper reproductive tract [67], and a causative role for M. genitalium in PID is biologically plausible, as indicated by studies in nonhuman primates in which endosalpingitis was induced after inoculation with M. genitalium [72].
In summary, existing data provide some support for the hypothesis that M. genitalium can cause PID but are conflicting. Supporting data include the observation that M. genitalium can be directly detected in the endometrium and fallopian tubes of women with PID and/or salpingitis, epidemiologic associations with endometritis and PID in studies assessing M. genitalium by PCR, and animal model studies. However, the frequency with which M. genitalium–infected women experience PID remains largely unknown, and serologic data in humans remain conflicting, perhaps partly because of the variety of serologic assays used and concerns about cross-reactivity in some assays between antibody for M. genitalium and Mycoplasma pneumoniae.
Infertility
Four studies—3 conducted in Denmark and 1 in Poland— evaluated whether antibody to M. genitalium is more common in women with tubal factor infertility (TFI) than in control groups of fertile women or women with other causes of infertility [73–76]. The 2 highest-quality studies observed a higher prevalence of M. genitalium antibody among women with TFI than among women with other causes of infertility, and in both, the association between M. genitalium and TFI was independent of a woman’s C. trachomatis antibody status [74, 75]. One of the other 2 studies found that infertile women were more likely to have antibody to M. genitalium than were fertile women, but this finding was not statistically significant and was isolated to infertile women without TFI [76]. Three studies evaluated the association of M. genitalium and male infertility [77–79], only 1 of which included a control group [77]. The controlled study, conducted among Danish men attending an infertility clinic, found no association between prevalent M. genitalium infection and male infertility assessed by sperm morphology, mobility, or ability to invade mucus substitute material in vitro. Two uncontrolled studies conducted in Tunisia found M. genitalium by PCR in 6 (4%) of 120 and 5 (5%) of 104 infertile men defined by sperm quality evaluation according to World Health Organization guidelines. We did not identify any studies involving men that used serologic testing. Thus, existing data provide some support for the hypothesis that M. genitalium can cause female infertility but are inconclusive. Supporting evidence comes from some, but not all, case-control studies that have measured M. genitalium exposure with use of serologic testing and from some clinical and animal model studies suggesting that M. genitalium can cause PID, which is thought to be a precursor to TFI. In contrast, the limited existing evidence does not support a role for M. genitalium as a cause of male infertility.
Ectopic Pregnancy
Only 1 study evaluated the potential association of M. genitalium with ectopic pregnancy. A case-control study among Swedish women compared the prevalence of M. genitalium antibody in women with ectopic pregnancies who received a diagnosis during 1984–1986 with that in a control group of healthy pregnant women tested for rubella in 1988. Antibody to M. genitalium was present in similar numbers of women with or without ectopic pregnancy (18% vs 15%; not statistically significant) [64]. M. genitalium was marginally associated with ectopic pregnancy in a subgroup of women aged 15–30 years (20% vs 11%; odds ratio, 1.6; 95% confidence interval, .6–4.0). Of note, antibody to C. trachomatis was also associated with ectopic pregnancy only among women aged 15–30 years. The very limited existing evidence is insufficient to conclude that M. genitalium is associated with ectopic pregnancy.
Adverse Birth Outcomes
Five published studies investigated the association of M. genitalium with any adverse birth outcome [80–84]. All studies evaluated preterm birth as an outcome. One also evaluated the association of M. genitalium with miscarriage [81]. The study end points were relatively uncommon in all 3 studies (1%–4%), with 1 exception [83], limiting their statistical power. Only 3 of the 5 studies tested a control group of women who did not experience adverse birth outcomes [81, 82, 84]. In 2 of the 3 controlled studies, none of the women with preterm birth had M. genitalium infection [81, 82]. However, a large case-control study involving pregnant women in Peru found a 2.5-fold increased risk for spontaneous preterm delivery among women positive for M. genitalium [84] (adjusted odds ratio, 2.5; 95% confidence interval, 1.20–5.02). In contrast, a case-control study conducted in Guinea-Bissau used PCR analysis to test 1014 women for M. genitalium 7 days after delivery and found no association of M. genitalium with a combined outcome of still birth, spontaneous abortion, premature birth, or small for gestational age (6.2% vs 6%) or with any of the adverse pregnancy outcomes individually [80]. Therefore, existing evidence is sparse and conflicting regarding a causal role for M. genitalium in adverse birth outcomes. On the basis of very limited data, M. genitalium does not seem to be common in women who experience preterm births in high-income countries. Data are conflicting in lower-income countries.
Comparison Between Azithromycin and Doxycycline for Treatment of M. genitalium Infection
We identified 7 studies that evaluated microbiologic cure rates achieved with a 7–8-day course of doxycycline [12, 20, 44, 45, 53, 85, 86], 9 that evaluated microbiologic cure rates with azithromycin (1 gram) [12, 41–45, 53, 86, 87], and 4 that evaluated the efficacy of azithromycin (500 milligrams once, followed by 250 milligrams once daily for 4 days) [44, 45, 85, 87] (Table 2). One additional study used a combination of azithromycin doses, and presentation of data did not allow efficacy to be evaluated separately, although all 20 patients were cured [53]. Only 2 randomized trials compared azithromycin with doxycycline [45, 86]. One trial included 78 men with M. genitalium urethritis and found that azithromycin was superior to doxycycline (microbiologic cure, 87% vs 45%; P = .002) [45]. The other trial included 54 men with M. genitalium urethritis and also found that azithromycin was superior to doxycycline (microbiologic cure, 67% vs 31%; P .002), but cure rates for both drugs were substantially lower than those observed in the prior trial [86].
Table 2.
Mycoplasma genitalium and Clinical Treatment
| Citation | Study design | Study population | Outcome definitionsa | Treatment regimen | Reported findings |
| Horner et al, 1993 [6] | Case series | 98 M. genitalium–positive British men with NGU attending STD clinic; aged 19–53 years | Microbiologic failure; follow-up (10–21 days) | Doxycycline (200 milligrams stat plus 100 mg/d × 13 days) | 4/14 (29%) had microbiologic failure |
| Gambini et al, 2000 [12] | Cohort | 52 M. genitalium–positive Italian men with NGU attending STD clinic; aged 17–70 years | Microbiologic failure; clinical failure; follow-up (7 days) | Doxycycline (200 mg/d × 7 days) or Azithromycin (1 gram stat) Failures: received alternate treatment regimen | Doxycycline: 2/35 (6%) had clinical and microbiologic failure Azithromycin: 3/17 (18%) had clinical and microbiologic failure Failures: 0/5 (0%) had clinical or microbiologic failure |
| Johannisson et al, 2000 [13] | Case series | 21 M. genitalium–positive Swedish men with urethritis (n = 18) and women (n = 3) attending STD clinics; aged 18–60 years | Microbiologic failure; clinical failure; follow-up (3–4 weeks) | Tetracycline (0.5 grams 2×/day × 10 days) | Tetracycline in men: 8/13 (61%) had microbiologic failure; 6/13 (46%) had clinical failure Women: 1/1 (100%) had microbiologic failure |
| Horner et al, 2001 [16] | Cohort | 109 M. genitalium–positive British men with NGU attending STD clinic; age range NR | Clinical failure; follow-up (2, 6, 12 weeks) | Doxycycline (200 milligrams stat plus 100 milligrams/days × 13 days) or Erythromycin (500 milligrams 4×/day × 14 days) Persistent urethritis: Erythromycin (500 milligrams 4×/day × 14 days) plus metronidazole (400 milligrams 2×/day × 5 days) | Doxycycline-erythromycin (combined): 7/7 (100%) had clinical failure |
| Maeda et al, 2001 [40] | Cohort | 12 M. genitalium–positive Japanese men with NGU attending urology clinic; aged 17–69 years | Microbiologic failure; clinical failure; follow-up (14 days) | Levofloxacin (100 milligrams 3×/day × 14 days) | Levofloxacin: 8/12 (67%) had microbiologic and 1/12 (8%) had clinical failure 5/7 (71%) with microbiologic failure but clinical cure had recurrent NGU at 4 weeks |
| Falk et al, 2003 [85] | Cohort | 60 M. genitalium–positive Swedish men (n = 34) and women (n = 26) attending STD clinic; age range NR | Microbiologic failure; follow-up (4–5 weeks) | Doxycycline (200 milligrams stat plus 100 milligrams × 8 days) or Lymecycline (300 miligrams 2×/day × 10 days) Asymptomatic M. genitalium–positive: azithromycin (500 milligrams stat plus 250 mg/d × 4 days) | Doxycycline-lymecycline (combined): 10/16 men (63%) and 10/14 women (71%) had microbiologic failure Azithromycin: 0/8 men and women (0%) had microbiologic failure |
| Dupin et al, 2003 [20] | Cohort | 9 M. genitalium–positive French men with urethritis attending STD clinic; age range NR | Microbiologic failure; clinical failure; follow-up (15–28 days) | Doxycycline (100 mg/d × 7 days) or Minocycline (100 mg/d × 7 days) or Spectinomycin (2 grams) and minocycline (100 mg/d × 7 days) | Doxycycline: 1/1 (100%) had microbiologic and clinical failure Minocycline: 4/7 (57%) had microbiologic and 2/7 (29%) had clinical failure Spectinomycin-minocycline: 0/1 (0%) had microbiologic or clinic failure |
| Bradshaw et al, 2006 [41] | Case series | 34 M. genitalium–positive Australian men with NGU attending STD clinic; aged 22–54 years | Microbiologic failure; clinical failure; follow-up (1 month) | Azithromycin (1 gram stat) Failures: Azithromycin (1 gram weekly × 3) Azithromycin failures: moxifloxacin (400 milligrams 2×/days × 10 days) | Azithromycin (stat): 9/32 (28%) had microbiologic failure, and 8/32 (25%) had partial clinical failure and recurrence Azithromycin (weekly): 3/3 (100%) had microbiologic failure Moxifloxacin: 0/9 (0%) had microbiologic failure |
| Wikstrom et al, 2006 [53] | Cohort | 38 M. genitalium–positive Swedish men with persistent urethritis (n = 32) and female partners (n = 6) attending STD clinic, initially treated with doxycycline (200 milligrams stat plus 100 mg/d × 8 days); aged 19–47 years | Microbiologic failure; clinical failure; follow-up (3 weeks) | Azithromycin (1 gram stat or 500 milligrams stat plus 250 mg/d × 4 days) or Erythromycin (500 milligrams 2x/d × 10 days) Female partners: azithromycin (1.5 gram × 5 days) | Azithromycin: 0/20 (0%) of men had microbiologic and 2/20 (10%) had clinical failure; 0/4 (0%) women had microbiologic failure; clinical failure NR Erythromycin: 3/5 (60%) of men had microbiologic and 9/11 (82%) had clinical failure |
| Ross et al, 2006 [88] | Randomized double-blind multisite controlled trial | 4 M. genitalium–positive European and South African women with PID; age range NR | Microbiologic failure; follow-up (5–24 and 28–42 days) | Moxifloxacin (400 mg/d × 14 days) or Ofloxacin (400 milligrams 2×/day) plus metronidazole (500 milligrams 2×/day × 14 days) | Moxifloxacin: 0/3 (0%) had microbiologic failure Ofloxacin-metronidazole: 0/1 (0%) had microbiologic failure |
| Stamm et al, 2007 [42] | Randomized double-blind multisite controlled trial | 42 M. genitalium–positive US men with NGU attending STD clinics; aged 18–45 years | Microbiologic failure; clinical failure; follow-up (5 weeks) | Rifalazil (2.5, 12.5, or 25 milligrams stat) or Azithromycin (1 gram stat) | Rifalazil, 2.5 milligrams: 5/5 (100%) had microbiologic and 6/8 (75%) had clinical failure Rifalazil,12.5 milligrams: 7/7 (100%) had microbiologic and 8/8 (100%) had clinical failure Rifalazil, 25 milligrams: 5/5 (100%) had microbiologic and 3/5 (60%) had clinical failure Azithromycin: 1/7 (14%) had microbiologic and clinical failure |
| Haggerty et al, 2008 [68] | Cohort | 88 M. genitalium–positive US girls and women with PID attending outpatient clinics; aged 14–37 years | Microbiologic failure; clinical failure; follow-up (30 days) | Inpatient: cefoxitin (2 gram parenterally every 6 hours) plus Doxycycline (100 milligrams 2×/day × 14 days) Outpatient: Cefoxitin (2 gram intramuscular) plus Probenecid (1 gram) plus Doxycycline (100 milligrams 2×/day × 14 days) | Endometrium and/or cervix: 23/56 (41%) had microbiologic failure Endometrium: 14/32 (44%) had microbiologic failure Greater likelihood of clinical failure among women with M. genitalium in the endometrium (adjusted relative risk, 4.6; 95% CI 1.1–20.1) |
| Björnelius et al, 2008 [44] | Cohort | 159 M. genitalium–positive Norwegian and Swedish men with urethritis (n = 115) and women with cervicitis (n = 44) attending STD clinics; aged 18–61 years | Microbiologic failure; clinical failure; follow-up (20–56 days) | Doxycycline (200 milligrams stat plus 100 milligrams × 8 days) or Azithromycin (1 gram stat) Doxycycline failures: Extended Azithromycin (500 milligrams stat plus 250 milligrams × 4 days); Azithromycin failures: extended doxycycline (100 milligrams 2×/ day× 15 days) | Doxycycline: 63/76 (83%) of men and 17/27 (63%) of women had microbiologic failure; 54/75 (72%) of men had persisting signs and 45/67 (67%) had persisting symptoms, and 15/20 (75%) of women had clinical failure Azithromycin: 6/39 (15%) of men and 2/17 (12%) of women had microbiologic failure; 20/37 (54%) of men and 5/8 (63%) of women had persisting signs; 7/31 (23%) of men and 6/10 (60%) of women had persisting symptoms Extended Azithromycin: 2/47 (4%) of men and 0/6 (0%) of women had microbiologic failure Extended Doxycycline: 1/3 men (33%) and 1/1 woman (100%) had microbiologic failure |
| Jernberg et al, 2008 [87] | Cohort | 452 M. genitalium–positive Norwegian men with NGU (n = 234) and women with cervicitis (n = 218) attending STD clinics; age range NR | Microbiologic failure; follow-up (4–5 weeks) | Azithromycin (1 gram stat) or Azithromycin (1 gram stat plus 1 gram stat 5–7 days after 1st dose) or Ofloxacin (200 milligrams 2×/day × 10 days) or Moxifloxacin (400 milligrams × 7 days) Asymptomatic M. genitalium–positive: Azithromycin (500 milligrams plus 250 milligrams × 4 days) | Azithromycin, 1 gram: 39/183 (21%) had microbiologic failure Aazithromycin, 1 gram × 2: 10/38 (26%) had microbiologic failure Azithromycin for asymptomatic patients: 22/98 (22%) had microbiologic failure Ofloxacin: 5/9 (55%) had microbiologic failure Moxifloxacin: 0/3 (0%) had microbiologic failure |
| Bradshaw et al, 2008 [43] | Cohort | 120 M. genitalium–positive Australian men with urethritis (n = 102) and women with cervicitis (n = 18) attending STD clinic; age range NR | Microbiologic failure; follow-up (1 month) | Azithromycin (1 gram stat) Failures: moxifloxacin (400 milligrams × 10 days) | Azithromycin: 19/120 (16%) had microbiologic failure Moxifloxacin: 0/11 (0%) had microbiologic failure |
| Mena et al, 2009 [45] | Randomized trial | 78 M. genitalium–positive US men with NGU attending STD clinic; age range NR | Microbiologic failure; clinical failure; follow-up (1st: 10–17 days; 2nd: 31–41 days) | Azithromycin (1 gram stat) or Doxycycline (100 milligrams 2×/day × 7 days) Failures: Extended Azithromycin (500 milligrams stat plus 250 mg/d × 4 days) | Azithromycin: 3/23 (13%) had microbiologic and 6/23 (26%) had clinical failure Doxycycline: 17/31 (55%) had microbiologic and 10/31 (20%) had clinical failure Extended azithromycin: 2/5 (40%) had microbiologic and 1/5 (20%) had clinical failure |
| Schwebke et al, 2011 [86] | Randomized trial (double-blind) | 54 M. genitalium–positive US men attending 4 urban STD clinics; aged 16–45 years | Microbiologic failureb; follow-up (1st: 15–19 days; 2nd: 35–40 days) | Azithromycin (1 g stat; with or without tinidazole) or Doxycycline (100 milligrams 2×/d × 7 days with or without tinidazole) | Azithromycin: 15/45 (33.3%) had microbiologic failure; clinical failure NR Doxycycline: 27/39 (69.2%) had microbiologic failure; clinical failure NR |
| Takahashi et al, 2011 [89] | Cohort | 4 M. genitalium–positive Japanese men attending urology clinics; aged ≥18 years | Microbiologic failure; clinical failure; follow-up (1–3 weeks) | Levofloxacin (500 milligrams × 7 days) | Levofloxacin: 2/5 (40%) had microbiologic and 2/4 (50%) had clinical failure |
| Hamasuna et al, 2011 [90] | Cohort | 18 M. genitalium–positive Japanese outpatient men; aged ≥20 years | Microbiologic failure; clinical failure; follow-up (2–3 weeks) | Gatifloxacin (200 milligrams 2×/day × 7 days) | Gatifloxacin: 3/18 (17%) had microbiologic and 0/43 (0%) had clinical failure |
Abbreviations: NGU, nongonococcal urethritis; NR, not reported; PID, pelvic inflammatory disease; STD, sexually transmitted disease.
Except where otherwise noted, microbiologic failure was defined as detection of DNA by means of polymerase chain reaction in urine, urethral or cervical swab samples, or biopsy specimens at follow-up. Clinical failure was defined as partial clinical response to therapy [12], signs at follow-up [13], signs and/or symptoms at follow-up [16, 44], symptoms at follow-up [20, 40, 53, 90], ≥5 polymorphonuclear (PMN) leukocytes/high-power field (HPF) at follow-up [41, 89], persistent symptoms or ≥5 PMN leukocytes/HPF at follow-up [42], continued endometritis and pelvic pain at follow-up [68], or symptoms and/or discharge at examination plus ≥5 PMN leukocytes/HPF at follow-up [45].
In this study, microbiologic failure was defined as detection of RNA by Transcription Mediated Amplification in urine at follow-up.
Microbiologic cure rates achieved with doxycycline varied substantially across studies (17%–94%), whereas cure rates achieved with azithromycin (1 g) were higher and more consistent (67%–100%). Pooling data from all studies, doxycycline resulted in microbiologic cures in 88 (42%) of 212 treated men, compared with 371 (80%) of 466 men treated with a 1-gram dose of azithromycin. On the basis of existing published data, we conclude that a 1-gram dose of azithromycin is superior to 7 days of doxycycline in the treatment of M. genitalium infection. However, several recent studies have documented treatment failures in men with azithromycin-resistant M. genitalium infection [41, 43, 91]. Azithromycin may not be superior to doxycycline in areas that have a high prevalence of azithromycin-resistant M. genitalium. Unfortunately, little data currently exist on the epidemiology of azithromycin-resistant M. genitalium infection.
Longer Course Versus Single 1-gram Dose of Azithromycin for Treatment of M. genitalium Infection
No randomized trials have compared different azithromycin regimens. However, 2 observational studies have evaluated this issue [44, 87]. One conducted in Norway compared 3 different dosing regimens: 1 g once, 1 g followed by a second 1-gram dose in 5–7 days, and 500 milligrams once followed by 250 milligrams daily for 4 days [87]. Microbiologic cure occurred in 144 (79%) of 189, 20 (74%) of 30, and 72 (78%) of 98 patients given the 3 regimens, respectively. Another study conducted in Sweden compared a 1-gram dose of azithromycin in previously untreated men with 500 milligrams once followed by 250 milligrams daily for 4 days in men in whom doxycycline therapy had first failed [44]. Microbiologic cure occurred in 45 (96%) of 47 men given the extended regimen, compared with 33 (85%) of 39 men given the single dose (P = .11). Of note, a study of azithromycin-resistant M. genitalium (minimum inhibitory concentration, >8 μg/mL) found that, among 9 paired M. genitalium strains obtained before and after failed therapy with 1 gram of azithromycin, 7 expressed a resistance mutation only in the isolates obtained following treatment failure [91], suggesting that selective pressure may have resulted in the emergence of resistant organisms. Similar findings were reported from Japan [92–94] and New Zealand [95]. Existing data are insufficient to conclude that one azithromycin regimen is superior to another. However, at least one knowledgeable authority recommends that a 1.5-gram regimen given over the course of 5 days is preferable to a single 1-gram dose because of a possibly diminished risk of resistance associated with a longer course of treatment [96].
Effectiveness of Quinolones in the Treatment of M. genitalium Infection
Clinical and minimum inhibitory concentration data suggest that neither levofloxacin nor ofloxacin, alternative treatment regimens for NGU and cervicitis, are highly active against M. genitalium. Likewise, ciprofloxacin is not active against M. genitalium [40, 87, 91, 97–109]. Gatifloxacin seemed promising but was removed from the market because of serious adverse effects [110]. Moxifloxacin (400 milligrams for 7 or 10 days) has been used in at least 14 cases in which azithromycin and/or doxycycline treatment failed to eradicate the infection [41, 43, 87, 88], and no cases of clinical or microbiologic treatment failure have been reported. However, no clinical trials have evaluated moxifloxacin as a therapy for M. genitalium. Thus, on the basis of very limited observational data, moxifloxacin seems to be superior to both azithromycin and doxycycline for the treatment of diagnosed M. genitalium infection. In defining the role of moxifloxicin as a therapy for M. genitalium, this apparent superiority must be weighed against the high cost of the drug, the need for relatively prolonged therapy, and the relative paucity of data on the drug for this indication. We conclude that the quinolone class of antibiotics overall is not superior to azithromycin and doxycycline; levofloxacin, ofloxacin, and ciprofloxacin have lower cure rates than azithromycin. Moxifloxacin seems to be superior to other treatments for M. genitalium, but this conclusion is based on a small number of cases and the drug has not been tested in clinical trials.
Preferred Therapy for M. genitalium Infection
Based on small numbers of persons, the highest cure rates for M. genitalium urethritis seem to occur with moxifloxacin (400 milligrams daily for 7–10 days; cure rates, 100%). Azithromycin regimens, either a 1-gram dose or 500 milligrams once followed by 250 milligrams daily for 4 days, seem to be equivalent, although somewhat less effective than moxifloxacin (azithromycin cure rates range from 67% to 95%). All 3 of these regimens are superior to doxycycline. Treatment of M. genitalium urethritis is complicated by the absence of a commercially available assay; thus, in most cases, clinicians will only be able to treat discharge syndromes syndromically. However, in settings with the capacity to test for M. genitalium, we conclude that either azithromycin regimen is preferred over doxycycline. Because of the recent concerns about the emergence of azithromycin resistance, all regimens should be followed by clinical evaluation and a test of cure at 3–4 weeks. Azithromycin treatment failures should be treated with moxifloxacin.
Therapy for Discharge Syndromes and Pelvic Inflammatory Disease and the Potential Role Played by M. genitalium
M. genitalium seems to be responsible for 15%–20% of cases of NGU. If doxycycline and azithromycin are 50% and 80% effective, respectively, NGU microbiologic treatment failures would occur in an estimated 7.5%–10% and 3%–4.5%, respectively, of men with NGU receiving each drug. (Not all microbiologic treatment failures would lead to clinical failures; thus, this number would be somewhat lower when considering clinical failures.) Whether a difference of this magnitude justifies a change in empirical therapy is uncertain. Consultation with experts on M. genitalium undertaken in 2010 led to conflicting recommendations. One expert recommended that azithromycin should be the preferred agent for NGU on the basis of its superiority to doxycycline in the treatment of M. genitalium infection, whereas another expressed concern about single-dose azithromycin inducing resistance in M. genitalium, recommending that doxycycline should be the preferred therapy for NGU and extended-dose azithromycin should be given to persons with clinical treatment failure. There is little evidence about the clinical efficacy of moxifloxacin in the treatment of other causes of NGU, such as C. trachomatis [88], but existing data suggest that it is effective, and in vitro studies indicate that the drug is consistently active with a minimum inhibitory concentration of 0.015μg/ml–1 and a 90% minimum inhibitory concentration of 0.06μg/ml—values that are comparable to or lower than those of levofloxicin [109, 111–114]. We conclude that there is insufficient evidence to recommend any changes to the preferred regimens for NGU in recognition of the role played by M. genitalium. However, the alternative regimens should include moxifloxacin (400 milligrams daily for 7 days), and we recommend treatment with moxifloxacin in cases of M. genitalium urethritis that do not respond to first-line regimens (over currently specified alternate regimens).
Cross-sectional studies involving men seeking evaluation for persistent or recurrent NGU suggest that M. genitalium is responsible for 12%–41% of cases, and numerous clinical studies have documented that failure to eradicate M. genitalium is associated with persistent urethritis [12, 20, 40–45]. The 2006 guidelines suggest that men in whom NGU treatment fails should be evaluated for treatment adherence and reexposure, tested for Trichomonas vaginalis, treated for that pathogen, and receive azithromycin (1 g) if they were originally treated with doxycycline. We believe that it is common practice to treat persons in whom azithromycin fails with doxycycline, levofloxacin, or ofloxacin, which are of uncertain efficacy against M. genitalium. Because of the strong evidence that M. genitalium is associated with NGU in men and that failure to eradicate M. genitalium is associated with persistent urethritis, we conclude that men treated with azithromycin who have persistent NGU in the absence of nonadherence or reexposure should receive moxifloxicin (400 milligrams daily for 7) in addition to therapy for T. vaginalis.
The currently recommended therapies for cervicitis are azithromycin (single 1-gram dose) or doxycycline (100 milligrams twice daily for 7 days). Although the existing evidence suggests M. genitalium may play a role in cases of cervicitis [23, 47, 54–58, 60], it remains somewhat conflicting, and the prevalence of M. genitalium infection among women with cervicitis has been variable (ranging from 8% to 27%, with 1 exception [46]). Furthermore, in some settings, M. genitalium is significantly more common among women infected with C. trachomatis [49, 115], suggesting that the 2 organisms may be found together. The sole study that has directly examined treatment outcomes in M. genitalium–infected women with cervicitis showed that 55%–63% of women treated with doxycycline experienced microbiologic or clinical failure, compared with only 12% of women given azithromycin [44], suggesting that azithromycin is more effective against cervicitis associated with M. genitalium. Nevertheless, because the causative agent most commonly detected in cases of cervicitis remains C. trachomatis, the currently recommended therapies for cervicitis are likely to be adequate in most cases.
We conclude that existing evidence does not support a recommendation to alter the currently recommended therapies for cervicitis in recognition of the potential role played by M. genitalium. M. genitalium may be considered in cases of cervicitis that persist after treatment with doxycycline or azithromycin. Moxifloxacin should be considered for cases of clinically significant cervicitis that persist after azithromycin or doxycycline therapy in which reexposure to an infected partner or medical nonadherence are unlikely. As with persistent NGU, it is uncertain whether women with persistent cervicitis should first be treated with azithromycin or doxycycline (ie, the drug they did not initially receive) or whether clinicians should immediately use moxifloxicin in women in whom first-line therapy fails. When possible, women with persistent cervicitis should be tested for M. genitalium with the decision to treat with moxifloxicin based on the results of diagnostic testing. Because of the multiple causes of female urethritis and the limited amount of data on its association with M. genitalium, we do not recommend treatment with moxifloxacin for persistent cases of female urethritis in the absence of a positive diagnostic test result.
The currently recommended therapy for PID consists of cefotetan (2 grams intravenously every 12 hours), cefoxitin (2 grams intramuscularly single dose or intravenously every 6 hours), or ceftriaxone (250 milligrams intramuscularly) plus doxycycline (100 milligrams twice daily for14 days) with probenicid (1 gram) when cefoxitin is delivered intramuscularly. The PID Evaluation and Clinical Health (PEACH) study provides the sole evaluation of the effectiveness of this regimen against M. genitalium infection in women with clinically suspected PID; 41% of M. genitalium–positive women experienced microbiologic failure, and 44% experienced clinical failure [68], suggesting that the standard regimens are ineffective against M. genitalium. Nevertheless, the prevalence of M. genitalium infection among women with clinically suspected PID enrolled in the PEACH study was only 6.5%, less than half of the prevalence of C. trachomatis (14%) or N. gonorrhoeae (15%). Prevalence of M. genitalium infection was even lower (3.6%) among women in a multicenter study conducted in Europe and South Africa, whereas prevalences of C. trachomatis (42%) and N. gonorrhoeae (31.3%) infection were substantially higher [88]. Therefore, we conclude that, in the absence of an etiologic diagnosis for M. genitalium infection, the existing evidence does not support a recommendation to change the current therapies for PID. Persistent cases may respond to moxifloxacin (400 milligrams daily for 14 days).
DISCUSSION
M. genitalium is clearly associated with both acute and persistent/chronic urethritis in men, and men with urethritis who receive a diagnosis of M. genitalium infection should be treated for the infection. Available observational and randomized trial data have shown that azithromycin is superior to doxycycline in treating M. genitalium–associated urethritis, and M. genitalium should be suspected in cases of NGU that persist or recur after therapy with doxycycline. Despite the apparent superiority of azithromycin, there are increasing concerns over emerging resistance to this therapeutic agent, and only moxifloxacin has demonstrated 100% cure rates. This apparent high efficacy, however, must be balanced against the very limited amount of data available on treatment with moxifloxacin, the higher cost, and the longer duration of therapy (7–10 days, compared with a single dose for azithromycin).
Whether M. genitalium infection is associated with significant morbidity among women and requires therapy is the more pertinent and more difficult question to answer. The data associating cervicitis with M. genitalium remain conflicting. More importantly, although some data suggest that M. genitalium may cause PID and the sequelae of that condition (ectopic pregnancy, infertility, and adverse pregnancy outcomes), existing evidence is inconclusive. More definitive studies of the natural history of M. genitalium infection in women are required before we can determine that serious reproductive health outcomes occur, in whom they occur, and how often. This final issue—how often M. genitalium leads to adverse sequelae—is critical, because future decisions about the importance of screening for the infection will need to be based on cost-effectiveness analyses, and the results of these analyses will depend on the magnitude of the health risks associated with the infection. The POPI trial suggests that M. genitalium plays a small role in PID in high-income countries, but this may not be the case in lower-income countries with higher prevalence of M. genitalium infection [70].
The question of when to treat for M. genitalium is complicated by the lack of a commercially available diagnostic test. Although some laboratories offer M. genitalium tests, in the vast majority of settings, clinicians are forced to make decisions about M. genitalium treatment based on clinical syndromes without specific diagnostic testing. Because of the prevalence of M. genitalium infection among persons with STD syndromes and current evidence on the effectiveness of standard therapies, we believe that currently recommended first-line treatments for NGU, cervicitis, and PID should not be altered on the basis of considerations related to M. genitalium. However, moxifloxacin should be considered in persistent or recurrent cases of these syndromes in settings where M. genitalium testing is not available. In settings with access to testing, M. genitalium infection should be treated with azithromycin, followed by a test of cure. If persistent infection is documented or clinical treatment failure is apparent, patients should receive moxifloxacin therapy.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support.
This work was supported in part by the National Institutes of Health (NIAID U19 AI31448 and NIAID R01 AI072728).
Supplement sponsorship.
This article was published as part of a supplement entitled “Sexually Transmitted Disease Treatment Guidelines” sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest.
L. E. M. and M. R. G. have received study drugs from Pfizer and reagents and test kits from Gen-Probe. L. E. M. has received conference support from Bio-Rad. J. M. B. reports no conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1:1288–91. doi: 10.1016/s0140-6736(81)92461-2. [DOI] [PubMed] [Google Scholar]
- 2.Fraser CM, Gocayne JD, White O, et al. The minimal gene complement of Mycoplasma genitalium. Science. 1995;270:397–403. doi: 10.1126/science.270.5235.397. [DOI] [PubMed] [Google Scholar]
- 3.Gibson DG, Benders GA, Andrews-Pfannkoch C, et al. Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome. Science. 2008;319:1215–20. doi: 10.1126/science.1151721. [DOI] [PubMed] [Google Scholar]
- 4.Jensen JS, Uldum SA, Sondergard-Andersen J, Vuust J, Lind K. Polymerase chain reaction for detection of Mycoplasma genitalium in clinical samples. J Clin Microbiol. 1991;29:46–50. doi: 10.1128/jcm.29.1.46-50.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer HM, Gilroy CB, Furr PM, Taylor-Robinson D. Development and evaluation of the polymerase chain reaction to detect Mycoplasma genitalium. FEMS Microbiol Lett. 1991;61:199–203. doi: 10.1016/0378-1097(91)90551-k. [DOI] [PubMed] [Google Scholar]
- 6.Horner PJ, Gilroy CB, Thomas BJ, Naidoo RO, Taylor-Robinson D. Association of Mycoplasma genitalium with acute non-gonococcal urethritis. Lancet. 1993;342:582–5. doi: 10.1016/0140-6736(93)91411-e. [DOI] [PubMed] [Google Scholar]
- 7.Jensen JS, Orsum R, Dohn B, Uldum S, Worm AM, Lind K. Mycoplasma genitalium: a cause of male urethritis? Genitourin Med. 1993;69:265–9. doi: 10.1136/sti.69.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janier M, Lassau F, Casin I, et al. Male urethritis with and without discharge: a clinical and microbiological study. Sex Transm Dis. 1995;22:244–52. doi: 10.1097/00007435-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Deguchi T, Komeda H, Yasuda M, et al. Mycoplasma genitalium in non-gonococcal urethritis. Int J STD AIDS. 1995;6:144–5. doi: 10.1177/095646249500600219. [DOI] [PubMed] [Google Scholar]
- 10.Busolo F, Camposampiero D, Bordignon G, Bertollo G. Detection of Mycoplasma genitalium and Chlamydia trachomatis DNAs in male patients with urethritis using the polymerase chain reaction. New Microbiol. 1997;20:325–32. [PubMed] [Google Scholar]
- 11.Maeda S, Tamaki M, Nakano M, Uno M, Deguchi T, Kawada Y. Detection of Mycoplasma genitalium in patients with urethritis. J Urol. 1998;159:405–7. doi: 10.1016/s0022-5347(01)63933-8. [DOI] [PubMed] [Google Scholar]
- 12.Gambini D, Decleva I, Lupica L, Ghislanzoni M, Cusini M, Alessi E. Mycoplasma genitalium in males with nongonococcal urethritis: prevalence and clinical efficacy of eradication. Sex Transm Dis. 2000;27:226–9. doi: 10.1097/00007435-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Johannisson G, Enstrom Y, Lowhagen GB, et al. Occurrence and treatment of Mycoplasma genitalium in patients visiting STD clinics in Sweden. Int J STD AIDS. 2000;11:324–6. doi: 10.1177/095646240001100508. [DOI] [PubMed] [Google Scholar]
- 14.Keane FE, Thomas BJ, Gilroy CB, Renton A, Taylor-Robinson D. The association of Mycoplasma hominis, Ureaplasma urealyticum and Mycoplasma genitalium with bacterial vaginosis: observations on heterosexual women and their male partners. Int J STD AIDS. 2000;11:356–60. doi: 10.1258/0956462001916056. [DOI] [PubMed] [Google Scholar]
- 15.Björnelius E, Lidbrink P, Jensen JS. Mycoplasma genitalium in non-gonococcal urethritis–a study in Swedish male STD patients. Int J STD AIDS. 2000;11:292–6. doi: 10.1177/095646240001100504. [DOI] [PubMed] [Google Scholar]
- 16.Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and Ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995–1003. doi: 10.1086/319594. [DOI] [PubMed] [Google Scholar]
- 17.Totten PA, Schwartz MA, Sjostrom KE, et al. Association of Mycoplasma genitalium with nongonococcal urethritis in heterosexual men. J Infect Dis. 2001;183:269–76. doi: 10.1086/317942. [DOI] [PubMed] [Google Scholar]
- 18.Mena L, Wang X, Mroczkowski TF, Martin DH. Mycoplasma genitalium infections in asymptomatic men and men with urethritis attending a sexually transmitted diseases clinic in New Orleans. Clin Infect Dis. 2002;35:1167–73. doi: 10.1086/343829. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida T, Deguchi T, Ito M, Maeda S, Tamaki M, Ishiko H. Quantitative detection of Mycoplasma genitalium from first-pass urine of men with urethritis and asymptomatic men by real-time PCR. J Clin Microbiol. 2002;40:1451–5. doi: 10.1128/JCM.40.4.1451-1455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dupin N, Bijaoui G, Schwarzinger M, et al. Detection and quantification of Mycoplasma genitalium in male patients with urethritis. Clin Infect Dis. 2003;37:602–5. doi: 10.1086/376990. [DOI] [PubMed] [Google Scholar]
- 21.Falk L, Fredlund H, Jensen JS. Symptomatic urethritis is more prevalent in men infected with Mycoplasma genitalium than with Chlamydia trachomatis. Sex Transm Infect. 2004;80:289–93. doi: 10.1136/sti.2003.006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iser P, Read TH, Tabrizi S, et al. Symptoms of non-gonococcal urethritis in heterosexual men: a case control study. Sex Transm Infect. 2005;81:163–5. doi: 10.1136/sti.2004.010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anagrius C, Lore B, Jensen JS. Mycoplasma genitalium: prevalence, clinical significance, and transmission. Sex Transm Infect. 2005;81:458–62. doi: 10.1136/sti.2004.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradshaw CS, Tabrizi SN, Read TR, et al. Etiologies of nongonococcal urethritis: bacteria, viruses, and the association with orogenital exposure. J Infect Dis. 2006;193:336–45. doi: 10.1086/499434. [DOI] [PubMed] [Google Scholar]
- 25.Leung A, Eastick K, Haddon LE, Horn CK, Ahuja D, Horner PJ. Mycoplasma genitalium is associated with symptomatic urethritis. Int J STD AIDS. 2006;17:285–8. doi: 10.1258/095646206776790231. [DOI] [PubMed] [Google Scholar]
- 26.Yu JT, Tang WY, Lau KH, Chong LY, Lo KK. Asymptomatic urethral infection in male sexually transmitted disease clinic attendees. Int J STD AIDS. 2008;19:155–8. doi: 10.1258/ijsa.2007.007199. [DOI] [PubMed] [Google Scholar]
- 27.Moi H, Reinton N, Moghaddam A. Mycoplasma genitalium is associated with symptomatic and asymptomatic non-gonococcal urethritis in men. Sex Transm Infect. 2009;85:15–8. doi: 10.1136/sti.2008.032730. [DOI] [PubMed] [Google Scholar]
- 28.Gaydos C, Maldeis NE, Hardick A, Hardick J, Quinn TC. Mycoplasma genitalium compared to chlamydia, gonorrhoea and trichomonas as an aetiological agent of urethritis in men attending STD clinics. Sex Transm Infect. 2009;85:438–40. doi: 10.1136/sti.2008.035477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soni S, Alexander S, Verlander N, et al. The prevalence of urethral and rectal Mycoplasma genitalium and its associations in men who have sex with men attending a genitourinary medicine clinic. Sex Transm Infect. 2010;86:21–4. doi: 10.1136/sti.2009.038190. [DOI] [PubMed] [Google Scholar]
- 30.Taylor-Robinson D, Renton A, Jensen JS, et al. Association of Mycoplasma genitalium with acute non-gonococcal urethritis in Russian men: a comparison with gonococcal and chlamydial urethritis. Int J STD AIDS. 2009;20:234–7. doi: 10.1258/ijsa.2008.008298. [DOI] [PubMed] [Google Scholar]
- 31.Couldwell DL, Gidding HF, Freedman EV, et al. Ureaplasma urealyticum is significantly associated with non-gonococcal urethritis in heterosexual Sydney men. Int J STD AIDS. 2010;21:337–41. doi: 10.1258/ijsa.2009.009499. [DOI] [PubMed] [Google Scholar]
- 32.Berntsson M, Lowhagen GB, Bergstrom T, et al. Viral and bacterial aetiologies of male urethritis: findings of a high prevalence of Epstein-Barr virus. Int J STD AIDS. 2010;21:191–4. doi: 10.1258/ijsa.2009.009262. [DOI] [PubMed] [Google Scholar]
- 33.Hilton J, Azariah S, Reid M. A case-control study of men with non-gonococcal urethritis at Auckland Sexual Health Service: rates of detection of Mycoplasma genitalium. Sex Health. 2010;7:77–81. doi: 10.1071/SH09092. [DOI] [PubMed] [Google Scholar]
- 34.Pepin J, Sobela F, Deslandes S, et al. Etiology of urethral discharge in West Africa: the role of Mycoplasma genitalium and Trichomonas vaginalis. Bull World Health Organ. 2001;79:118–26. [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor-Robinson D, Jensen JS, Fehler G, Radebe F, Ballard RC. Observations on the microbiology of urethritis in black South African men. Int J STD AIDS. 2002;13:323–5. doi: 10.1258/0956462021925144. [DOI] [PubMed] [Google Scholar]
- 36.Sturm PD, Moodley P, Khan N, et al. Aetiology of male urethritis in patients recruited from a population with a high HIV prevalence. Int J Antimicrob Agents. 2004;24(Suppl 1):S8–14. doi: 10.1016/j.ijantimicag.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Black V, Magooa P, Radebe F, Myers M, Pillay C, Lewis DA. The detection of urethritis pathogens among patients with the male urethritis syndrome, genital ulcer syndrome and HIV voluntary counselling and testing clients: should South Africa's syndromic management approach be revised? Sex Transm Infect. 2008;84:254–8. doi: 10.1136/sti.2007.028464. [DOI] [PubMed] [Google Scholar]
- 38.Morency P, Dubois MJ, Gresenguet G, et al. Aetiology of urethral discharge in Bangui, Central African Republic. Sex Transm Infect. 2001;77:125–9. doi: 10.1136/sti.77.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo D, Zhu W, Zhang X, et al. Molecular epidemiologic study of Mycoplasma genitalium infection in high risk populations of sexually transmitted diseases in China. Chin Med J (Engl) 2000;113:1015–8. [PubMed] [Google Scholar]
- 40.Maeda SI, Tamaki M, Kojima K, et al. Association of Mycoplasma genitalium persistence in the urethra with recurrence of nongonococcal urethritis. Sex Transm Dis. 2001;28:472–6. doi: 10.1097/00007435-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Bradshaw CS, Jensen JS, Tabrizi SN, et al. Azithromycin failure in Mycoplasma genitalium urethritis. Emerg Infect Dis. 2006;12:1149–52. doi: 10.3201/eid1207.051558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamm WE, Batteiger BE, McCormack WM, Totten PA, Sternlicht A, Kivel NM. A randomized, double-blind study comparing single-dose rifalazil with single-dose azithromycin for the empirical treatment of nongonococcal urethritis in men. Sex Transm Dis. 2007;34:542–52. doi: 10.1097/01.olq.0000253348.44308.8c. [DOI] [PubMed] [Google Scholar]
- 43.Bradshaw CS, Chen MY, Fairley CK. Persistence of Mycoplasma genitalium following azithromycin therapy. PLoS One. 2008;3:e3618. doi: 10.1371/journal.pone.0003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Björnelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: a controlled clinical trial. Sex Transm Infect. 2008;84:72–6. doi: 10.1136/sti.2007.027375. [DOI] [PubMed] [Google Scholar]
- 45.Mena LA, Mroczkowski TF, Malanda N, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium positive urethritis in men. Clin Infect Dis. 2009;48:1649–54. doi: 10.1086/599033. [DOI] [PubMed] [Google Scholar]
- 46.Casin I, Vexiau-Robert D, De La Salmoniere P, Eche A, Grandry B, Janier M. High prevalence of Mycoplasma genitalium in the lower genitourinary tract of women attending a sexually transmitted disease clinic in Paris, France. Sex Transm Dis. 2002;29:353–9. doi: 10.1097/00007435-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Falk L, Fredlund H, Jensen JS. Signs and symptoms of urethritis and cervicitis among women with or without Mycoplasma genitalium or Chlamydia trachomatis infection. Sex Transm Infect. 2005;81:73–8. doi: 10.1136/sti.2004.010439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hogdahl M, Kihlstrom E. Leucocyte esterase testing of first-voided urine and urethral and cervical smears to identify Mycoplasma genitalium-infected men and women. Int J STD AIDS. 2007;18:835–8. doi: 10.1258/095646207782716983. [DOI] [PubMed] [Google Scholar]
- 49.Huppert JS, Mortensen JE, Reed JL, Kahn JA, Rich KD, Hobbs MM. Mycoplasma genitalium detected by transcription-mediated amplification is associated with Chlamydia trachomatis in adolescent women. Sex Transm Dis. 2008;35:250–4. doi: 10.1097/OLQ.0b013e31815abac6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Korte JE, Baseman JB, Cagle MP, et al. Cervicitis and genitourinary symptoms in women culture positive for Mycoplasma genitalium. Am J Reprod Immunol. 2006;55:265–75. doi: 10.1111/j.1600-0897.2005.00359.x. [DOI] [PubMed] [Google Scholar]
- 51.Hooton TM, Roberts MC, Roberts PL, Holmes KK, Stamm WE, Kenny GE. Prevalence of Mycoplasma genitalium determined by DNA probe in men with urethritis. Lancet. 1988;1:266–8. doi: 10.1016/s0140-6736(88)90350-9. [DOI] [PubMed] [Google Scholar]
- 52.Taylor-Robinson D, Gilroy CB, Thomas BJ, Hay PE. Mycoplasma genitalium in chronic non-gonococcal urethritis. Int J STD AIDS. 2004;15:21–5. doi: 10.1258/095646204322637209. [DOI] [PubMed] [Google Scholar]
- 53.Wikstrom A, Jensen JS. Mycoplasma genitalium: a common cause of persistent urethritis among men treated with doxycycline. Sex Transm Infect. 2006;82:276–9. doi: 10.1136/sti.2005.018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gaydos C, Maldeis NE, Hardick A, Hardick J, Quinn TC. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36:598–606. doi: 10.1097/OLQ.0b013e3181b01948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manhart LE, Critchlow CW, Holmes KK, et al. Mucopurulent cervicitis and Mycoplasma genitalium. J Infect Dis. 2003;187:650–7. doi: 10.1086/367992. [DOI] [PubMed] [Google Scholar]
- 56.Pepin J, Labbe AC, Khonde N, et al. Mycoplasma genitalium: an organism commonly associated with cervicitis among west African sex workers. Sex Transm Infect. 2005;81:67–72. doi: 10.1136/sti.2003.009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pepin J, Sobela F, Khonde N, et al. The syndromic management of vaginal discharge using single-dose treatments: a randomized controlled trial in West Africa. Bull World Health Organ. 2006;84:729–38. doi: 10.2471/blt.06.029819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uno M, Deguchi T, Komeda H, et al. Mycoplasma genitalium in the cervices of Japanese women. Sex Transm Dis. 1997;24:284–6. doi: 10.1097/00007435-199705000-00009. [DOI] [PubMed] [Google Scholar]
- 59.Palmer HM, Gilroy CB, Claydon EJ, Taylor-Robinson D. Detection of Mycoplasma genitalium in the genitourinary tract of women by the polymerase chain reaction. Int J STD AIDS. 1991;2:261–3. doi: 10.1177/095646249100200407. [DOI] [PubMed] [Google Scholar]
- 60.Moi H, Reinton N, Moghaddam A. Mycoplasma genitalium in women with lower genital tract inflammation. Sex Transm Infect. 2009;85:10–4. doi: 10.1136/sti.2008.032748. [DOI] [PubMed] [Google Scholar]
- 61.Lusk MJ, Konecny P, Naing ZW, Garden FL, Cumming RG, Rawlinson WD. Mycoplasma genitalium is associated with cervicitis and HIV infection in an urban Australian STI clinic population. Sex Transm Infect. 2011;87:107–9. doi: 10.1136/sti.2010.045138. [DOI] [PubMed] [Google Scholar]
- 62.Moller BR, Taylor-Robinson D, Furr PM. Serological evidence implicating Mycoplasma genitalium in pelvic inflammatory disease. Lancet. 1984;1:1102–3. doi: 10.1016/s0140-6736(84)92511-x. [DOI] [PubMed] [Google Scholar]
- 63.Lind K, Kristensen GB. Significance of antibodies to Mycoplasma genitalium in salpingitis. Eur J Clin Microbiol. 1987;6:205–7. doi: 10.1007/BF02018216. [DOI] [PubMed] [Google Scholar]
- 64.Jurstrand M, Jensen JS, Magnuson A, Kamwendo F, Fredlund H. A serological study of the role of Mycoplasma genitalium in pelvic inflammatory disease and ectopic pregnancy. Sex Transm Infect. 2007;83:319–23. doi: 10.1136/sti.2007.024752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen CR, Manhart LE, Bukusi EA, et al. Association between Mycoplasma genitalium and acute endometritis. Lancet. 2002;359:765–6. doi: 10.1016/S0140-6736(02)07848-0. [DOI] [PubMed] [Google Scholar]
- 66.Simms I, Eastick K, Mallinson H, et al. Associations between Mycoplasma genitalium, Chlamydia trachomatis, and pelvic inflammatory disease. Sex Transm Infect. 2003;79:154–6. doi: 10.1136/sti.79.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohen CR, Mugo NR, Astete SG, et al. Detection of Mycoplasma genitalium in women with laparoscopically diagnosed acute salpingitis. Sex Transm Infect. 2005;81:463–6. doi: 10.1136/sti.2005.015701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haggerty CL, Totten PA, Astete SG, et al. Failure of cefoxitin and doxycycline to eradicate endometrial Mycoplasma genitalium and the consequence for clinical cure of pelvic inflammatory disease. Sex Transm Infect. 2008;84:338–42. doi: 10.1136/sti.2008.030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bjartling C, Osser S, Persson K. The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. BJOG. 117:361–4. doi: 10.1111/j.1471-0528.2009.02455.x. [DOI] [PubMed] [Google Scholar]
- 70.Oakeshott P, Aghaizu A, Hay P, et al. Is Mycoplasma genitalium in women the “new chlamydia?” A community-based prospective cohort study. Clin Infect Dis. 2010;51:1160–6. doi: 10.1086/656739. [DOI] [PubMed] [Google Scholar]
- 71.Wiesenfeld HC, Martin DH, Mancuso M, Hillier SL, Amortegui A, Sweet RL. The association between Mycoplasma genitalium and subclinical pelvic inflammatory disease. London, United Kingdom: International Society for Sexually Transmitted Disease Reseach (ISSTDR); 2009. Abstract #P3.36. [Google Scholar]
- 72.Moller BR, Taylor-Robinson D, Furr PM, Freundt EA. Acute upper genital-tract disease in female monkeys provoked experimentally by Mycoplasma genitalium. Br J Exp Pathol. 1985;66:417–26. [PMC free article] [PubMed] [Google Scholar]
- 73.Moller BR, Taylor-Robinson D, Furr PM, Toft B, Allen J. Serological evidence that chlamydiae and mycoplasmas are involved in infertility of women. J Reprod Fertil. 1985;73:237–40. doi: 10.1530/jrf.0.0730237. [DOI] [PubMed] [Google Scholar]
- 74.Clausen HF, Fedder J, Drasbek M, et al. Serological investigation of Mycoplasma genitalium in infertile women. Hum Reprod. 2001;16:1866–74. doi: 10.1093/humrep/16.9.1866. [DOI] [PubMed] [Google Scholar]
- 75.Svenstrup HF, Fedder J, Kristoffersen SE, Trolle B, Birkelund S, Christiansen G. Mycoplasma genitalium, Chlamydia trachomatis, and tubal factor infertility–a prospective study. Fertil Steril. 2008;90:513–20. doi: 10.1016/j.fertnstert.2006.12.056. [DOI] [PubMed] [Google Scholar]
- 76.Grzesko J, Elias M, Maczynska B, Kasprzykowska U, Tlaczala M, Goluda M. Occurrence of Mycoplasma genitalium in fertile and infertile women. Fertil Steril. 2009;91:2376–80. doi: 10.1016/j.fertnstert.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 77.Kjaergaard N, Kristensen B, Hansen ES, et al. Microbiology of semen specimens from males attending a fertility clinic. APMIS. 1997;105:566–70. doi: 10.1111/j.1699-0463.1997.tb05054.x. [DOI] [PubMed] [Google Scholar]
- 78.Gdoura R, Kchaou W, Chaari C, et al. Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis and Mycoplasma genitalium infections and semen quality of infertile men. BMC Infect Dis. 2007;7:129. doi: 10.1186/1471-2334-7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gdoura R, Kchaou W, Ammar-Keskes L, et al. Assessment of Chlamydia trachomatis, Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis, and Mycoplasma genitalium in semen and first void urine specimens of asymptomatic male partners of infertile couples. J Androl. 2008;29:198–206. doi: 10.2164/jandrol.107.003566. [DOI] [PubMed] [Google Scholar]
- 80.Labbe AC, Frost E, Deslandes S, Mendonca AP, Alves AC, Pepin J. Mycoplasma genitalium is not associated with adverse outcomes of pregnancy in Guinea-Bissau. Sex Transm Infect. 2002;78:289–91. doi: 10.1136/sti.78.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oakeshott P, Hay P, Taylor-Robinson D, et al. Prevalence of Mycoplasma genitalium in early pregnancy and relationship between its presence and pregnancy outcome. BJOG. 2004;111:1464–7. doi: 10.1111/j.1471-0528.2004.00276.x. [DOI] [PubMed] [Google Scholar]
- 82.Kataoka S, Yamada T, Chou K, et al. Association between preterm birth and vaginal colonization by mycoplasmas in early pregnancy. J Clin Microbiol. 2006;44:51–5. doi: 10.1128/JCM.44.1.51-55.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Edwards RK, Ferguson RJ, Reyes L, Brown M, Theriaque DW, Duff P. Assessing the relationship between preterm delivery and various microorganisms recovered from the lower genital tract. J Matern Fetal Neonatal Med. 2006;19:357–63. doi: 10.1080/00207170600712071. [DOI] [PubMed] [Google Scholar]
- 84.Hitti J, Garcia P, Totten P, Paul K, Astete S, Holmes KK. Correlates of cervical Mycoplasma genitalium and risk of preterm birth among Peruvian women. Sex Transm Dis. 2010;37:81–5. doi: 10.1097/OLQ.0b013e3181bf5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Falk L, Fredlund H, Jensen JS. Tetracycline treatment does not eradicate Mycoplasma genitalium. Sex Transm Infect. 2003;79:318–9. doi: 10.1136/sti.79.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: emphasizing emerging pathogens–a randomized clinical trial. Clin Infect Dis. 2011;52:163–70. doi: 10.1093/cid/ciq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: an open study. Int J STD AIDS. 2008;19:676–9. doi: 10.1258/ijsa.2008.008038. [DOI] [PubMed] [Google Scholar]
- 88.Ross JD, Cronje HS, Paszkowski T, et al. Moxifloxacin versus ofloxacin plus metronidazole in uncomplicated pelvic inflammatory disease: results of a multicentre, double blind, randomised trial. Sex Transm Infect. 2006;82:446–51. doi: 10.1136/sti.2005.019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takahashi S, Ichihara K, Hashimoto J, et al. Clinical efficacy of levofloxacin 500 mg once daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2011;17:392–6. doi: 10.1007/s10156-010-0192-z. [DOI] [PubMed] [Google Scholar]
- 90.Hamasuna R, Takahashi S, Kiyota H, et al. Effect of gatifloxacin against Mycoplasma genitalium-related urethritis: an open clinical trial. Sex Transm Infect. 2011;87:389–90. doi: 10.1136/sti.2010.048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jensen JS, Bradshaw CS, Tabrizi SN, Fairley CK, Hamasuna R. Azithromycin treatment failure in Mycoplasma genitalium-positive patients with nongonococcal urethritis is associated with induced macrolide resistance. Clin Infect Dis. 2008;47:1546–53. doi: 10.1086/593188. [DOI] [PubMed] [Google Scholar]
- 92.Ito S, Shimada Y, Yamaguchi Y, et al. Selection of Mycoplasma genitalium strains harbouring macrolide resistance-associated 23S rRNA mutations by treatment with a single 1 g dose of azithromycin. Sex Transm Infect. 2011;87:412–4. doi: 10.1136/sextrans-2011-050035. [DOI] [PubMed] [Google Scholar]
- 93.Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255–8. doi: 10.1016/j.ijantimicag.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 94.Shimada Y, Deguchi T, Nakane K, et al. Macrolide resistance-associated 23S rRNA mutation in Mycoplasma genitalium, Japan. Emerg Infect Dis. 2011;17:1148–50. doi: 10.3201/eid1706.101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yew HS, Anderson T, Coughlan E, Werno A. Induced macrolide resistance in Mycoplasma genitalium isolates from patients with recurrent nongonococcal urethritis. J Clin Microbiol. 2011;49:1695–6. doi: 10.1128/JCM.02475-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jensen JC. Mycoplasma genitalium as an STI-consequences for patient management. London, United Kingdom: International Society for Sexually Transmitted Disease Research (ISSTDR); 2009. [Google Scholar]
- 97.Renaudin H, Tully JG, Bebear C. In vitro susceptibilities of Mycoplasma genitalium to antibiotics. Antimicrob Agents Chemother. 1992;36:870–2. doi: 10.1128/aac.36.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hannan PC. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol. 1998;47:1115–22. doi: 10.1099/00222615-47-12-1115. [DOI] [PubMed] [Google Scholar]
- 99.Bebear CM, Renaudin J, Charron A, et al. Mutations in the gyrA, parC, and parE genes associated with fluoroquinolone resistance in clinical isolates of Mycoplasma hominis. Antimicrob Agents Chemother. 1999;43:954–6. doi: 10.1128/aac.43.4.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hannan PC, Woodnutt G. In vitro activity of gemifloxacin (SB 265805; LB20304a) against human mycoplasmas. J Antimicrob Chemother. 2000;45:367–9. doi: 10.1093/jac/45.3.367. [DOI] [PubMed] [Google Scholar]
- 101.Bebear CM, Renaudin H, Bryskier A, Bebear C. Comparative activities of telithromycin (HMR 3647), levofloxacin, and other antimicrobial agents against human mycoplasmas. Antimicrob Agents Chemother. 2000;44:1980–2. doi: 10.1128/aac.44.7.1980-1982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bebear CM, Renaudin H, Charron A, Gruson D, Lefrancois M, Bebear C. In vitro activity of trovafloxacin compared to those of five antimicrobials against mycoplasmas including Mycoplasma hominis and Ureaplasma urealyticum fluoroquinolone-resistant isolates that have been genetically characterized. Antimicrob Agents Chemother. 2000;44:2557–60. doi: 10.1128/aac.44.9.2557-2560.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duffy LB, Crabb D, Searcey K, Kempf MC. Comparative potency of gemifloxacin, new quinolones, macrolides, tetracycline and clindamycin against Mycoplasma spp. J Antimicrob Chemother. 2000;45(Suppl 1):29–33. doi: 10.1093/jac/45.suppl_3.29. [DOI] [PubMed] [Google Scholar]
- 104.Deguchi T, Maeda S, Tamaki M, et al. Analysis of the gyrA and parC genes of Mycoplasma genitalium detected in first-pass urine of men with non-gonococcal urethritis before and after fluoroquinolone treatment. J Antimicrob Chemother. 2001;48:742–4. doi: 10.1093/jac/48.5.742. [DOI] [PubMed] [Google Scholar]
- 105.Pereyre S, Guyot C, Renaudin H, Charron A, Bebear C, Bebear CM. In vitro selection and characterization of resistance to macrolides and related antibiotics in Mycoplasma pneumoniae. Antimicrob Agents Chemother. 2004;48:460–5. doi: 10.1128/AAC.48.2.460-465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yasuda M, Maeda S, Deguchi T. In vitro activity of fluoroquinolones against Mycoplasma genitalium and their bacteriological efficacy for treatment of M. genitalium-positive nongonococcal urethritis in men. Clin Infect Dis. 2005;41:1357–9. doi: 10.1086/496983. [DOI] [PubMed] [Google Scholar]
- 107.Hamasuna R, Osada Y, Jensen JS. Antibiotic susceptibility testing of Mycoplasma genitalium by TaqMan 5′ nuclease real-time PCR. Antimicrob Agents Chemother. 2005;49:4993–8. doi: 10.1128/AAC.49.12.4993-4998.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Waites KB, Crabb DM, Duffy LB. Comparative in vitro activities of the investigational fluoroquinolone DC-159a and other antimicrobial agents against human mycoplasmas and ureaplasmas. Antimicrob Agents Chemother. 2008;52:3776–8. doi: 10.1128/AAC.00849-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bebear CM, de Barbeyrac B, Pereyre S, Renaudin H, Clerc M, Bebear C. Activity of moxifloxacin against the urogenital mycoplasmas Ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14:801–5. doi: 10.1111/j.1469-0691.2008.02027.x. [DOI] [PubMed] [Google Scholar]
- 110.Hamasuna R, Takahashi S, Kiyota H, et al. Effect of gatifloxacin against Mycoplasma genitalium-related urethritis: an open clinical trial. Sex Transm Infect. 2011;87:389–90. doi: 10.1136/sti.2010.048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Speciale A, Musumeci R, Blandino G, Milazzo I, Caccamo F, Nicoletti G. Minimal inhibitory concentrations and time-kill determination of moxifloxacin against aerobic and anaerobic isolates. Int J Antimicrob Agents. 2002;19:111–8. doi: 10.1016/s0924-8579(01)00486-1. [DOI] [PubMed] [Google Scholar]
- 112.Malay S, Roblin PM, Reznik T, Kutlin A, Hammerschlag MR. In vitro activities of BMS-284756 against Chlamydia trachomatis and recent clinical isolates of Chlamydia pneumoniae. Antimicrob Agents Chemother. 2002;46:517–8. doi: 10.1128/AAC.46.2.517-518.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miyashita N, Fukano H, Yoshida K, Niki Y, Matsushima T. In-vitro activity of moxifloxacin and other fluoroquinolones against Chlamydia species. J Infect Chemother. 2002;8:115–7. doi: 10.1007/s101560200019. [DOI] [PubMed] [Google Scholar]
- 114.Donati M, Rodriguez Fermepin M, Olmo A, D'Apote L, Cevenini R. Comparative in-vitro activity of moxifloxacin, minocycline and azithromycin against Chlamydia spp. J Antimicrob Chemother. 1999;43:825–7. doi: 10.1093/jac/43.6.825. [DOI] [PubMed] [Google Scholar]
- 115.Cohen CR, Nosek M, Meier A, et al. Mycoplasma genitalium infection and persistence in a cohort of female sex workers in Nairobi, Kenya. Sex Transm Dis. 2007;34:274–9. doi: 10.1097/01.olq.0000237860.61298.54. [DOI] [PubMed] [Google Scholar]
- 116.Haggerty CL, Totten PA, Astete SG, Ness RB. Mycoplasma genitalium among women with nongonococcal, nonchlamydial pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2006;2006:30184. doi: 10.1155/IDOG/2006/30184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.