Non-technical summary
Cardiovascular disease is responsible for 30% of deaths worldwide and epidemiological data demonstrate that poor growth before birth is associated with an increased risk of heart disease in adult life. We show that in response to reduced placental substrate supply there is an increase in cardiac insulin-like growth factor-2 (IGF-2) and the IGF-2 receptor (IGF-2R) in the fetus. Importantly, this effect is programmed because it is also present after birth in the lamb at 21 days of age. We also show that the increase in IGF-2 and IGF-2R gene expression is not epigenetically regulated through the IGF-2/H19 or IGF-2R methylation process. This study places the IGF-2 receptor signalling pathway as a prime candidate for mediating cardiac hypertrophy in fetal growth restriction before and after birth.
Abstract
Abstract
Reduced growth in fetal life together with accelerated growth in childhood, results in a ∼50% greater risk of coronary heart disease in adult life. It is unclear why changes in patterns of body and heart growth in early life can lead to an increased risk of cardiovascular disease in adulthood. We aimed to investigate the role of the insulin-like growth factors in heart growth in the growth-restricted fetus and lamb. Hearts were collected from control and placentally restricted (PR) fetuses at 137–144 days gestation and from average (ABW) and low (LBW) birth weight lambs at 21 days of age. We quantified cardiac mRNA expression of IGF-1, IGF-2 and their receptors, IGF-1R and IGF-2R, using real-time RT-PCR and protein expression of IGF-1R and IGF-2R using Western blotting. Combined bisulphite restriction analysis was used to assess DNA methylation in the differentially methylated region (DMR) of the IGF-2/H19 locus and of the IGF-2R gene. In PR fetal sheep, IGF-2, IGF-1R and IGF-2R mRNA expression was increased in the heart compared to controls. LBW lambs had a greater left ventricle weight relative to body weight as well as increased IGF-2 and IGF-2R mRNA expression in the heart, when compared to ABW lambs. No changes in the percentage of methylation of the DMRs of IGF-2/H19 or IGF-2R were found between PR and LBW when compared to their respective controls. In conclusion, a programmed increased in cardiac gene expression of IGF-2 and IGF-2R may represent an adaptive response to reduced substrate supply (e.g. glucose and/or oxygen) in order to maintain heart growth and may be the underlying cause for increased ventricular hypertrophy and the associated susceptibility of cardiomyocytes to ischaemic damage later in life.
Introduction
Reduced growth in fetal life together with accelerated growth in childhood, results in an increased risk of hypertension and a ∼50% greater risk of coronary heart disease in adult life (Fall et al. 1995; Barker et al. 2002, 2005). It is unclear why changes in growth patterns in early life can lead to a vulnerability to cardiovascular disease in adulthood (Kajantie et al. 2005; Rich-Edwards et al. 2005). It has therefore been proposed that a suboptimal intrauterine environment results in fetal adaptations, which act to maintain heart growth, but which result in permanent changes to cardiac structure and function (Barker, 1995; McMillen et al. 2005; Thornburg et al. 2008). In the human and sheep, heart growth in early gestation is predominantly due to the proliferation of mononucleated cardiomyocytes (Burrell et al. 2003). In late gestation, these cardiomyocytes differentiate to binucleated cardiomyocytes that then contribute to heart growth by hypertrophy (Zak, 1974; Smolich et al. 1989). Furthermore, Burrell et al. (2003) have shown that ∼90% of cardiomyocytes are binucleated in 4-week-old lambs. Thus, in the human and sheep, the heart contains most of the cardiomyocytes that it will have for life at birth (Woodcock & Matkovich, 2005). We (Morrison et al. 2007) and others (Bubb et al. 2007; Louey et al. 2007) have shown that fetal growth restriction is associated with a delay in binucleation of cardiomyocytes and in the presence of larger cardiomyocytes relative to heart size. Whilst left ventricular hypertrophy is the strongest predictor of progressive heart disease and poor cardiovascular outcomes in adult life (Levy et al. 1990), few studies have investigated the effects of fetal growth restriction on the expression of factors that regulate ventricular growth in early life.
Hyperplastic and hypertrophic growth of cardiomyocytes is regulated by a range of factors including the intra-cardiac insulin-like growth factors and their receptors (Han et al. 1988; Cheung et al. 1996). Insulin-like growth factors IGF-1 and IGF-2 promote growth predominantly through the IGF-1 receptor (IGF-1R) (Cohick & Clemmons, 1993). IGF-1 has been implicated in the initiation of ventricular hypertrophy in humans (Huang et al. 2003). In a range of in vivo (Lumbers et al. 2009) and in vitro (Huang et al. 2002a) experimental models, IGF-1 has been shown to act via IGF-1R to increase cardiomyocyte size (McMullen et al. 2004; Porrello et al. 2009). It has been shown that when the IGF-1R signalling pathway is blocked in vitro, the addition of IGF-2 also results in an increase in the size of cardiomyocytes (Huang et al. 2002b). This suggests that IGF-2 may act to stimulate hypertrophy through the IGF-2R, which is interesting considering that the IGF-2R has traditionally been viewed as a receptor which acts to clear IGF-2, rather than as a receptor that operates as part of a ligand-mediated growth pathway (Kornfeld, 1992).
Recent studies suggest that a suboptimal intrauterine environment can lead to epigenetic changes in the IGF-2/H19 and IGF-2R genes and associated changes in IGF-2 and IGF-2R gene expression (Li et al. 2010; Ollikainen et al. 2010; Zhang et al. 2010). To date, only one study has shown that fetal exposure to hypoxia leads to increased cardiac methylation and cardiac vulnerability in rats in adult life (Patterson et al. 2010). There have been no studies, however, which have investigated the epigenetic regulation of cardiac IGF-2 and IGF-2R in the growth-restricted sheep fetus.
In the sheep, experimental restriction of placental growth results in lower circulating fetal IGF-1 concentrations, but no change in IGF-1 and IGF-2 mRNA expression in the fetal heart at 120 days gestation (Kind et al. 1995). At this gestational age, however, there is relatively little fetal growth restriction present in the PR sheep as the impact of placental restriction occurs predominantly between 120 days gestation and term, when there is a doubling of body weight in the normally grown fetal sheep (Kind et al. 1995). We hypothesized that the adaptations that occur within the fetal heart in response to placental restriction include increased cardiac IGF-1R and IGF-2R gene and protein expression, and that increased cardiac IGF-2R expression is maintained into postnatal life, resulting in cardiomyocyte hypertrophy.
Methods
Ethical approval
All procedures were approved by The University of South Australia and University of Adelaide Animal Ethics Committees and complied with the Australian code of practice for the care and use of animals for scientific purposes. The authors have read, and the experiments comply with, the policies and regulations of The Journal of Physiology given by Drummond (2009).
Animals and surgery
Carunclectomy, surgical removal of the majority of the endometrial caruncles, was performed in 14 non-pregnant ewes as previously described (Edwards et al. 1999; Danielson et al. 2005; Morrison et al. 2007; Dyer et al. 2009) under aseptic conditions with general anaesthesia induced by an intravenous injection of sodium thiopentone (1.25 g ml−1, Boehringer Ingeheim, New South Wales, Australia) and maintained with 2–4% halothane in oxygen. At surgery, antibiotics were administered to the ewe (153.5 mg procaine penicillin, 393 mg benzathine penicillin, 500 mg dihydrostreptomycin, Lyppards, South Australia, Australia). After 10–12 weeks ewes entered a mating program. Pregnancy was confirmed by ultrasound at ∼60 days gestation.
Fetal study
Vascular surgery was performed in 11 of 16 control fetuses and in 11 of 11 PR fetuses at 105–115 days gestation as previously described (Edwards et al. 1999). Briefly, under anaesthesia (as above) catheters were implanted in the fetal carotid artery and jugular vein. A catheter was also inserted into the amniotic cavity. At surgery, antibiotics were administered to the ewe (as above) and fetus (150 mg procaine penicillin, 112.5 mg benzathine penicillin, 250 mg dihydrostreptomycin). Antibiotics were administered intramuscularly to each ewe for 3 days after surgery and to each fetus intra-amniotically (500 mg ampicillin; Lyppards) for 4 days after surgery. Animals were allowed to recover from surgery for at least 4 days prior to experimentation. Fetal arterial blood samples (0.5 ml) were collected daily to monitor pH, partial pressures of O2 and CO2 ( and
and  ), oxygen saturation (
), oxygen saturation ( ) and haemoglobin (Hb) content using an ABL520 analyser (Radiometer, Copenhagen, Denmark). The mean gestational arterial
) and haemoglobin (Hb) content using an ABL520 analyser (Radiometer, Copenhagen, Denmark). The mean gestational arterial  was calculated for each fetus as the mean of all arterial
was calculated for each fetus as the mean of all arterial  values collected from 4 days post surgery until 137–144 days. Fetuses were included in the control group if the ewe did not undergo carunclectomy surgery and they had a mean gestational
values collected from 4 days post surgery until 137–144 days. Fetuses were included in the control group if the ewe did not undergo carunclectomy surgery and they had a mean gestational  > 17 mmHg and in the PR group if they had a mean gestational
> 17 mmHg and in the PR group if they had a mean gestational  < 17 mmHg and their body weight was less than the 10th centile of the control group (Morrison et al. 2007; Dyer et al. 2009; Gentili et al. 2009).
< 17 mmHg and their body weight was less than the 10th centile of the control group (Morrison et al. 2007; Dyer et al. 2009; Gentili et al. 2009).
Lamb study
A frequency distribution curve of birth weights from 45 control Merino singleton lambs born during a 5 year period in our laboratory was used to categorise lamb birth weights as either average or low. The mean birth weight (±standard deviation (SD)) of the control singleton cohort was 5.63 ± 0.67 kg. Lambs in the current study were classified as average birth weight (ABW) if their birth weight was within 2 SD of this mean value (ABW: 4.9–6.7 kg, n = 13), or low birth weight (LBW) if their birth weight was more than 2 SD below the cohort mean (LBW: <4.9 kg, n = 8 (3 control and 5 PR); Table 1) (Duffield et al. 2009).
Table 1.
Mean blood gas, pH and Hb values across late gestation in control and PR fetuses
| Control (n = 11) | PR (n = 11) | |
|---|---|---|
 (mmHg) (mmHg) |
22.2 ± 0.7 | 13.8 ± 0.5 * |
 (mmHg) (mmHg) |
49.1 ± 0.7 | 52.8 ± 1.0 * |
| pH | 7.389 ± 0.007 | 7.361 ± 0.004 * |
 (%) (%) |
67.8 ± 2.0 | 35.5 ± 2.1 * |
| Hb (g dl−1) | 10.2 ± 0.3 | 11.4 ± 1.0 |
Values are means ± SEM.
Significant difference between control and PR groups (P < 0.05).
Post mortem
All animals were humanely killed with an overdose of sodium pentobarbitone (Lyppards) at 137–144 days gestation (fetal study) or at 21 days after birth (lamb study). Body and heart weights were recorded, and a sample of left ventricle was removed and snap frozen. In the fetal study, the remainder of the heart was perfused through the aorta with heparin and saturated potassium chloride, to prevent blood clotting and to arrest the heart in diastole. In lambs the left ventricle was weighed, flash frozen in liquid nitrogen and stored at −80°C. We defined relative left ventricle weight as left ventricle weight relative to body weight. Analyses were performed subject to tissue availability, and consequently sample size was not identical for all experiments.
Determination proportion of mononucleated cardiomyocytes and cardiomyocyte size
Cardiomyocytes were isolated from the heart as previously described (Barbera et al. 2000; Sundgren et al. 2003; Morrison et al. 2007) and fixed in 1% paraformaldehyde. Cardiomyocytes were stained with methylene blue (ProSiTech, Queensland, Australia) and examined using an Olympus VANOX-T microscope (Olympus Optical Co. Ltd, Tokyo, Japan). The relative proportion of mononucleated and binucleated cardiomyocytes was determined by counting a total of 300 cardiomyocytes. To determine cardiomyocyte size, the length and width of 50 mononucleated and 50 binucleated cardiomyocytes were assessed using AnalySIS software (Software Imaging System, South Australia, Australia) (Morrison et al. 2007). The area of the cardiomyocytes was calculated using the formula: Area = π× (0.5 × length) × (0.5 × width).
Marker of cardiomyocyte proliferation
Immunohistochemistry with a Ki67 antibody (DAKO, Dianova, Germany) was performed on fixed cells as previously described (Morrison et al. 2007). Random, non-repeating fields were analysed to determine the percentage of mononucleated cardiomyocytes that were positive for Ki67 in a population of 300 using an Olympus VANOX-T microscope.
RNA extraction and quantitative real-time RT-PCR
In a subset of samples for fetuses (control, n = 8; PR, n = 5) and for lambs (ABW, n = 12; LBW, n = 7), RNA was isolated from the left ventricle (∼100 mg) using Trizol reagent (Invitrogen Australia Pty Ltd, Victoria, Australia) and chloroform. RNA was treated for genomic DNA contamination using Ambion Turbo DNase (Ambion Inc., Austin, USA). The cDNA was synthesized from purified RNA (∼5 μg) with Superscript 3 reverse transcriptase (Invitrogen) and random hexamers.
Quantitative real-time RT-PCR was used to assess the relative abundance of IGF-1, IGF-2, IGF-1R and IGF-2R (Gentili et al. 2009). All real-time RT-PCR was carried out using the SYBR green fluorescence method in triplicate using an ABI Prism 7300 sequence detection system (PE Applied Biosystems, Foster City, USA). The 10 μl reaction mixture contained 5 μl Sybr Green Master Mix (PE Applied Biosystems), 1 μl of each primer (final concentration of 900 nm), 2.0 μl of molecular grade H2O and 1 μl of 50 ng μl−1 cDNA template or molecular grade H2O as a no-template control. The cycling conditions consisted of 40 cycles at 95°C for 15 s and 60°C for 1 min. The specificity of the products was documented with high-resolution gel electrophoresis and analysis of the melting temperatures. PCR products were purified with the QIAquick Spin Extraction Kit (Qiagen Pty Ltd, Australia, Victoria, Australia) and commercially sequenced (Sequencing Facility, Flinders Medical Centre, South Australia, Australia). Data are expressed as relative expression of target gene to acidic ribosomal-protein large subunit-P0 (RpP0) mRNA expression (Duffield et al. 2009; Gentili et al. 2009).
Protein extraction and IGF-1R and IGF-2R Western blotting
In a subset of samples (IGF-1R: control fetuses, n = 16; PR fetuses, n = 10; ABW lambs, n = 12; LBW lambs, n = 8; IGF-2R: control fetuses, n = 8; PR fetuses, n = 6; ABW lambs, n = 12; LBW lambs, n = 7), ∼50 mg of left ventricle tissue was homogenised (Kinematica PT-MR-3100, Lucerne, Switzerland) in 500 μl of homogenising buffer (MilliQ water; Millipore, New South Wales, Australia), 50 mm Tris pH 8, 150 mm NaCl, 1 mm sodium vanadate, 10 mm NaF, 0.6% Triton-X 100, a protease inhibitor tablet). The suspensions were centrifuged (Eppendorf Centrifuge 5415, Crown Scientific, Victoria, Australia) for 30 min at 15,700 g. Protein content of extracts was determined using a Micro BCA protein assay kit (Pierce, Thermo Fisher Scientific Inc., Rockford, USA) with bovine serum albumin (2 mg ml−1) to generate a standard curve.
The extracted protein was diluted in aliquots where 10× Reducing Agent (Invitrogen, pre-made) and 4× Sample Buffer (Invitrogen, pre-made) were added. The samples were then separated on NuPAGE Novex Bis-Tris 10% or 12% (15 wells Mini Gel, Invitrogen, USA) at 140 V for 1 h. Proteins were transferred onto a polyvinylidene difluoride membrane at 30 V for 1 h 15 min. The membranes were then washed 3 × 5 min with Tris-buffered saline (TBS) and blocked with 5% skimmed milk powder in Tris-buffered saline with 1% Tween (TBS-T) for 1 h at room temperature. The membranes were then washed 3 × 5 min with TBS-T and then incubated with the respective primary antibody: dilution of 1:1000 for anti-IGF-1R (Cell Signalling Technology, Inc., Massachusetts, USA) and 1:250 for anti-IGF-2R (BD Transduction Laboratories, New South Wales, Australia) overnight at 4°C with fast agitation. These membranes were washed 3 × 5 min in TBS-T. Membranes that were previously incubated with anti-IGF-1R were then incubated with a 1:2000 dilution of horse radish peroxidase (HRP)-labelled rabbit IgG (Cell Signalling Technology, Inc.), while membranes that were incubated with anti-IGF-2R were then incubated with a 1:2000 dilution HRP-labelled mouse IgG (Cell Signalling Technology, Inc.) for 1 h. The membranes were again washed with 3 × 5 min of TBS-T and the antigen-antibody complexes were detected by enhanced chemiluminescence. β-Actin (1:5000 dilution, Sigma Aldrich, New South Wales, Australia) was used as a loading control. Samples were re-run and analysed multiple times to avoid loading variability and there was no significant difference between protein abundance of β-actin loaded for control and PR in each gel. Each Western blot was stained with Ponceau S to visualize equal protein loading on each well. Bands were quantified by densitometry using software Quantity One (Bio-Rad, Hercules, USA).
Methylation analysis
In a subset of samples for fetuses (control, n = 8; PR, n = 5) and for lambs (ABW, n = 13; LBW, n = 8), DNA methylation within the DMRs of IGF-2/H19 and IGF-2R was analysed by combined bisulphite restriction assay (COBRA) (Xiong & Laird, 1997; Zhang et al. 2010). DNA (∼2 μg) from individual hearts was subjected to bisulphite conversion (Epitect, Qiagen, Basel, Switzerland). PCR was performed on 100 ng of bisulphite-converted DNA using primers and conditions that amplified methylated and unmethylated templates with no bias. For IGF-2/H19 we investigated three amplicons covering three proximal CCCTC-binding factor (CTCF) binding sites individually (GenBank Accession AJ566210, 1488-1778, Supplemental data: Table 1). For IGF-2R, the amplicon of a 148 bp fragment derived from intron 2 of the gene was examined (GenBank Accession AY182033, 1828-1976). COBRA was performed using restriction endonucleases that cleave only those amplicons derived from methylated templates. IGF-2/H19 and IGF-2R amplicons were digested with 20 U of either NruI, HinfI or MluI (New England Biolabs, Massachusetts, USA), for 2 h at 37°C. The intensity of uncut and cut fragments was quantified using an Experion automated electrophoresis system (Bio-Rad). Percentage of methylation was estimated by measuring the ratio of cut to uncut PCR product.
Statistical analysis
The effects of treatment and sex on the absolute and relative weights of organs and the relative expression of genes/proteins were determined using a 2-way ANOVA. In both the fetal and lamb studies, sex had no effect on any of the measured parameters; therefore data were analysed using Student's t test. Linear regression was used to determine the relationship between mean gestational  , fetal weight or heart weight with genes and proteins of interest. All analyses were performed using the program SPSS 18 for Windows (Statistical Package for Social Scientists Inc., Illinois, USA). Data are presented as the mean ± SEM. A probability of less than 5% (P < 0.05) was considered statistically significant.
, fetal weight or heart weight with genes and proteins of interest. All analyses were performed using the program SPSS 18 for Windows (Statistical Package for Social Scientists Inc., Illinois, USA). Data are presented as the mean ± SEM. A probability of less than 5% (P < 0.05) was considered statistically significant.
Results
Fetal study
Fetal growth outcomes
Placental restriction resulted in a significant reduction in fetal  and
and  during late gestation (Table 1) and a reduction in fetal body weight (Table 2), when compared to control fetuses. There was also a decrease in fetal heart weight in the PR fetuses, but no change in fetal heart weight relative to body weight between PR and control fetuses (Table 2). Fetal body weight and heart weight were each positively related to mean arterial
during late gestation (Table 1) and a reduction in fetal body weight (Table 2), when compared to control fetuses. There was also a decrease in fetal heart weight in the PR fetuses, but no change in fetal heart weight relative to body weight between PR and control fetuses (Table 2). Fetal body weight and heart weight were each positively related to mean arterial  (data not shown). Binucleated cardiomyocytes in the left ventricle were larger relative to heart weight in PR compared to control fetuses (Table 2). Fetal mean arterial
(data not shown). Binucleated cardiomyocytes in the left ventricle were larger relative to heart weight in PR compared to control fetuses (Table 2). Fetal mean arterial 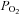 was significantly related to the size of binucleated cardiomyocytes relative to heart weight (Table 3).
was significantly related to the size of binucleated cardiomyocytes relative to heart weight (Table 3).
Table 2.
Morphometric measurements and cardiomyocyte proliferation and measurements in control and PR fetuses
| Control fetuses | PR fetuses | |
|---|---|---|
| Males:females | 10:6 | 5:6 |
| Age at post mortem (days) | 139.2 ± 0.7 (16) | 139.3 ± 0.5 (11) |
| Body weight at post mortem (kg) | 4.8 ± 0.2 (16) | 2.3 ± 0.2 * (11) |
| Crown rump length (cm) | 56.9 ± 0.7 (16) | 44.1 ± 1.3 * (11) |
| Abdominal circumference (cm) | 38.5 ± 0.8 (16) | 29.1 ± 1.3 * (9) |
| Heart weight (g) | 36.0 ± 1.3 (16) | 18.0 ± 1.2 * (11) |
| Relative heart weight (g kg−1) | 7.6 ± 0.3 (16) | 8.2 ± 0.5 (11) |
| Per cent mononucleated cardiomyocytes (%) | 43.4 ± 1.5 (15) | 57.9 ± 3.5 * (10) |
| Per cent Ki67+ mononucleated cardiomyocytes (%) | 1.7 ± 0.8 (7) | 2.0 ± 0.7 (9) |
| Relative binucleated cardiomyocyte size | ||
| Length/heart weight (μm g−1) | 2.4 ± 0.1 (12) | 4.7 ± 0.3 * (10) |
| Width/heart weight (μm g−1) | 0.3 ± 0.01(12) | 0.6 ± 0.04 * (10) |
| Area/heart weight (μm2 g−1) | 19.2 ± 0.6 (12) | 37.0 ± 3.0 * (10) |
Values are means ± SEM (n).
Significant difference between fetal groups (P < 0.05).
Table 3.
Relationships between mean gestational arterial  (mmHg) and IGF-1, IGF-2, IGF-1R, IGF-2R mRNA expression or the relative length, width and area of binucleated cardiomyocytes
(mmHg) and IGF-1, IGF-2, IGF-1R, IGF-2R mRNA expression or the relative length, width and area of binucleated cardiomyocytes
| Equation | r2 | P | |
|---|---|---|---|
| IGF-1 | y = −0.002x+ 0.403 | 0.077 | NS |
| IGF-2 | y = −0.206x+ 8.401 | 0.75 | 0.001 |
| IGF-1R | y = −0.038x+ 1.549 | 0.78 | 0.001 |
| IGF-2R | y = −0.028x+ 1.401 | 0.62 | 0.007 |
| Relative binucleated cardiomyocyte length | y = −0.234x+ 7.841 | 0.713 | 0.0001 |
| Relative binucleated cardiomyocyte width | y = −0.029x+ 0.967 | 0.68 | 0.0001 |
| Relative binucleated cardiomyocyte area | y = −1.761x+ 60.146 | 0.619 | 0.0001 |
IGF-1, IGF-2, IGF-1R and IGF-2R mRNA expression and protein abundance in the fetal heart
The expression of IGF-1 mRNA in the heart of PR fetuses was not significantly different from that of control fetuses (control, 0.355 ± 0.017; PR, 0.369 ± 0.023); however, the expression of IGF-2 was significantly higher in the heart of PR fetuses, when compared to controls (Fig. 1A). Cardiac IGF-2 mRNA expression was positively related to both the percentage of mononucleated cardiomyocytes (Fig. 1B) and the relative length of the binucleated cardiomyocytes (Fig. 1C). There was an increase in both cardiac IGF-1R mRNA expression (Fig. 2A) and protein abundance (Fig. 2B) in the PR compared to the control fetuses. Cardiac IGF-1R mRNA was positively related to the percentage of mononucleated cardiomyocyte (Fig. 2C). Furthermore, IGF-1R protein abundance was positively related to the relative length of the binucleated cardiomyocytes (Fig. 2D). There was an increase in cardiac IGF-2R mRNA expression (Fig. 3A), but not protein abundance (Fig. 3B), for PR compared to control fetuses. The IGF-2R mRNA expression was positively related to the relative length of the binucleated cardiomyocytes (Fig. 3C) but was not related to the per cent of mononucleated cardiomyocytes (y = 21.32x+ 29.94, r2 = 0.161, P = NS).
Figure 1. Cardiac IGF-2 mRNA expression in control and PR fetuses.
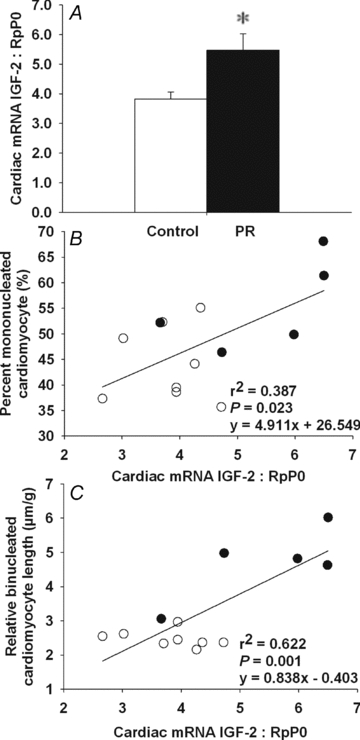
Cardiac IGF-2 mRNA expression normalized to RpP0 of PR and control fetuses (A). Relationship between cardiac IGF-2 mRNA expression and both the per cent mononucleated cardiomyocyte (B) and left ventricle relative binucleated cardiomyocyte length (C). Values are mean ± SEM. *Significantly different from control fetuses (P < 0.05). Control, open bars/circles; PR, filled bars/circles.
Figure 2. Cardiac IGF-1R mRNA and protein expression in control and PR fetuses.
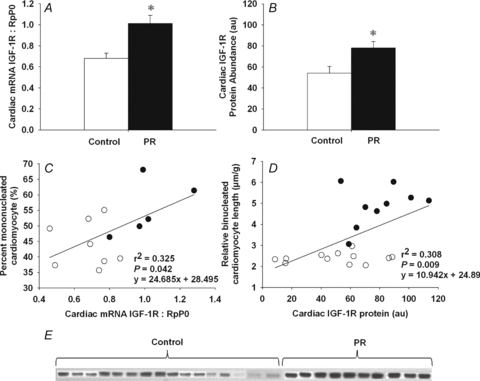
Cardiac IGF-1R mRNA expression normalized to RpP0 (A) and IGF-1R protein abundance (B) in PR and control fetuses. Relationship between IGF-1R protein abundance and both the per cent mononucleated cardiomyocyte (C) and left ventricle relative binucleated cardiomyocyte length (D). Western blot of cardiac IGF-1R protein in control and PR fetuses (E). Values are mean ± SEM. *Significantly different from control fetuses (P < 0.05). Control, open bars/circles; PR, filled bars/circles.
Figure 3. Cardiac IGF-2R mRNA and protein expression in control and PR fetuses.
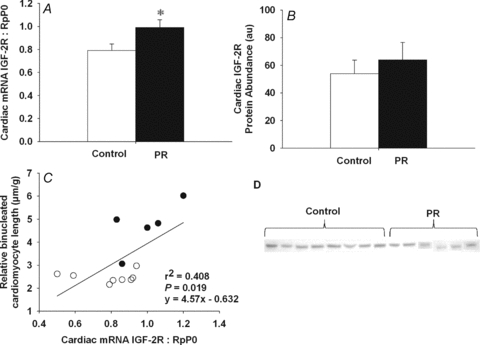
Cardiac IGF-2R mRNA expression normalized to RpP0 (A) and IGF-2R protein abundance (B) in PR and control fetuses. Relationship of cardiac IGF-2R mRNA expression to the relative length of binucleated cardiomyocytes (C). Western blot of cardiac IGF-2R protein in control and PR fetuses (D). Values are mean ± SEM. *Significantly different from control fetuses (P < 0.05). Control, open bars/circles; PR, filled bars/circles.
Lamb study
Lamb heart growth outcomes
LBW lambs had a lower body weight at 21 days after birth when compared to ABW lambs. Absolute heart and left ventricle weights of LBW lambs were lower while left ventricle weight relative to the body weight was greater when compared to ABW lambs at 21 days (Table 4).
Table 4.
Body weight, heart and ventricular weights ABW and LBW lambs at 21 days of age
| ABW lambs | LBW lambs | |
|---|---|---|
| Males:females | 8:5 | 3:5 |
| Birth weight (kg) | 5.85 ± 0.13 (13) | 3.80 ± 0.15* (8) |
| Age at post mortem (days) | 21 ± 0 | 21 ± 0 |
| Body weight at post mortem (kg) | 13.20 ± 0.18 (13) | 9.73 ± 0.40* (8) |
| Heart weight (g) | 79.80 ± 2.30 (13) | 63.98 ± 2.69* (8) |
| Relative heart weight (g kg−1) | 6.06 ± 0.18 (13) | 6.60 ± 0.21 (7) |
| Left ventricle weight (g) | 44.19 ± 1.82 (13) | 36.87 ± 0.87* (7) |
| Left ventricle weight relative to body weight (g kg−1× 103) | 3.34 ± 0.12 (13) | 3.78 ± 0.12* (7) |
Values are means ± SEM (n).
Significant difference between lamb groups (P < 0.05).
IGF-1, IGF-2, IGF-1R and IGF-2R mRNA expression and protein abundance in the lamb heart at 21 days
There was no difference in cardiac IGF-1 mRNA expression between LBW and ABW lambs (ABW, 0.146 ± 0.018; LBW, 0.145 ± 0.031), but cardiac IGF-2 mRNA expression was significantly higher in the LBW compared to ABW lambs (Fig. 4A). Cardiac IGF-2 mRNA expression was directly related to relative left ventricle weight (Fig. 4B). There was no difference in cardiac IGF-1R protein abundance between the LBW and ABW lambs (ABW, 73.3 ± 0.018; LBW, 95.8 ± 8.85) and there was no relationship between IGF-1R protein abundance and relative left ventricle weight in lambs (y = −0.07x+ 47.52, r2 = 0.153, P = NS). IGF-2R mRNA expression was higher in hearts from LBW compared to ABW lambs (Fig. 5A), but there was no significant difference in the abundance of IGF-2R protein between the two groups (Fig. 5B). The relationship between relative left ventricle weight and cardiac IGF-2R protein abundance was negative in the ABW lambs (Fig. 5C), but positive in the LBW lambs (Fig. 5D).
Figure 4. Cardiac IGF-2 mRNA expression in ABW and LBW lambs.
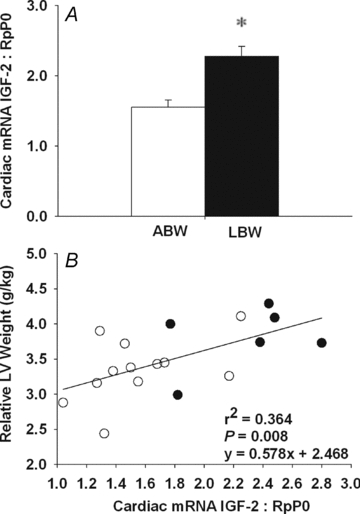
A, cardiac IGF-2 mRNA expression normalized to RpP0 in LBW and ABW lambs. B, relationship between cardiac IGF-2 mRNA expression and the relative weight of the left ventricle. Values are mean ± SEM. *Significantly different from ABW lambs (P < 0.05). ABW, open bars/circles; LBW, filled bars/circles.
Figure 5. Cardiac IGF-2R mRNA and protein expression in ABW and LBW lambs.
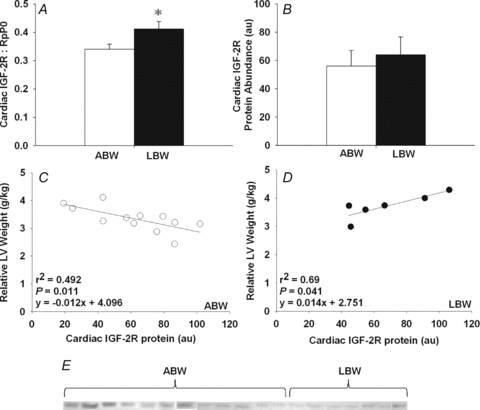
Cardiac IGF-2R mRNA expression normalized to RpP0 (A) and IGF-2R protein abundance (B) in LBW and ABW lambs. Relationship between cardiac IGF-2R protein abundance and relative left ventricle weight in ABW (C) and LBW lambs (D). Western blot of cardiac IGF-2R protein in ABW and LBW lambs (E). Values are mean ± SEM. *Significantly different from ABW lambs (P < 0.05). ABW, open bars/circles; LBW, filled bars/circles.
Methylation of IGF-2 and IGF-2R differentially methylated regions (DMR) before and after birth
There was no significant difference in the percentage of methylation of the DMRs investigated for IGF-2/H19 or IGF-2R between the control and PR groups, either before or after birth (Table 5).
Table 5.
The percentage of methylation of the IGF-2/H19 DMR and IGF-2R DMR in hearts of control and PR fetuses or ABW and LBW lambs
| CTCF binding site | Restriction enzyme | Control (n = 8) | PR (n = 5) | ABW (n = 13) | LBW (n = 8) |
|---|---|---|---|---|---|
| IGF-2/H19 2nd CTCF region | NruI | 17.4 ± 2.7 | 21.2 ± 2.1 | 20.7 ± 3.0 | 12.7 ± 1.5 |
| IGF-2/H19 3rd CTCF region | HinfI | 39.1 ± 9.8 | 63.1 ± 14.2 | 47.2 ± 6.7 | 50.7 ± 11.0 |
| IGF-2/H19 4th CTCF region | NruI | 29.8 ± 1.0 | 30.3 ± 0.9 | 23.2 ± 2.0 | 28.5 ± 1.7 |
| IGF-2R intron 2 DMR | MluI | 29.2 ± 0.7 | 33.8 ± 4.9 | 36.2 ± 3.8 | 32.2 ± 4.4 |
Values are mean ± SEM.
Discussion
Reduced placental substrate supply and cardiac growth
Reduced substrate supply leading to fetal growth restriction has been shown to delay cardiomyocyte binucleation (Bubb et al. 2007; Louey et al. 2007; Morrison et al. 2007) and result in an increased relative length, width and area of binucleated cardiomyocytes (Morrison et al. 2007). These data are consistent with studies in a number of sheep models of fetal growth restriction including placental insufficiency (Bubb et al. 2007; Louey et al. 2007; Morrison et al. 2007), maternal undernutrition (Dong et al. 2005) and fetal anaemia (Olson et al. 2006). Importantly, the current study shows that in the 21-day-old lamb there is also an increase in relative left ventricular weight.
IGFs in cardiac hypertrophy
Left ventricular hypertrophy is a prognostic indicator of poor cardiovascular outcomes (Levy et al. 1990; Brown et al. 2000). Left ventricular hypertrophy, which can improve cardiac pump function, can be either physiological (Rohini et al. 2009), such as the response to pregnancy and exercise, or pathological, if prolonged. IGF-1 causes physiological hypertrophy (McMullen, 2008) through its action on the IGF-1R and the phophoinositol-3 kinase subunit 110α (McMullen et al. 2004; Rohini et al. 2009), which have important roles in regulating cell growth (Evans-Anderson et al. 2008). In a range of experimental models, IGF-1 acts both in vivo (Lumbers et al. 2009) and in vitro (Huang et al. 2002a) to increase the size of cardiomyocytes (McMullen et al. 2004; Porrello et al. 2009) through IGF-1R activation although, in the fetus, activation of IGF-1R has been shown to increase either proliferation (Sundgren et al. 2003) or hypertrophy (Lumbers et al. 2009). Together, these data suggest that IGF-1 and IGF-1R both play an important role in both heart development and left ventricular hypertrophy during fetal life.
Reduced placental substrate supply and cardiac IGFs
Circulating levels of IGF-1 fall in fetuses exposed to periods of fetal substrate or nutrient restriction, irrespective of the cause or nature of the substrate deficit (Straus et al. 1991; Owens et al. 1994). Similarly, in fetal rats (Straus et al. 1991) and sheep (Kind et al. 1995), a decreased abundance of IGF-1 mRNA relative to IGF-2 mRNA has been observed in tissues such as liver, skeletal muscle, kidney and lung, during periods of nutrient or substrate restriction. While we have shown that there was no change in cardiac IGF-1 mRNA in the PR fetus or the LBW lamb, we did find a significant increase in IGF-2 mRNA expression in the hearts of the PR fetuses and LBW lambs. This is in contrast to a previous report in PR fetuses at 120 days gestation (Kind et al. 1995). The difference between the two studies may relate, in part, to the emergence of fetal growth restriction in the PR fetus between 120 days and 137–144 days gestation. In this and previous studies, the PR fetuses were half the weight of control fetuses at 140 days gestation (Edwards et al. 1999; Danielson et al. 2005), whereas only relatively small differences are present between fetal weights of PR and control fetuses at 120 days gestation (Kind et al. 1995). Together, these findings suggest that the increase in IGF-2 expression may be initiated in the heart of the growth-restricted fetus between 120 and 137–144 days gestation.
IGF-2 has been described as the dominant growth factor in fetal life due to the high abundance of IGF-2 mRNA and protein in many fetal tissues (Brown et al. 1986; Han et al. 1988; Hill, 1990) and high protein concentration in fetal serum (Gluckman et al. 1983; Owens et al. 1994). In our study, PR resulted in an increase in IGF-2, in the absence of changes in IGF-1 mRNA expression in the fetal heart, and the increase in IGF-2 was inversely related to the mean gestational fetal  in arterial blood. Given that IGF-2 mRNA expression normally decreases in the fetal heart between 60 days gestation and term (Cheung et al. 1996), it is possible that exposure of the fetus to chronic substrate restriction may act to delay or slow the normal physiological decline in IGF-2 mRNA expression in the fetal heart. The PR fetus is chronically hypoxaemic in late gestation and hypoxia has been shown to stimulate IGF-2 gene expression in endothelial progenitor cells (Maeng et al. 2009), consistent with the findings of the present study. It is interesting that increased cardiac IGF-2 mRNA expression was maintained in the LBW lamb because the hypoxaemia experienced by the PR fetus does not persist after the transition into postnatal life when the LBW lamb undergoes accelerated growth compared to the ABW lamb (Duffield et al. 2009).
in arterial blood. Given that IGF-2 mRNA expression normally decreases in the fetal heart between 60 days gestation and term (Cheung et al. 1996), it is possible that exposure of the fetus to chronic substrate restriction may act to delay or slow the normal physiological decline in IGF-2 mRNA expression in the fetal heart. The PR fetus is chronically hypoxaemic in late gestation and hypoxia has been shown to stimulate IGF-2 gene expression in endothelial progenitor cells (Maeng et al. 2009), consistent with the findings of the present study. It is interesting that increased cardiac IGF-2 mRNA expression was maintained in the LBW lamb because the hypoxaemia experienced by the PR fetus does not persist after the transition into postnatal life when the LBW lamb undergoes accelerated growth compared to the ABW lamb (Duffield et al. 2009).
Reduced placental substrate supply and cardiac IGF-1R
Increased IGF-2 mRNA expression in the heart may result in activation of the IGF-1R and/or the IGF-2R signalling pathways. In this study, we showed an increase in cardiac IGF-1R mRNA expression and protein abundance in the PR fetus but not in the LBW lamb when compared to control fetuses and ABW lamb, respectively. In vivo stimulation of IGF-1R in the fetal sheep heart by administration of an IGF-1 analogue, LONG™R3IGF-1, stimulates cardiomyocyte division and hyperplasia, but not hypertrophic growth (Sundgren et al. 2003). As IGF-2 also mediates its metabolic effects through the IGF-1R, an increase in cardiac IGF-2 mRNA might be expected to promote the proliferation of mononucleated cardiomyocytes and thus increase the number of these cells. It is of note that we found that there was an increased proportion of mononucleated cardiomyocytes present in both the left and right ventricles from the PR fetus, when compared to normally grown fetuses (Morrison et al. 2007). Dong et al. (2005) also reported that maternal nutrient restriction between 28–78 days gestation in the sheep resulted in an increase in IGF-1R protein levels in fetal heart tissue at 78 days, but not 135 days gestation. In contrast, in a model of fetal anaemia where there was fetal hypoxaemia, there was no change in protein expression for downstream proteins in the IGF-1R signalling pathway in the heart (Olson et al. 2006). Interestingly, in the present study, there was an inverse relationship between the expression of either IGF-1R in the heart and mean gestational fetal 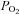 . This suggested that restriction of placental substrate supply acts directly or indirectly to regulate the expression of the IGF receptors within the fetal heart. Since placental restriction has been shown to result in a decrease in fetal plasma IGF-1 concentrations (Kind et al. 1995), the up-regulation of IGF-1R mRNA expression within the fetal heart may be a compensatory mechanism to maintain heart growth in PR fetuses. Therefore, it appears that another signalling pathway, rather than IGF-1R, must be involved in the cardiac hypertrophy effects of intrauterine growth restriction. (Rohini et al. 2009).
. This suggested that restriction of placental substrate supply acts directly or indirectly to regulate the expression of the IGF receptors within the fetal heart. Since placental restriction has been shown to result in a decrease in fetal plasma IGF-1 concentrations (Kind et al. 1995), the up-regulation of IGF-1R mRNA expression within the fetal heart may be a compensatory mechanism to maintain heart growth in PR fetuses. Therefore, it appears that another signalling pathway, rather than IGF-1R, must be involved in the cardiac hypertrophy effects of intrauterine growth restriction. (Rohini et al. 2009).
Reduced placental substrate supply, IGF-2R and cardiac hypertrophy
It has been shown in vitro that when the IGF-1R signalling pathway is blocked, addition of IGF-2 results in an increase in the size of cardiomyocytes (Huang et al. 2002b). Thus, IGF-2 may act to stimulate heart cell growth through the IGF-2R. This is interesting as IGF-2R has traditionally been viewed as a receptor which acts to clear IGF-2, rather than as a receptor that is part of a ligand-mediated growth pathway (Kornfeld, 1992). Pathological hypertrophy induced by endothelin-1 and angiotensin is mediated by their action on G protein-coupled (Gαq) receptors (McMullen, 2008; Rohini et al. 2009). IGF-2R is also a Gαq receptor which activates phospholipase C-β. This results in phosphorylation of PKC-α and Ca2+–calmodulin-dependent protein kinase II and can lead to pathological hypertrophy (Chu et al. 2008). Pathological ventricular hypertrophy begins as an adaptive response to increase cardiac function, but if this response is prolonged, it can lead to dilated cardiomyopathy, heart failure and sudden death. We have shown that PR increases IGF-2R mRNA, but not protein, expression in the fetus and that this is maintained in the heart of the LBW lamb. It is possible that a high turnover of IGF-2R protein limits the capacity to measure an increase in the abundance of IGF-2R protein in the PR or LBW groups compared to controls. This could suggest an up-regulation of lysosomal biogenesis and therefore increased degradation of the IGF-2R protein. We did, however, observe a negative relationship between IGF-2R protein abundance and relative left ventricle weight in the ABW lambs, consistent with other studies (Powell et al. 2005), suggesting that IGF-2R plays a traditional clearance role in ABW lamb. Importantly, however, there was a positive relationship between IGF-2R protein abundance and relative left ventricle weight in the LBW lamb, suggesting that the IGF-2R signalling pathway may be activated to result in ventricular hypertrophy in the LBW lamb.
Methylation of the DMR in the promoter region of IGF-2 and IGF-2R
IGF-2 and IGF-2R are imprinted genes in the sheep and are expressed in a parent-of-origin-specific manner from the paternal and maternal allele, respectively (Young et al. 2001; Reik et al. 2003; Dolinoy et al. 2007). Epigenetic modification plays a key role in the transmission of the parental identity of particular alleles through the germline by complex mechanisms typified by cytosine methylation of ‘imprinting control regions’ (ICR). For IGF-2, the ICR resides within a DMR 4 kb upstream of the neighbouring non-protein coding H19 gene. When the DMR is methylated, IGF-2 is expressed and when the DMR is unmethylated, IGF-2 expression is inhibited by H19 activity (Wood & Oakey, 2006). IGF-2R expression is tightly linked to the methylation state of a DMR located in intron 2 of the gene. When the DMR is methylated, IGF-2R is expressed and when unmethylated IGF-2R is silent (Wood & Oakey, 2006). Despite the increases in cardiac IGF-2 and IGF-2R mRNA expression present in the current study, we did not find evidence of epigenetic changes in classical sites for these genes in the heart of the PR fetus and LBW lamb. This suggests that mechanisms other than those related to the methylation of the relevant DMR regions are involved in the changes in expression of these genes, which are initiated in the growth-restricted fetus during late gestation.
Summary
Our study has demonstrated, for the first time that placental restriction results in increased IGF-2 and IGF-2R mRNA expression in the sheep heart, before and after birth. We speculate that in fetal life, an increase in cardiac IGF-2 activates both the IGF-1R and IGF-2R signalling pathways, because we observed delayed binucleation (IGF-1R-mediated event) and increased hypertrophy (IGF-2R-mediated event) of fetal cardiomyocytes. Given that IGFs play important roles in both proliferation and hypertrophy of cardiomyocytes, adaptive changes to maintain heart growth before birth in the PR fetus may have long-lasting consequences for the cardiomyocytes. The relevance of these changes to the etiology of intrauterine growth restriction-associated cardiovascular disease requires further exploration.
Acknowledgments
We are grateful to Laura O'Carroll and Jayne Skinner for their expert assistance during sheep surgery and the conduct of the protocols using the pregnant ewes and lambs in this study. We thank Lisa Nicholas for the validation of the methylation analyses at the three proximal CTCF binding sites in the IGF-2/H19 gene. We are also grateful to Darran Tosh for performing the COBRA assays. The authors have nothing to disclose. J.L.M. was supported by a Career Development Award from the NHF of Australia (CR07A3328) and the NHMRC (511341) and a South Australian Cardiovascular Research Network Fellowship (CR10A4988). D.A.B. was supported by a Senior Research Fellowship from the National Health and Medical Research Council (NHMRC) of Australia (349405). This work was funded by an NHMRC Program Grant Award (to I.C.M.).
Glossary
Abbreviations
- ABW
average birth weight
- COBRA
combined bisulphite restriction assay
- DMR
differentially methylated regions
- Gαq
G protein-coupled
- Hb
haemoglobin
- HRP
horse radish peroxidase
- ICR
imprinting control regions
- IGF-1
insulin-like growth factor-1
- IGF-1R
insulin-like growth factor-1 receptor
- IGF-2
insulin-like growth factor-2
- IGF-2R
insulin-like growth factor-2 receptor
- KB buffer
Kraft–Brühe buffer
- LBW
low birth weight
- PR
placentally restricted

oxygen saturation
- TBS
Tris-buffered saline
- TBS-T
Tris-buffered saline with 1% Tween
Author contributions
I.C.M., D.A.B. and J.L.M. were responsible for the conception and design of the experiments. K.W.C.W., L.Z., I.C.M., K.J.B., J.A.D., S.Z., C.M.S. and J.L.M. were each involved in data acquisition. K.W.C.W., L.Z., I.C.M., K.J.B., J.A.D., S.Z., C.M.S., D.A.B. and J.L.M. were involved in analysis and interpretation of the data. K.W.C.W, L.Z, I.C.M., D.A.B. and J.L.M. drafted the article and all authors contributed to and approved the final version.
References
- Barbera A, Giraud GD, Reller MD, Maylie J, Morton MJ, Thornburg KL. Right ventricular systolic pressure load alters myocyte maturation in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1157–R1164. doi: 10.1152/ajpregu.2000.279.4.R1157. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- Brown AL, Graham DE, Nissley SP, Hill DJ, Strain AJ, Rechler MM. Developmental regulation of insulin-like growth factor II mRNA in different rat tissues. J Biol Chem. 1986;261:13144–13150. [PubMed] [Google Scholar]
- Brown DW, Giles WH, Croft JB. Left ventricular hypertrophy as a predictor of coronary heart disease mortality and the effect of hypertension. Am Heart J. 2000;140:848–856. doi: 10.1067/mhj.2000.111112. [DOI] [PubMed] [Google Scholar]
- Bubb KJ, Cock ML, Black MJ, Dodic M, Boon WM, Parkington HC, Harding R, Tare M. Intrauterine growth restriction delays cardiomyocyte maturation and alters coronary artery function in the fetal sheep. J Physiol. 2007;578:871–881. doi: 10.1113/jphysiol.2006.121160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell JH, Boyn AM, Kumarasamy V, Hsieh A, Head SI, Lumbers ER. Growth and maturation of cardiac myocytes in fetal sheep in the second half of gestation. Anat Rec. 2003;274A:952–961. doi: 10.1002/ar.a.10110. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Johnson DD, Reyes V. Ontogeny of insulin-like growth factor-I and -II gene expression in ovine fetal heart. J Soc Gynecol Investig. 1996;3:309–315. [PubMed] [Google Scholar]
- Chu CH, Tzang BS, Chen LM, Kuo CH, Cheng YC, Chen LY, Tsai FJ, Tsai CH, Kuo WW, Huang CY. IGF-II/mannose-6-phosphate receptor signaling induced cell hypertrophy and atrial natriuretic peptide/BNP expression via Gαq interaction and protein kinase C-α/CaMKII activation in H9c2 cardiomyoblast cells. J Endocrinol. 2008;197:381–390. doi: 10.1677/JOE-07-0619. [DOI] [PubMed] [Google Scholar]
- Cohick WS, Clemmons DR. The insulin-like growth factors. Annu Rev Physiol. 1993;55:131–153. doi: 10.1146/annurev.ph.55.030193.001023. [DOI] [PubMed] [Google Scholar]
- Danielson L, McMillen IC, Dyer JL, Morrison JL. Restriction of placental growth results in greater hypotensive response to α-adrenergic blockade in fetal sheep during late gestation. J Physiol. 2005;563:611–620. doi: 10.1113/jphysiol.2004.080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Weidman JR, Jirtle RL. Epigenetic gene regulation: linking early developmental environment to adult disease. Reprod Toxicol. 2007;23:297–307. doi: 10.1016/j.reprotox.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Dong F, Ford SP, Fang CX, Nijland MJ, Nathanielsz PW, Ren J. Maternal nutrient restriction during early to mid gestation up-regulates cardiac insulin-like growth factor (IGF) receptors associated with enlarged ventricular size in fetal sheep. Growth Horm IGF Res. 2005;15:291–299. doi: 10.1016/j.ghir.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Drummond G. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JA, Vuocolo T, Tellam R, McFarlane JR, Kauter KG, Muhlhausler BS, McMillen IC. Intrauterine growth restriction and the sex specific programming of leptin and peroxisome proliferator-activated receptor γ (PPARγ) mRNA expression in visceral fat in the lamb. Pediatr Res. 2009;66:59–65. doi: 10.1203/PDR.0b013e3181a7c121. [DOI] [PubMed] [Google Scholar]
- Dyer JL, McMillen IC, Warnes KE, Morrison JL. No evidence for an enhanced role of endothelial nitric oxide in the maintenance of arterial blood pressure in the IUGR sheep fetus. Placenta. 2009;30:705–710. doi: 10.1016/j.placenta.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, Simonetta G, Owens JA, Robinson JS, McMillen IC. Restriction of placental and fetal growth in sheep alters fetal blood pressure responses to angiotensin II and captopril. J Physiol. 1999;515:897–904. doi: 10.1111/j.1469-7793.1999.897ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans-Anderson HJ, Alfieri CM, Yutzey KE. Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ Res. 2008;102:686–694. doi: 10.1161/CIRCRESAHA.107.163428. [DOI] [PubMed] [Google Scholar]
- Fall CH, Vijayakumar M, Barker DJ, Osmond C, Duggleby S. Weight in infancy and prevalence of coronary heart disease in adult life. BMJ. 1995;310:17–19. doi: 10.1136/bmj.310.6971.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili S, Morrison JL, McMillen IC. Intrauterine growth restriction and differential patterns of hepatic growth and expression of IGF1, PCK2, and HSDL1 mRNA in the sheep fetus in late gestation. Biol Reprod. 2009;80:1121–1127. doi: 10.1095/biolreprod.108.073569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Johnson-Barrett JJ, Butler JH, Edgar BW, Gunn TR. Studies of insulin-like growth factor -I and -II by specific radioligand assays in umbilical cord blood. Clin Endocrinol (Oxf) 1983;19:405–413. doi: 10.1111/j.1365-2265.1983.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Han VK, Lund PK, Lee DC, D'Ercole AJ. Expression of somatomedin/insulin-like growth factor messenger ribonucleic acids in the human fetus: identification, characterization, and tissue distribution. J Clin Endocrinol Metab. 1988;66:422–429. doi: 10.1210/jcem-66-2-422. [DOI] [PubMed] [Google Scholar]
- Hill DJ. Relative abundance and molecular size of immunoreactive insulin-like growth factors I and II in human fetal tissues. Early Hum Dev. 1990;21:49–58. doi: 10.1016/0378-3782(90)90110-5. [DOI] [PubMed] [Google Scholar]
- Huang CY, Buchanan DL, Gordon RL, Jr, Sherman MJ, Razzaq J, White K, Buetow DE. Increased insulin-like growth factor-I gene expression precedes left ventricular cardiomyocyte hypertrophy in a rapidly-hypertrophying rat model system. Cell Biochem Funct. 2003;21:355–361. doi: 10.1002/cbf.1040. [DOI] [PubMed] [Google Scholar]
- Huang CY, Hao LY, Buetow DE. Hypertrophy of cultured adult rat ventricular cardiomyocytes induced by antibodies against the insulin-like growth factor (IGF)-I or the IGF-I receptor is IGF-II-dependent. Mol Cell Biochem. 2002a;233:65–72. doi: 10.1023/a:1015514324328. [DOI] [PubMed] [Google Scholar]
- Huang CY, Hao LY, Buetow DE. Insulin-like growth factor-II induces hypertrophy of adult cardiomyocytes via two alternative pathways. Cell Biol Int. 2002b;26:737–739. doi: 10.1006/cbir.2002.0919. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Osmond C, Barker DJ, Forsen T, Phillips DI, Eriksson JG. Size at birth as a predictor of mortality in adulthood: a follow-up of 350 000 person-years. Int J Epidemiol. 2005;34:655–663. doi: 10.1093/ije/dyi048. [DOI] [PubMed] [Google Scholar]
- Kind KL, Owens JA, Robinson JS, Quinn KJ, Grant PA, Walton PE, Gilmour RS, Owens PC. Effect of restriction of placental growth on expression of IGFs in fetal sheep: relationship to fetal growth, circulating IGFs and binding proteins. J Endocrinol. 1995;146:23–34. doi: 10.1677/joe.0.1460023. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- Li CCY, Maloney CA, Cropley JE, Suter CM. Epigenetic programming by maternal nutritions: shaping future generations. Epigenomics. 2010;2:539–549. doi: 10.2217/epi.10.33. [DOI] [PubMed] [Google Scholar]
- Louey S, Jonker SS, Giraud GD, Thornburg KL. Placental insufficiency decreases cell cycle activity and terminal maturation in fetal sheep cardiomyocytes. J Physiol. 2007;580:639–648. doi: 10.1113/jphysiol.2006.122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumbers ER, Kim MY, Burrell JH, Kumarasamy V, Boyce AC, Gibson KJ, Gatford KL, Owens JA. Effects of intrafetal IGF-I on growth of cardiac myocytes in late-gestation fetal sheep. Am J Physiol Endocrinol Metab. 2009;296:E513–E519. doi: 10.1152/ajpendo.90497.2008. [DOI] [PubMed] [Google Scholar]
- McMillen IC, Adam CL, Muhlhausler BS. Early origins of obesity: programming the appetite regulatory system. J Physiol. 2005;565:9–17. doi: 10.1113/jphysiol.2004.081992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen JR. Role of insulin-like growth factor 1 and phosphoinositide 3-kinase in a setting of heart disease. Clin Exp Pharmacol Physiol. 2008;35:349–354. doi: 10.1111/j.1440-1681.2007.04873.x. [DOI] [PubMed] [Google Scholar]
- McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, Schinke M, Kong S, Sherwood MC, Brown J, Riggi L, Kang PM, Izumo S. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110α) pathway. J Biol Chem. 2004;279:4782–4793. doi: 10.1074/jbc.M310405200. [DOI] [PubMed] [Google Scholar]
- Maeng YS, Choi HJ, Kwon JY, Park YW, Choi KS, Min JK, Kim YH, Suh PG, Kang KS, Won MH, Kim YM, Kwon YG. Endothelial progenitor cell homing: prominent role of the IGF2-IGF2R-PLCβ2 axis. Blood. 2009;113:233–243. doi: 10.1182/blood-2008-06-162891. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Botting KJ, Dyer JL, Williams SJ, Thornburg KL, McMillen IC. Restriction of placental function alters heart development in the sheep fetus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R306–R313. doi: 10.1152/ajpregu.00798.2006. [DOI] [PubMed] [Google Scholar]
- Ollikainen M, Smith KR, Joo EJ, Ng HK, Andronikos R, Novakovic B, Abdul Aziz NK, Carlin JB, Morley R, Saffery R, Craig JM. DNA methylation analysis of multiple tissues from newborn twins reveals both genetic and intrauterine components to variation in the human neonatal epigenome. Hum Mol Genet. 2010;19:4176–4188. doi: 10.1093/hmg/ddq336. [DOI] [PubMed] [Google Scholar]
- Olson AK, Protheroe KN, Scholz TD, Segar JL. The mitogen-activated protein kinases and Akt are developmentally regulated in the chronically anemic fetal sheep heart. J Soc Gynecol Investig. 2006;13:157–165. doi: 10.1016/j.jsgi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Owens JA, Kind KL, Carbone F, Robinson JS, Owens PC. Circulating insulin-like growth factors-I and -II and substrates in fetal sheep following restriction of placental growth. J Endocrinol. 1994;140:5–13. doi: 10.1677/joe.0.1400005. [DOI] [PubMed] [Google Scholar]
- Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of PKC{ɛ} gene repression in rat hearts. Circ Res. 2010;107:365–373. doi: 10.1161/CIRCRESAHA.110.221259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Bell JR, Schertzer JD, Curl CL, McMullen JR, Mellor KM, Ritchie RH, Lynch GS, Harrap SB, Thomas WG, Delbridge LM. Heritable pathologic cardiac hypertrophy in adulthood is preceded by neonatal cardiac growth restriction. Am J Physiol Regul Integr Comp Physiol. 2009;296:R672–R680. doi: 10.1152/ajpregu.90919.2008. [DOI] [PubMed] [Google Scholar]
- Powell K, Mackie K, McEvoy T, Sinclair K, Robinson J, Ashworth C, Young L, Wilmut I, Rooke J. Effect of in vitro culture treatment and donor ewe diet on IGF2R expression in fetal tissues. Reprod Fertil Dev. 2005;17:265–265. [Google Scholar]
- Reik W, Constancia M, Fowden A, Anderson N, Dean W, Ferguson-Smith A, Tycko B, Sibley C. Regulation of supply and demand for maternal nutrients in mammals by imprinted genes. J Physiol. 2003;547:35–44. doi: 10.1113/jphysiol.2002.033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Edwards JW, Kleinman K, Michels KB, Stampfer MJ, Manson JE, Rexrode KM, Hibert EN, Willett WC. Longitudinal study of birth weight and adult body mass index in predicting risk of coronary heart disease and stroke in women. BMJ. 2005;330:1115. doi: 10.1136/bmj.38434.629630.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohini A, Agrawal N, Koyani CN, Singh R. Molecular targets and regulators of cardiac hypertrophy. Pharmacol Res. 2009;61:269–280. doi: 10.1016/j.phrs.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Smolich JJ, Walker AM, Campbell GR, Adamson TM. Left and right ventricular myocardial morphometry in fetal, neonatal, and adult sheep. Am J Physiol Heart Circ Physiol. 1989;257:H1–H9. doi: 10.1152/ajpheart.1989.257.1.H1. [DOI] [PubMed] [Google Scholar]
- Straus DS, Ooi GT, Orlowski CC, Rechler MM. Expression of the genes for insulin-like growth factor-I (IGF-I), IGF-II, and IGF-binding proteins-1 and -2 in fetal rat under conditions of intrauterine growth retardation caused by maternal fasting. Endocrinology. 1991;128:518–525. doi: 10.1210/endo-128-1-518. [DOI] [PubMed] [Google Scholar]
- Sundgren NC, Giraud GD, Schultz JM, Lasarev MR, Stork PJ, Thornburg KL. Extracellular signal-regulated kinase and phosphoinositol-3 kinase mediate IGF-1 induced proliferation of fetal sheep cardiomyocytes. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1481–R1489. doi: 10.1152/ajpregu.00232.2003. [DOI] [PubMed] [Google Scholar]
- Thornburg KL, Louey S, Giraud GD. The role of growth in heart development. Nestle Nutr Workshop Ser Pediatr Program. 2008;61:39–51. doi: 10.1159/000113169. [DOI] [PubMed] [Google Scholar]
- Wood AJ, Oakey RJ. Genomic imprinting in mammals: emerging themes and established theories. PLoS Genet. 2006;2:1677–1685. doi: 10.1371/journal.pgen.0020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock EA, Matkovich SJ. Cardiomyocytes structure, function and associated pathologies. Int J Biochem Cell Biol. 2005;37:1746–1751. doi: 10.1016/j.biocel.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Xiong Z, Laird PW. COBRA: a sensitive and quantitative DNA methylation assay. Nucl Acids Res. 1997;25:2532–2534. doi: 10.1093/nar/25.12.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LE, Fernandes K, McEvoy TG, Butterwith SC, Gutierrez CG, Carolan C, Broadbent PJ, Robinson JJ, Wilmut I, Sinclair KD. Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat Genet. 2001;27:153–154. doi: 10.1038/84769. [DOI] [PubMed] [Google Scholar]
- Zak R. Development and proliferative capacity of cardiac muscle cells. Circ Res. 1974;35:17–26. [PubMed] [Google Scholar]
- Zhang S, Rattanatray L, MacLaughlin SM, Cropley JE, Suter CM, Molloy L, Kleemann D, Walker SK, Muhlhausler BS, Morrison JL, McMillen IC. Periconceptional undernutrition in normal and overweight ewes leads to increased adrenal growth and epigenetic changes in adrenal IGF2/H19 gene in offspring. FASEB J. 2010;24:2772–2782. doi: 10.1096/fj.09-154294. [DOI] [PubMed] [Google Scholar]


