Non-technical summary
Although the core feature in polycystic ovary syndrome (PCOS) is a hormonal imbalance, this syndrome is associated with obesity, insulin resistance, hypertension and endothelial dysfunction. This constellation of factors leads to greater risks for infertility, diabetes and cardiovascular disease. In this study we examine two important receptors involved in endothelial function, ET-A and ET-B, using the skin microcirculation to examine small blood vessel responses. We demonstrate that the ET-B receptor is likely to play an important role in the pathophysiology of cardiovascular disease in women with PCOS.
Abstract
Abstract
Endothelin-1 is elevated in women with polycystic ovary syndrome (PCOS), and may play a role in the endothelial dysfunction associated with PCOS. Endothelin-1 binds two receptor subtypes, endothelin A (ET-A) and endothelin B (ET-B). We hypothesized that ET-A mediates vasoconstriction in the cutaneous microvasculature in women with and without PCOS. We further hypothesized that while the ET-B receptors mediate vasodilatation in both groups of women, this response would be blunted in women with PCOS. During local skin warming, we used laser Doppler flowmetry combined with intradermal microdialysis to measure skin blood flow (SkBF) during graded ET-A (BQ-123) and ET-B (BQ-788) antagonist infusions in women with (n = 6) and without (n = 8) PCOS. In both groups, SkBF increased during local heating. The percentage of maximal SkBF–[BQ123] sigmoidal dose–response curve indicated a vasodilatory response as the concentration of the antagonist increased (Hill slope 4.96 ± 4.77, 4.74 ± 5.01; logED50 2.53 ± 0.09, 2.49 ± 0.09 nm, for PCOS and Control, respectively). In contrast, the % max SkBF–[BQ788] curve indicated a vasoconstrictive response (Hill slope –4.69 ± 3.85, –4.03 ± 3.85; logED50, 2.56 ± 0.09, 2.41 ± 0.12 nm, in PCOS and Control). Moreover, the SkBF–[BQ788] curve shifted to the right in women with PCOS, suggesting attenuated ET-B receptor mediated vasodilatation during local skin warming compared to Controls. Thus, the endothelium located ET-B receptors function similarly in women with and without PCOS, although with blunted responsiveness in women with PCOS. Our studies suggest that the lower ET-B receptor responsiveness associated with PCOS may reflect lower endothelial-mediated vasodilatation independent of generally lower vascular reactivity.
Introduction
Polycystic ovary syndrome (PCOS) is the most common reproductive endocrinopathy in young women, affecting 6–10% of women of reproductive age, and over 100 million women worldwide (Padmanabhan, 2009). At its core, PCOS is a hormonal imbalance, characterized by hyperandroidism and chronic amenorrhoea or oligomenorrhoea. Polycystic ovary syndrome is associated with metabolic disturbances including insulin resistance, dyslipidaemia, type II diabetes and android type obesity (Gambineri et al. 2002). One-third of non-diabetic women with PCOS develop the metabolic syndrome by 49 years old, although most of these women develop this before they reach the age of 40.
Endothelial dysfunction is common in women with PCOS, increasing their risk for atherosclerosis (Abbott et al. 2002). In women with PCOS, flow-mediated and nitrate-mediated vasodilatation of conduit arteries are attenuated compared to control women (Kravariti et al. 2005), and both insulin resistance and total testosterone (a primary androgen elevated in PCOS) are independent predictors of the reduced vasodilatation (Kravariti et al. 2005). Testosterone may exert its influence in both the vascular smooth muscle and the endothelium through actions on substances in the blood such as endothelin-1 (ET-1) (Kravariti et al. 2005), a vasoactive substance involved in endothelial function. Endothelin-1 is one of several circulating molecules of endothelial injury, and binds two receptor subtypes, endothelin A (ET-A) and endothelin B (ET-B). Endothelin A receptors are located in vascular smooth muscle and mediate vasoconstriction (Ariai et al. 1990; Lin et al. 1991). Although ET-B receptors are found in the vascular smooth muscle where they mediate vasoconstriction (Haynes et al. 1995), these receptors are also found in the endothelium where they can mediate vasodilatation (Ishikawa et al. 1994; Haynes, 1995).
Sex hormones can determine the localization of expression of ET receptor subtypes in the peripheral microvasculature (Kellogg et al. 2001). Kellogg et al. (2001) demonstrated that the ET-B receptor is involved in the control of resting tonic skin blood flow (SkBF) in both men and women. However, the ET-B receptor antagonist BQ-788 induced vasodilatation in men (Kellogg et al. 2001; Leslie et al. 2004) but vasoconstriction in women (Kellogg et al. 2001). These authors interpreted these findings to indicate that in cutaneous vessels the ET-B receptors in men are located primarily on the vascular smooth muscle and mediate vasoconstriction, while these same receptors in women are located primarily in the endothelium and mediate vasodilatation. In the case of hyperandrogenic women with PCOS, because of their unique hormonal profile (static elevations in oestrogens and testosterone, low progesterone exposure), the activities of the ET-A and ET-B subtype receptors are likely to be even more complex.
The skin is the largest and most accessible organ in humans, and the cutaneous circulation can be used as a surrogate of generalized microvascular function in varied populations (Holowatz et al. 2008). Several pathologies such as hypertension, insulin resistance, diabetes and renal disease, which are associated with endothelial dysfunction, manifest impaired microvascular function (Creager et al. 1990; Kruger et al. 2006; Holowatz et al. 2008). Recent data support the combined use of laser Doppler flowmetry and cutaneous microdialysis methodologies to study mechanisms involved in microvascular function (Holowatz et al. 2008). In the present studies, we measured SkBF using laser Doppler flowmetry combined with cutaneous microdialysis (Holowatz et al. 2008) to examine the receptor mechanism by which ET-1 influences peripheral microvascular function in women with and without PCOS. We hypothesized that infusing the ET-B competitive antagonist BQ-788 into the skin would decrease SkBF in a dose-dependent manner in women both with and without PCOS. We further hypothesized that the SkBF-[ET-B antagonist] dose–response curve would be shifted to the right in women with PCOS, indicating reduced endothelial ET-B receptor mediated vasodilatation. Finally, we hypothesized that PCOS would have little effect on the SkBF-[ET-A antagonist] dose–response curve indicating the changes in endothelial vasodilatation are independent of overall microvascular responsiveness.
Methods
Subjects
Ethical approval
We recruited six women with PCOS and eight women without PCOS (control). All women were non-smoking, and when interviewed to obtain their medical history indicated excellent health other than PCOS. Women without PCOS reported regular menstrual cycles (26–32 days) with no gynaecological abnormalities. All subjects gave written informed consent to participate in the study, which conformed to the guidelines contained in the Declaration of Helsinki and had prior approval by the Human Investigation Committee of Yale University School of Medicine.
All women underwent transvaginal ultrasound to either confirm the diagnosis of PCOS or exclude PCOS and polycystic appearing ovaries in the control women. Potential control women were also excluded if they had any of the symptoms or signs of PCOS (see below). Polycystic ovary syndrome subjects were admitted into the study based on the presence of hyperandrogenism in addition to one out of two cardinal characteristics of PCOS based on the Rotterdam Criteria (Trivax & Azziz, 2007). In order to be defined as having PCOS, in addition to androgen excess, at least one of the two following criteria was present: oligo/anovulation, defined as intermenstrual period of ≥45 days, or a total of ≤8 menses per year; or have polycystic ovaries. Polycystic ovaries were defined by the morphological appearance of 12 small follicles in the range of 3–9 mm mean diameter in the ovary on day 3 as determined by transvaginal ultrasound. We also excluded other disorders of the ovaries, adrenal and pituitary. Criteria were evaluated by an obstetrician/gynaecologist with over 18 years experience in this area (Dr Taylor). Subjects in both groups were not taking any medications.
Experimental protocol
To keep hormone exposure level consistent, all women without PCOS were tested in the first 5 days of a normal menstrual cycle. None of the women with PCOS were cycling, so they were tested at their convenience. Subjects participated in two experiments on separate days, one time for each ET receptor subtype antagonist (i.e. ET-A (BQ-123), ET-B (BQ-788)). The order of the tests was alternated among the subjects based on the order of entry into the investigation within their respective group.
Skin blood flow and microdialysis protocol
Skin blood flow tests for dose–response curves were conducted in an environmental chamber (Ta = 28°C). Subjects ate a diet controlled for water and sodium the night before and the morning of the skin blood flow test under each experimental condition (∼13 kcal (kg body weight)−1). Upon arrival, hydration state was immediately assessed from urine specific gravity, which was between 1.003 and 1.026 in all subjects. Following the urine sample, the subject was weighed to the nearest 10 g on a beam balance and positioned in the semi recumbent position in a dental chair modified to support the forearm. During a resting period the subjects were instrumented for the measurement of beat-to-beat arterial blood pressure (Pinaz method, Finometer, Finapress Medical Systems, Affligem, Belgium) and skin microdialysis (see below). After 1 h of seated rest, a blood sample was drawn to measure serum concentrations of 17β-oestradiol ([E2]S), progesterone ([P4]S), and total and free testosterone ([Ttotal]S) and ([Tfree]S).
To determine peripheral microvascular function, we measured red blood cell flux, an index of SkBF, at five different sites on the forearm using laser Doppler flowmetry (Doppler Monitor, PF 5020 LDPM Unit, Perimed AB, Stockholm, Sweden). The Doppler probes both measure SkBF and control local skin temperature. We chose to study SkBF coupled with cutaneous microdialysis because this method permits the study of a local and immediate SkBF response to the drug infusions. Moreover, because the responses are local, microdialysis substantially reduces the risks associated with venous infusion, which would require whole body exposure to the drugs.
Microdialysis probe placement
Under sterile conditions, five 27-gauge needles were inserted on the dorsal aspect of the forearm (intradermal). The entrance and exit sites were 2 cm apart, and the needles were 2 cm apart. Microdialysis probes were threaded through the lumen of the needle, after which the needle was removed, leaving the hollow fibre portion of the microdialysis probe in place under the skin. All five microdialysis probes were infused with 0.9% saline (2 μl min−1; Harvard microinfusion pump, Harvard Apparatus, Holliston, MA, USA) for 120 min after placement to allow for recovery from the microdialysis probe placement (Minson et al. 2001; Holowatz et al. 2008).
ET-A and ET-B dose–response curves
Following recovery, we measured resting SkBF for 15 min at all sites. Immediately after the resting measurements, BQ-123 or BQ-788 was infused (5 μl min−1) into the five sites at 75, 150, 300, 500 and 750 nm for 45 min. Following infusion of the blocking agents, resting SkBF was measured again for 15 min. At the end of the second resting period, the local heating devices (within the laser Doppler probes) were increased to 42°C to induce vasodilatation. Local heating of the skin is commonly used to evaluate microvascular function because skin warming induces rapid endothelium-dependent vasodilatation (Minson et al. 2001; Sokolnicki et al. 2007; Kellogg et al. 2008), and is indicative of changes in other vascular beds and of systemic diseases (Holowatz et al. 2008). While the probe temperatures were maintained at 42°C, we continued to infuse all microdialysis probes with their respective blocking agents. During local heating, the temperature in the probes was held constant until a clear plateau in SkBF was achieved (usually ∼35 min). After the plateau in SkBF had been achieved, we maintained this temperature for 5 min while continuously measuring SkBF, beat-to-beat blood pressure and heart rate. Each series of infusions was followed by a sodium nitroprusside (SNP) infusion (28 mM, 10 μl min−1) to determine maximal SkBF.
Blood analysis
An aliquot was transferred to a tube without anticoagulant for the determination of [E2]S, [P4]S, [Ttotal]S and [Tfree]S. The samples were centrifuged, frozen immediately and stored at –80°C until analysis. Serum concentrations of E2, P4, Ttotal and Tfree were measured using competitive binding radioimmunoassay methods. Intra- and interassay coefficients of variation for the mid-range standards for [E2]S (180 ± 13.1 pg ml−1) were 2.7% and 4.3% (Siemens Healthcare Diagnostics, Los Angeles, CA, USA), for [P4]S (3.5 ± 0.2 ng ml−1) were 2.4% and 2.6% (Siemens Healthcare Diagnostics). Intra- and interassay coefficients of variation for the low-range standards for [Ttotal]S (145 ± 15 ng dl−1) were 3.3% and 4.3% and [Tfree]S (3.5 ± 0.6 pg ml−1) were 7.0% and 6.0% (Siemens Healthcare Diagnostics).
Data analysis and statistics
Laser Doppler flowmetry data were recorded at 1000 Hz using PowerLab (ADInstruments, Bella Vista, NSW, Australia). After a plateau was attained within each probe following the local heating, the final 2 min of SkBF and mean arterial blood pressure (MAP) at each antagonist dose were used for analysis. Skin blood flow at each dose of blocking agent was calculated based on a percentage of the maximal vasodilatory response (heating with SNP) to normalize the data. Percentage of maximal SkBF was our indicator of peripheral microvascular responsiveness. To determine cutaneous microvascular response to the various levels of ET receptor subtype blocking, individual subject's standard log plots of %max SkBF versus ET receptor subtype antagonist infusion concentration were fitted to a sigmoidal curve (Wenner et al. 2011). BQ-123 and BQ-788 doses were transformed to logarithmic concentrations, and SkBF normalized so that baseline SkBF = 0%, and SkBF at the highest antagonist concentration (i.e. 1000 nm) = 100% for BQ-123, whereas baseline SkBF = 100%, and SkBF at the highest antagonist concentration = 0% for BQ-788 (Wenner et al. 2011). Dose–response curves were generated using a non-linear regression with variable slope. Because our primary variables of interest were Hill slope and logED50, constraints were set for the bottom (zero) and top (100) parameters of the curves (Prism v4, GraphPad, San Diego, CA, USA) to best fit parameters of the model. The ED50 and logED50 (dose where 50% of the drug has its maximal effect) were determined by non-linear regression curve fitting of dose–response data fitted to the equation Y = Ymin+ (Ymax–Ymin)/(1 +[ED50/X]n), where Ymin and Ymax are the minimal and maximal responses, respectively, X is the BQ-123 or BQ-788 concentration, and n is the Hill slope (Prism, GraphPad). Because our data were normally distributed, we analysed the differences between groups for the logED50 using Student's t test for unpaired data (Prism, GraphPad). Group comparisons were made using PASW Statistics 18 (IBM SPSS, Chicago, IL, USA). We also performed separate analyses with either BMI or kg body weight as a covariate (ANCOVA) in multivariate analysis because these variables were statistically different between the groups. Differences were considered statistically significant when P < 0.05. All data are presented as means ± SEM in graphs and means (SD) in tables.
Sample size calculation
Sample size calculations were based on our primary outcome variable of interest: % max SkBF. The desired statistical test is two-sided and we assumed an α level of 0.01 to account for multiple comparisons. No other studies have examined the impact of these antagonists on dose–response curves in women with or without PCOS. However, Kellogg et al. (2001) reported a percentage difference between men and women in resting SkBF using BQ-788 during microdialysis with laser Doppler techniques of 6 (3)%; our pilot data indicated similar effect size and error terms within subjects. Given six to eight women per group and α = 0.01, this effect size would allow us >80% power (0.883) for ANOVA to differentiate these changes from chance. For the BMI ANCOVA analysis, given six to eight women per group and α = 0.05, this effect size would allow us >80% power (0.830) to differentiate these changes from chance (G-Power 3.1, Franz Faul, Edgar Erdfelder, Albert-Georg Lang and Axel Buchner, Heinrich-Heine-University, Düsseldorf, Germany).
Results
Subjects
General subject characteristics were similar in control and PCOS groups (Table 1), although women with PCOS were heavier and had greater BMI. As expected, [Ttotal]S and [Tfree]S were greater in women with PCOS compared to control (Table 1). However [E2]S and [P4]S were similar between groups.
Table 1.
Subject characteristics
| PCOS | Control | |
|---|---|---|
| Age (years) | 24 (6) | 29 (11) |
| Weight (km) | 85.4 (25.2) | 70.3 (11.6)* |
| Height (cm) | 1.63 (0.03) | 1.65 (0.05) |
| BMI (kg m−2) | 32.1 (9.2) | 25.8 (3.2)* |
| [E2]S (pg ml−1) | 47.2 (19.9) | 38.7 (22.6) |
| [P4]S (ng ml−1) | 0.7 (0.3) | 1.4 (1.7) |
| [Ttotal]S (ng dl−1) | 43.1 (15.1) | 20.1 (13.1)* |
| [Tfree]S (pg ml−1) | 1.6 (1.0) | 0.2 (0.2)* |
Body mass index (BMI) and serum concentrations of 17β-oestradiol ([E2]S), progesterone ([P4]S), total [Ttotal]S) and free testosterone ([Tfree]S) for women with (PCOS) and without (Control) polycystic ovary syndrome. Data are presented as means (SD).
Difference between PCOS and control.
Skin blood flow studies
Baseline SkBF was lower in women with PCOS, but only on the day preceding BQ-788 infusions (Fig. 1). When we tested this effect using a multivariate ANCOVA with BMI as a covariate, there was no correlation between BMI with baseline SkBF, but the probability value increased to 0.132, and so it appears that the group difference was, at least in part, explained by BMI. Forty-five minutes of BQ-123 infusion increased pre-heating SkBF in control but not in women with PCOS (P < 0.05). However, a similar infusion of BQ-788 had little effect on pre-heating SkBF in either group (Fig. 1). Blood pressure, as determined by our beat-to-beat blood pressure monitor (Finometer) was unaffected by group or infusion days (PCOS, 72.1 (6.4) and 72.3 (9.2), for BQ-123 and BQ-788, respectively; Control, 67.6 (13.8) and 70.2 (7.2), for BQ-123 and BQ-788). Maximal blood flow was similar across infusion concentrations and between groups. While there was some variability across specific sites, this is to be expected with laser Doppler measurements (Table 3).
Figure 1. Percentage maximal skin blood flow (SkBF) prior to and following 45 min of infusion of either ET-A receptor antagonist (BQ-123) or ET-B receptor antagonist (BQ-788) via microdialysis in women with (PCOS) and without (Control) polycystic ovary syndrome.
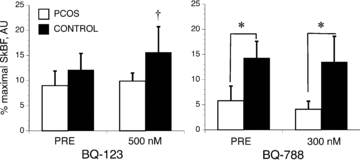
*Different between PCOS and Control. †Different between pre- and post-antagonist infusion. Data are presented as means ± SEM.
Table 3.
Maximal skin blood flow (AU) responses induced with combined heating and sodium nitroprusside infusion following stepwise infusion of the ET-B receptor antagonist (BQ-788) and the ET-A receptor antagonist (BQ-123) in women with (PCOS) and without (Control) polycystic ovary syndrome
| BQ788 750 nm | BQ788 500 nm | BQ788 300 nm | BQ788 150 nm | BQ788 75 nm | BQ123 750 nm | BQ123 500 nm | BQ123 300 nm | BQ123 150 nm | BQ123 75 nm | |
|---|---|---|---|---|---|---|---|---|---|---|
| PCOS | 1.0 (0.2) | 1.3 (0.6) | 1.5 (0.3) | 0.9 (0.6) | 2.3 (3.1) | 1.0 (0.7) | 1.4 (0.5) | 1.2 (0.5) | 0.7 (0.4) | 1.4 (0.7) |
| Control | 1.0 (0.6) | 1.0 (0.5) | 2.0 (3.3) | 0.9 (0.4) | 1.1 (0.9) | 0.7 (0.4) | 0.9 (0.6) | 1.5 (1.2) | 0.6 (0.4) | 1.8 (2.4) |
Data are presented as means (SD).
During BQ-123 infusion, blood flow increased in a sigmoidal fashion as the concentration of the blocker increased indicating that, as expected, the ET-A receptor mediates vasoconstriction in both groups of women (Fig. 2, Table 2). Moreover, the dose–response curves for the two groups were superimposed indicating that SkBF responses were similar between the two groups. In contrast, during BQ-788 infusion, blood flow decreased in a sigmoidal fashion as the concentration of the blocker increased indicating that the ET-B receptor mediates vasodilatation in both groups (Fig. 2, Table 2). In this case, the curve is shifted to the right in women with PCOS relative to the control group, suggesting an attenuated response to ET-B blockade during the local heating stimulus compared to the control group. Moreover, during the ET-B antagonist studies, SkBF responses at 300 nm were greater in the PCOS subjects (Fig. 3, P = 0.05), further demonstrating a blunted response to the ET-B receptor blockade. At the optimal infusion dose for ET-A blockade (500 nm of BQ-123), SkBF responses were similar between the groups.
Figure 2. Percentage of the maximal concentration of cutaneous vascular conductance (% SkBF max) as a function of the log concentration of the ET-A receptor antagonist (BQ-123) and the ET-B receptor antagonist (BQ-788) in women with (PCOS) and without (Control) polycystic ovary syndrome.
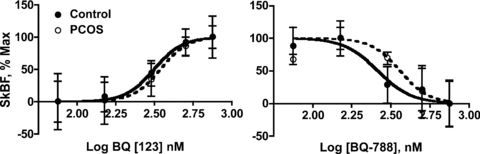
Data are presented as means ± SEM.
Table 2.
Dose–response curve parameters for skin blood flow (SkBF) during stepwise infusion of the ET-B receptor antagonist (BQ-788) and the ET-A receptor antagonist (BQ-123) in women with (PCOS) and without (Control) polycystic ovary syndrome
| ET-A | ET-B | |||
|---|---|---|---|---|
| PCOS | Control | PCOS | Control | |
| logED50 (nm) | 2.53 ± 0.09 | 2.49 ± 0.09 | 2.56 ± 0.09 | 2.41 ± 0.12 |
| ED50 (nm) | 342.3 | 312.1 | 358.8 | 258.8 |
| 95% CI | 221.6–528.8 | 204.9–475.5 | 141.0–473.8 | 233.5–551.4 |
| Slope (%max SkBF/log[ET]) | 4.96 ± 4.77 | 4.74 ± 5.01 | −4.69 ± 3.85 | −4.03 ± 3.85 |
Data are presented as mean (SD).
Figure 3. Percentage maximal skin blood flow (SkBF) with infusion of 500 nm of ET-A receptor antagonist [BQ-123] (left) or 300 nm of ET-B receptor antagonist [BQ-788] (right) via microdialysis in women with (PCOS) and without (Control) polycystic ovary syndrome.
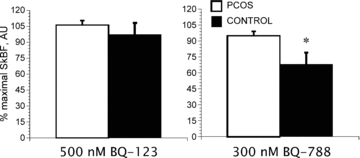
*Different between PCOS and Control. Data are presented as means ± SEM.
Discussion
Ours is the first study to demonstrate that in women with and without PCOS, administration of an ET-B antagonist attenuated vasodilatation in a dose–response manner. Thus, ET-B receptor-mediated vasodilatory actions in the endothelium predominate over ET-B-mediated vasoconstrictor actions in vascular smooth muscle in both groups of women in the cutaneous circulation. These data extend previous findings demonstrating a resting tonic dilator effect of ET-B in the cutaneous vasculature in normal healthy women (Kellogg et al. 2001). The BQ-788 decrease in SkBF during heating was attenuated in women with PCOS compared to controls, suggesting a reduction in the vasodilatory actions of ET-1 via the ET-B receptor. In contrast, ET-A receptor antagonist administration caused a dose-dependent vasodilatation during local heating similarly in both groups. As described in the Introduction, while both ET-A and ET-B subtype receptors are located in vascular smooth muscle where they mediate vasoconstriction (Ariai et al. 1990; Lin et al. 1991), only ET-B subtype receptors are present in the endothelium where they can mediate vasodilatation (Ishikawa et al. 1994). Our data suggest either a predominance of ET-B receptors or heightened sensitivity of these receptors in the endothelium in both groups of women, although with lower microvascular responsiveness in the women with PCOS. Previous data indicate that women have a greater proportion of ET-B receptors compared to men, suggesting sex hormones may modulate ET-B receptors (Ergul et al. 1998), which supports our current findings. Taking our ET-B and ET-A receptor blockade studies together, this attenuated cutaneous ET-B receptor responsiveness associated with PCOS may reflect lower endothelial-mediated vasodilatation relative to control subjects, independent of generally lower vascular reactivity.
The present data indicate an important contribution of the ET-B receptor to microvascular function in women both with and without PCOS. Microvascular dysfunction can impact both vascular resistance and insulin mediated glucose disposal in the periphery, thereby contributing to both hypertension and insulin resistance. The rightward shift in the SkBF-BQ 788 dose–response curve together with the attenuated vasoconstriction responses during 300 nm BQ-788 infusions suggests some level of peripheral microvascular dysfunction in women with PCOS, which is consistent with earlier findings in conduit arteries in this population (Kravariti et al. 2005) and is a key feature of cardiovascular disease risk associated with PCOS (Abbott et al. 2002). These findings in the peripheral circulation are especially important because these vessels impact glucose uptake so play an important role in insulin resistance, a primary pathology associated with PCOS.
We used local heating to examine the contribution of the ET system to microvascular function. Changes in SkBF in response local heating are biphasic, with the initial rapid phase predominantly mediated by local sensory nerves (Minson et al. 2001; Sokolnicki et al. 2007; Kellogg et al. 2008). When local heating is maintained, the 20–30 min plateau that results is mediated predominantly by nitric oxide generated from the endothelial nitric oxide synthase isoform (Kellogg et al. 2008) in normal subjects. However, the cutaneous response to local heating is complex, does not always involve nitric oxide, and integrates a number of systems including the sympathetic neurotransmitters noradrenaline and neuropeptide Y in both the initial peak and sustained plateau phases (Minson, 2010). Moreover, nitric oxide synthase inhibition does not fully suppress the plateau phase, indicating other vasodilator systems contribute to local heating induced vasodilatation. Although details of the mechanism of the local warming response in the skin have not been fully resolved, data support the combined use of laser Doppler flowmetry and microdialysis methodologies during local skin warming to describe microvascular function in varied populations, including healthy younger (Minson et al. 2001) and older adults (Holowatz, 2008), insulin resistance and diabetes (Sokolnicki et al. 2007), hypercholesterolaemia (Creager et al. 1990), renal disease (Kruger et al. 2006) and atherosclerosis (see Holowatz 2008 (Holowatz et al. 2008) for review).
Although we did not measure plasma ET-1 concentrations in this study, we previously found greater P[ET-1] in women with PCOS (1.45 versus 3.58 fmol ml−1 for control and PCOS, respectively) and that androgen administration increased ET-1 to a greater extent in women with PCOS than in controls (3.68 versus 7.10 fmol ml−1 for control and PCOS, respectively, following methyl testosterone administration) (Stachenfeld et al. 2010). Although a number of studies have demonstrated testosterone exposure may be involved in endothelial dysfunction in women (McCrohon et al. 1999; Orio et al. 2004), other studies have failed to demonstrate this relationship (Mather et al. 2000). Nonetheless, there is evidence to suggest that the hyperandrogenic state in PCOS is a risk factor for the development of early endothelial dysfunction (Orio et al. 2004). Endothelin-1 is lowest in the later follicular and the midluteal phases (Polderman et al. 2000) relative to the early follicular phase of a normal menstrual cycle suggesting that oestradiol exposure may impact plasma ET-1 concentration. Both oestrogen (Levin, 2001; Kim et al. 2008) and androgen (Kimura et al. 1993) receptors have been identified in vascular cells, and these receptors mediate reproductive hormone actions in endothelial and vascular smooth muscle cells. Moreover, plasma levels of ET-1 are higher in men than in women (Polderman et al. 1993). Taken together with the greater [Tfree]S in the present investigation, our data strengthen the earlier findings suggesting androgen exposure in women with PCOS is a contributing factor to changes in endothelial function in women with PCOS (Orio et al. 2004). While more studies are required to indicate the extent of involvement of either of these hormones on our data in the microcirculation, clearly the regulation by sex hormones of ET receptor subtypes plays an important role the peripheral vascular responses in women. Finally, while it is important to note the differences in P[ET-1], they are not necessarily representative of levels of ET-1 in the microvaculature, so future studies should include ET-1 dose–response curves with the ET-A and ET-B inhibitors.
These are the first data to define an optimal infusion dose for the endothelium receptor subtype antagonists during microdialysis studies to examine ET receptor subtypes or microvascular function in women with or without PCOS. The optimal dose to study these receptors using these antagonists is 500 and 300 nm for ET-A and ET-B, respectively, because we saw no further changes in SkBF after these concentrations indicating maximal inhibition of these receptors. These dose–response curves were necessary to determine the existence of a SkBF–ET-A/B relationship and also to define differences in the nature of this relationship between women with and without PCOS. We found that despite the shift of the ET-B dose–response curve to the right in women with PCOS, the optimal dose needed for maximal suppression did not differ between women with and without PCOS.
One limitation of laser Doppler flowmetry is its semi-quantitative nature, and therefore quantification and comparison of SkBF between groups can be challenging. Moreover, due to anatomical differences, there is variability in the level of SkBF across measurement sites, even when the sites are close to each other on the forearm (see Cracowski et al. 2006). For this reason, a percentage of a maximal value is often used to normalize the data. We used a standard method of combining local heating to 42°C while simultaneously administering sodium nitroprusside. It is important also to avoid using this measurement in patient populations in which maximal vasodilatation is inhibited. This was not the case in our PCOS subjects in the present study (Table 3).
Another limitation of our study is that we did not evaluate conduit artery endothelial function. Earlier studies examining endothelial function in women with PCOS using flow-mediated dilatation, a technique that measures conduit artery endothelial function by determining changes in flow during reactive hyperaemia (Raja-Khan et al. 2010), found endothelial function is compromised in women with PCOS, and this compromise was independent of insulin resistance, BMI and dyslipidaemia. Our study extends these earlier findings to the microcirculation and indicates that the ET-B receptor may be the primary receptor involved in endothelial function in women with PCOS. A third limitation is that the women in our PCOS group were heavier with higher BMI than our control group. We recruited our subjects irrespective of body weight, and there was a wide range of BMI in women with PCOS (20.6–42.7 kg m−2) and in the control (21.7–30.8 kg m−2) group, and both groups included obese and lean women. However, more of the women with PCOS were obese than in the control group, accounting for their greater mean body weight and BMI. To account for these differences statistically, we performed separate analyses with either BMI or body weight as a covariate in multivariate analysis when comparing key outcome variables. The use of BMI or body weight as covariates impacted only the comparisons of baseline SkBF suggesting our findings between the groups were, for the most part, independent of BMI. However, the use of the statistical device (ANCOVA) does not entirely remove the possibility that greater BMI may have influenced our findings. Thus, future studies examining these systems should consider body weight or adiposity.
Perspectives
The total cost of evaluating and providing care to reproductive-aged women with PCOS in the United States has been estimated at $4.36 billion, with much of the costs due to treatment related to the metabolic syndrome and consequent cardiovascular disease. While much of the treatment for PCOS focuses on infertility, it is clear that the cardiovascular, metabolic and psychological impact of this syndrome is profound. Thus understanding the extent to which their unique hormonal profile is involved in these clinical outcomes for women with PCOS is critical to reducing them. Endothelial dysfunction in the peripheral vascular system has been suggested as a primary mechanism for the cardiovascular and metabolic diseases in women with PCOS. Our data helped to define the receptor through which ET-1 acts within the microvasculature in women with PCOS and so may narrow the focus for treatment.
Acknowledgments
We gratefully acknowledge Cheryl Leone, MA and Andy Grabarek, BS for technical assistance, Gary Mack, PhD for input on study design and analysis, Osama Abdelghany, PharmD, BCOP, for microdialysis drug preparations, and the subjects for their time. This research was supported by NIH Grant R21 HL093450.
Glossary
Abbreviations
- BMI
body mass index
- ET-A
endothelin A
- ET-B
endothelin B
- ET-1
endothelin 1
- E2
oestradiol
- MAP
mean arterial blood pressure
- PCOS
polycystic ovary syndrome
- P4
progesterone
- SkBF
skin blood flow
- SNP
sodium nitroprusside
- Tfree
free testosterone
- Ttotal
total testosterone
Author contributions
M.M.W. participated in developing the concepts underlying this work, data collection and analysis, and the writing of the manuscript. H.S.T. provided medical supervision of the subjects, participated in developing the concepts underlying this work and the writing of the manuscript. N.S.S. participated in developing the concepts underlying this work, data collection, supervision and data analysis, and the writing of the manuscript. All authors approved the final version of the manuscript. All experiments were performed at The John B. Pierce Laboratory.
References
- Abbott DH, Dumesic DA, Franks S. Developmental origin of polycystic ovary syndrome. J Endocrinol. 2002;174:1–5. doi: 10.1677/joe.0.1740001. [DOI] [PubMed] [Google Scholar]
- Ariai H, Seiji H, Aramori I, Nakanishi O, Nakanishi S. Cloning and expression of a cDNA encoding endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci. 2006;27:503–508. doi: 10.1016/j.tips.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Creager MA, Cooke JP, Mendelsohn ME, Gallagher SJ, Coleman SM, Loscalzo J, Dzau VJ. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990;86:228–234. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergul A, Shoemaker K, Puett D, Tackett RL. Gender differences in the expression of endothelin receptors in human saphenous veins in vitro. J Pharmacol Exp Ther. 1998;285:511–517. [PubMed] [Google Scholar]
- Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord. 2002;26:883–896. doi: 10.1038/sj.ijo.0801994. [DOI] [PubMed] [Google Scholar]
- Haynes MP, Strachan FE, Webb DJ. Coronary heart disease/myocardial infarction/systemic vascular responses: endothelin ET sub A and ET sub B receptors cause vasoconstriction of human resistance and capacitance vessels in vivo. Circulation. 1995;92:357–363. doi: 10.1161/01.cir.92.3.357. [DOI] [PubMed] [Google Scholar]
- Haynes WG. Endothelins as regulators of vascular tone in man. Clin Sci (Colch) 1995;88:509–517. doi: 10.1042/cs0880509. [DOI] [PubMed] [Google Scholar]
- Holowatz LA. Human cutaneous microvascular ageing: potential insights into underlying physiological mechanisms of endothelial function and dysfunction. J Physiol. 2008;586:3301. doi: 10.1113/jphysiol.2008.157594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol. 2008;105:370–372. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Ihara M, Noguchi K, Mase T, Mino N, Saeki T, Furokoda T, Fukami T, Ozaki S, Nagase T, Nishikibe M, Yano M. Biochemical and pharmacological profile of a potent and selective endothelin β-receptor antagonist, BQ-788. Proc Natl Acad Sci U S A. 1994;91:4892–4896. doi: 10.1073/pnas.91.11.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Zhao JL, Wu Y. Endothelial nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. Am J Physiol Heart Circ Physiol. 2008;295:H123–H129. doi: 10.1152/ajpheart.00082.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DL, Liu Y, Pergola PE. Gender differences in the endothelin-B receptor contribution to basal cutaneous vascular tone in humans. J Appl Physiol. 2001;91:2407–2411. doi: 10.1152/jappl.2001.91.5.2407. [DOI] [PubMed] [Google Scholar]
- Kim KH, Moriarty K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids. 2008;73:864–869. doi: 10.1016/j.steroids.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura N, Mizokami A, Oonuma T, Sasano H, Nagura H. Immunocytochemical localization of androgen receptor with polyclonal antibody in paraffin-embedded human tissues. J Histochem Cytochem. 1993;41:671–678. doi: 10.1177/41.5.8468448. [DOI] [PubMed] [Google Scholar]
- Kravariti M, Naka KK, Kalantaridou SN, Kazakos N, Katsouras CS, Makrigiannakis A, Paraskevaidis EA, Chrousos GP, Tsatsoulis A, Michalis LK. Predictors of endothelial dysfunction in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:5088–5095. doi: 10.1210/jc.2005-0151. [DOI] [PubMed] [Google Scholar]
- Kruger A, Stewart J, Sahityani R, O'Riordan E, Thompson C, Adler S, Garrick R, Vallance P, Goligorsky MS. Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: correlation with cardiovascular risk. Kidney Int. 2006;70:157–164. doi: 10.1038/sj.ki.5001511. [DOI] [PubMed] [Google Scholar]
- Leslie SJ, Rahman MQ, Denvir MA, Newby DE, Webb DJ. Endothelins and their inhibition in the human skin microcirculation: ET[1-31], a new vasoconstrictor peptide. British J Clin Pharmacol. 2004;57:720–725. doi: 10.1111/j.1365-2125.2004.02074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Cell localization, physiology, and nongenomic actions of estrogen receptors. J Appl Physiol. 2001;91:1860–1867. doi: 10.1152/jappl.2001.91.4.1860. [DOI] [PubMed] [Google Scholar]
- Lin HY, Kaji EH, Winkel GK, Ives HE, Lodish HF. Cloning and functional expression of a vascular smooth muscle endothelin 1 receptor. Proc Natl Acad Sci U S A. 1991;88:3185–3189. doi: 10.1073/pnas.88.8.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather JK, Veram S, Corenblum B, Anderson T. Normal endothelial function despite insulin resistance in healthy women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 2000;85:1851–1856. doi: 10.1210/jcem.85.5.6587. [DOI] [PubMed] [Google Scholar]
- McCrohon JA, Jessup W, Handelsman DJ, Celermajer DS. Androgen exposure increases human monocyte adhesion to vascular endothelium and endothelial cell expression of vascular cell adhesion molecule-1. Circulation. 1999;99:2317–2322. doi: 10.1161/01.cir.99.17.2317. [DOI] [PubMed] [Google Scholar]
- Minson CT. Thermal provocation to evaluate microvascular reactivity in human skin. J Appl Physiol. 2010;109:1239–1246. doi: 10.1152/japplphysiol.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- Orio F, Palomba S, Cascella R, De Simone B, Di Biase S, Russo T, Laballa D, Zullo F, Lomardi G, Colao A. Early impairment of endothelial structure and function in young normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:4588–4593. doi: 10.1210/jc.2003-031867. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V. Polycystic ovary syndrome –“A riddle wrapped in a mystery inside an enigma”. J Clin Endocrinol Metab. 2009;94:1883–1885. doi: 10.1210/jc.2009-0492. [DOI] [PubMed] [Google Scholar]
- Polderman KH, Stehouwer CD, van Kamp GJ, Dekker GA, Verheught FWA, Gooren LJG. Influence of sex hormones on plasma endothelin levels. Ann Intern Med. 1993;118:429–432. doi: 10.7326/0003-4819-118-6-199303150-00006. [DOI] [PubMed] [Google Scholar]
- Polderman KH, Stehouwer CD, van Kamp GJ, Schalkwijk CG, Gooren LJ. Modulation of plasma endothelin levels by the menstrual cycle. Metabolism. 2000;49:648–650. doi: 10.1016/s0026-0495(00)80042-6. [DOI] [PubMed] [Google Scholar]
- Raja-Khan N, Shuja SA, Kunselman AR, Hogeman CS, Demers LM, Gnatuk CL, Legro RS. Brachial artery conductance during reactive hyperemia is increased in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2010;155:49–53. doi: 10.1016/j.ejogrb.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolnicki LA, Roberts SK, Wilkins BW, Basu A, Charkoudian N. Contribution of nitric oxide to cutaneous microvascular dilation in individuals with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2007;292:E314–318. doi: 10.1152/ajpendo.00365.2006. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS, Yeckel CW, Taylor HS. Greater exercise sweating in obese women with Polycystic Ovary Syndrome compared with obese controls. Med Sci Sports Exerc. 2010;42:1660–1668. doi: 10.1249/MSS.0b013e3181d8cf68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivax B, Azziz R. Diagnosis of polycystic ovary syndrome. Clin Obstet Gynecol. 2007;50:168–177. doi: 10.1097/GRF.0b013e31802f351b. [DOI] [PubMed] [Google Scholar]
- Wenner MM, Taylor HS, Stachenfeld NS. Progesterone enhances adrenergic control of skin blood flow in women with high but not low orthostatic tolerance. J Physiol. 2011;589:975–986. doi: 10.1113/jphysiol.2010.194563. [DOI] [PMC free article] [PubMed] [Google Scholar]


