Abstract
We previously produced human monoclonal antibody Fab fragments specific to Entamoeba histolytica in Escherichia coli. In order to use these Fab fragments for diagnostic purposes, an expression vector to produce a fusion protein of Fab and alkaline phosphatase (PhoA) in E. coli was designed and constructed. The E. coli PhoA gene was fused to the 3′ terminus of the gene encoding the heavy-chain Fd region. The kappa and Fd genes from a previously prepared antibody clone, CP33, which is specific for the 260-kDa lectin of E. histolytica, were used as human antibody genes. When the fusion protein of CP33 and PhoA was incubated with paraformaldehyde-fixed trophozoites of E. histolytica and developed with a substrate, the trophozoites appeared to be stained. These results demonstrate the feasibility of bacterial expression of a human monoclonal antibody-PhoA conjugate specific for E. histolytica and that the antibody can be used to detect E. histolytica antigen without the use of chemically conjugated secondary antibodies.
Amebiasis caused by infection with Entamoeba histolytica is one of the most important parasitic diseases not only in developing countries but also in developed countries. It has been estimated that 50 million people develop amebic colitis and extraintestinal abscesses, resulting in 40,000 to 100,000 deaths annually (3). Laboratory diagnosis of intestinal amebiasis is usually based on the microscopic detection of the organism in stool samples. However, nonpathogenic commensal Entamoeba dispar, which is morphologically identical with but genetically distinct from E. histolytica, has been identified recently as a separate species (9). Since treatment of E. dispar infection is not required, accurate diagnostic tools to discriminate between the two species are needed (3).
The application of monoclonal antibodies (MAbs) is one of several strategies for specific and sensitive diagnoses of infectious diseases. A number of MAbs which react specifically with E. histolytica or E. dispar have been produced by hybridoma technology (14, 16, 19-21). It has been reported that some MAbs were useful for detecting E. histolytica antigen in fecal and serum samples by sandwich enzyme-linked immunosorbent assay (1, 2, 11, 12). Recently, a new technology to produce a Fab fragment or single-chain Fv fragment in Escherichia coli has been established (4, 6, 15). The construction of vectors for the production of Fab in E. coli has also been reported (22, 24). When mouse immunoglobulin genes derived from a hybridoma producing E. histolytica-specific MAbs were expressed in this system, the specificity of the recombinant mouse Fab was comparable to that of the parent antibody (22). More recently, recombinant human MAb Fab fragments specific for E. histolytica have also been prepared from peripheral lymphocytes of a patient with an amebic liver abscess and of an asymptomatic cyst carrier (8, 18, 23). In order to use these human Fabs for diagnostic purposes, we report here the bacterial expression of a human Fab-alkaline phosphatase (PhoA) conjugate specific for E. histolytica.
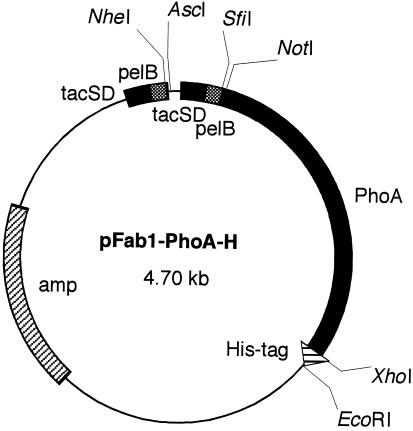
The phagemid vector pRPLS/Fab1 (24) was digested with restriction enzymes NotI and EcoRI. A synthetic DNA linker consisting of two oligonucleotides (5′-GGCCGCAGGTGGCGGAGGTTCTGGTGGCGGAGGTTCTGGTGGCGGAGGTTCTAGACTCGAGTAAG-3′ and 5′-AATTCTTACTCGAGTCTAGAACCTCCGCCACCAGAACCTCCGCCACCAGAACCTCCGCCACCTGC-3′) was inserted into the NotI/EcoRI site of pRPLS/Fab1, thus creating a 15-mer Gly4Ser linker and recognition site for XbaI and XhoI. The resulting phagemid, pFab1-L, was digested with restriction enzymes XbaI and XhoI. To clone the E. coli PhoA gene, E. coli XL1-Blue was alkali lysed, neutralized, and subjected to PCR amplification. Two synthetic primers (5′-CCTCTAGAGGTACCCCAGAAATGCCTGTTCTAGAAA-3′ and 5′-GGCTCGAGTTTAAGCCCCAGAGCGGC-3′) were used to amplify 1.45 kb of the PhoA gene (17). The amplified gene was digested with XbaI and XhoI and subcloned into the XbaI/XhoI site of pFab1-L, resulting in phagemid pFab1-PhoA. This phagemid, pFab1-PhoA, was digested with XhoI and EcoRI. A synthetic DNA linker consisting of two oligonucleotides (5′-TCGAGGGTGGCGGAGGTTCTCATCACCATCACCATCACTAAG-3′ and 5′-AATTCATGGTGATGGTGATGGTGATGAGAACCTCCGCCACCC-3′) was inserted into the XhoI/EcoRI site of pFab1-PhoA, thus creating a 15-mer Gly4Ser linker and a His6 tag. The resulting plasmid was named pFab1-PhoA-H (Fig. 1).
FIG. 1.
Structure of plasmid vector pFab1-PhoA-H used for expression of the fusion protein of Fab and alkaline phosphatase. Genes encoding the light chain and the Fd region of the heavy chain are ligated into the NheI/AscI and SfiI/NotI sites, respectively. tacSD, tac promoter Shine-Dalgarno sequence; pelB, signal sequence of pectate lyase of Erwinia carotovora; PhoA, gene for alkaline phosphatase; His-tag, gene for hexahistidine tag; amp, gene for ampicillin resistance.
As the source of human immunoglobulin genes, the kappa and Fd genes from a previously prepared antibody clone, CP33 (23), which is specific for the 260-kDa lectin of E. histolytica, were used for production of the fusion protein. The DNA fragment containing light- and heavy-chain genes was obtained by NheI/NotI digestion of pFab1-His2. The fragment was ligated with pFab1-PhoA-H and then introduced into competent E. coli JM109. The bacteria were spread on Luria-Bertani plates containing 50 μg of ampicillin per ml, and the vector with the inserts was selected. The positive clone was cultured in 1 liter of super broth (30 g of tryptone, 20 g of yeast extract, 10 g of MOPS [morpholinepropanesulfonic acid] per liter [pH 7]) containing ampicillin at 37°C until an optical density at 600 nm of 0.5 was achieved. Isopropyl-β-d-thiogalactopyranoside was added to the cultures to a final concentration of 100 μM, and the cultures were then incubated at 30°C for 12 h to achieve optimal expression. The bacteria were pelleted by centrifugation at 6,000 × g for 20 min, suspended in 20 ml of phosphate-buffered saline (PBS) containing 1 mM phenylmethylsulfonyl fluoride, and then sonicated. The lysates were centrifuged at 12,000 × g for 30 min, and the supernatant was filtered through 0.2-μm-pore-size syringe filters (Iwaki, Tokyo, Japan). Purification of the fusion protein from the supernatant was performed by affinity chromatography with His•Bind resin (Novagen, Madison, Wis.) in accordance with the manufacturer's instructions. Purified fusion protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as previously described (22). Western immunoblot analysis was also performed as previously described (22). The horseradish peroxidase (HRP)-conjugated goat immunoglobulin G (IgG) fraction specific to the human kappa chain (Organon Teknica, Durham, N.C.) and HRP-conjugated rabbit IgG fraction specific to alkaline phosphatase (Rockland, Gilbertsville, Pa.) were used for detection.
Approximately 2 × 105 trophozoites of E. histolytica HM-1:IMSS cultured axenically in BI-S-33 medium (10) were incubated on acetone-washed coverslips at 37°C for 30 min. The trophozoites were fixed with 4% paraformaldehyde in PBS for 30 min and then washed three times with PBS. After blocking with 5% bovine serum albumin was conducted for 15 min, the cells were incubated with the recombinant protein (50 μg/ml) for 30 min. After the cells were washed with PBS, development was conducted with a Vector red alkaline phosphatase substrate kit I (Vector Laboratories, Burlingame, Calif.) for 30 min in accordance with the manufacturer's instructions. Microscopic observation of the cells was performed under bright-field and fluorescent conditions by using a Nikon (Tokyo, Japan) XF-EFD2 fluorescence microscope.
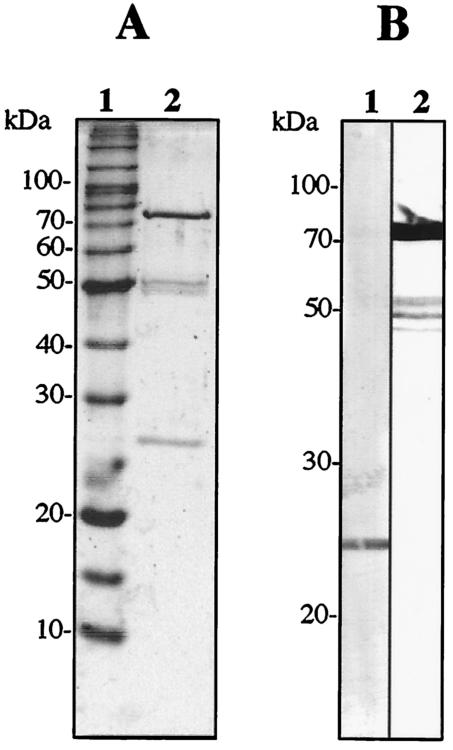
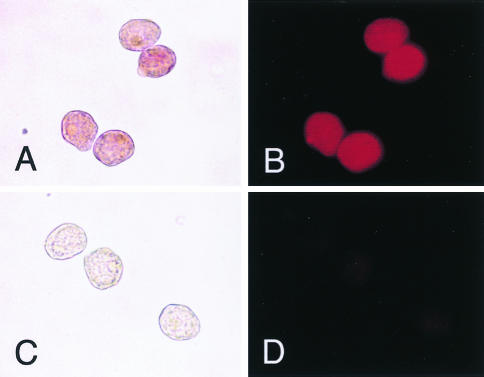
SDS-PAGE analysis of the purified fusion protein of CP33 and PhoA revealed the expected sizes of two bands with apparent molecular masses of 25 and 75 kDa, although minor bands with apparent molecular masses of 50 kDa were also present (Fig. 2A). With Western immunoblot analysis, the 25-kDa band was recognized by an anti-human kappa chain goat antibody (Fig. 2B, lane 1). On the other hand, the 75-kDa band was detected by an anti-PhoA rabbit antibody, indicating that the molecule was a fusion protein of the Fd fragment and PhoA (Fig. 2B, lane 2). When the fusion protein of Fab-PhoA was incubated with paraformaldehyde-fixed trophozoites of E. histolytica and developed with the substrate, the surfaces of the trophozoites were stained clearly under both bright-field and fluorescent conditions (Fig. 3).
FIG. 2.
SDS-PAGE (A) and Western immunoblot (B) analyses of a purified fusion protein of human Fab CP33 and alkaline phosphatase. (A) Two micrograms of the protein was subjected to analysis in 10% gel under reducing conditions and then stained with Coomassie brilliant blue. Lane 1, molecular size markers (BenchMark protein ladder; Life Technologies, Gaithersburg, Md.); lane 2, purified CP33-PhoA. Numbers to the left indicate molecular masses of the markers (in kilodaltons). (B) Protein bands were transferred to a polyvinylidene difluoride membrane. Lane 1 was treated with HRP-labeled anti-human kappa chain goat antibody. Lane 2 was treated with HRP-labeled anti-PhoA rabbit antibody.
FIG. 3.
Immunocytochemistry of E. histolytica with a fusion protein of human Fab CP33 and alkaline phosphatase. Paraformaldehyde-fixed trophozoites were treated with the fusion protein (A and B) and then with the substrate Vector red. As the controls (C and D), trophozoites were treated with a supernatant of E. coli lysates (vector control). (A and C) Bright-field microscopy; (B and D) fluorescence microscopy with a green filter. Magnification, ×360.
Recombinant human antibodies have been developed recently for application in immunoprophylaxis, or the treatment of infectious diseases. Although such human antibodies would also be useful for diagnostic purposes, one of the disadvantages of the use of human antibodies for the detection of pathogens in human samples might be reactivity of endogenous immunoglobulins with the secondary anti-human antibodies used in indirect methods. Therefore, direct labeling of the human antibody with enzymes is needed to reduce nonspecific binding of the second antibody. It was reported recently that immunoglobulin genes derived from murine hybridoma cells could be expressed in E. coli as fusion protein Fab-PhoA (7, 25) or scFv-PhoA (5, 7, 13, 17). The present study demonstrates that the bacterial expression of a human MAb-PhoA conjugate specific for E. histolytica is also possible. In addition to the advantage of using the antibody to detect the E. histolytica antigen without the need for chemically conjugated secondary antibodies, there is no requirement for experimental animals or reagents and equipment for the culture and cryopreservation of hybridoma cells. Accordingly, the use of this human recombinant antibody also provides an economic benefit.
The antigen recognized with CP33 was the heavy subunit of the galactose- and N-acetyl-d-galactosamine-inhibitable lectin of E. histolytica (23). It is well known that this lectin molecule is suitable as a target antigen for the detection of E. histolytica in fecal and serum samples (1, 2, 11, 12). In conclusion, we propose here that the human Fab-PhoA fusion protein can be used in the diagnosis of amebiasis.
Acknowledgments
This work was supported by a grant-in-aid for scientific research from the Japanese Society for the Promotion of Science and grants from the Ministry of Health, Labor, and Welfare of Japan.
REFERENCES
- 1.Abd-Alla, M. D., T. F. H. G. Jackson, V. Gathiram, A. M. El-Hawey, and J. I. Ravdin. 1993. Differentiation of pathogenic Entamoeba histolytica infections from nonpathogenic infections by detection of galactose-inhibitable adherence protein antigen in sera and feces. J. Clin. Microbiol. 31:2845-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abd-Alla, M. D., A. A. Wahib, and J. I. Ravdin. 2000. Comparison of antigen-capture ELISA to stool-culture methods for the detection of asymptomatic Entamoeba species infection in Kafer Daoud, Egypt. Am. J. Trop. Med. Hyg. 62:579-582. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 1997. WHO/PAHO/UNESCO report. A consultation with experts on amoebiasis. Mexico City, Mexico 28-29 January, 1997. Epidemiol. Bull. 18:13-14. [PubMed] [Google Scholar]
- 4.Better, M., C. P. Chang, R. R. Robinson, and A. H. Horwitz. 1988. Escherichia coli secretion of an active chimeric antibody fragment. Science 240:1041-1043. [DOI] [PubMed] [Google Scholar]
- 5.Bourin, P., A. Servat, J. J. Lataillade, M. Goyffon, D. Vaux, and P. Billiald. 2000. Immunolabeling of CD3-positive lymphocytes with a recombinant single-chain antibody/alkaline phosphatase conjugate. Biol. Chem. 381:173-178. [DOI] [PubMed] [Google Scholar]
- 6.Burton, D. R., and C. F. Barbas III. 1994. Human antibodies from combinatorial libraries. Adv. Immunol. 57:191-280. [DOI] [PubMed] [Google Scholar]
- 7.Carrier, A., F. Ducancel, N. B. Settiawan, L. Cattolico, B. Maillere, M. Leonetti, P. Drevet, A. Menez, and J. C. Boulain. 1995. Recombinant antibody-alkaline phosphatase conjugates for diagnosis of human IgGs: application to anti-HBsAg detection. J. Immunol. Methods 181:177-186. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, X.-J., S. Ihara, M. Takekoshi, and H. Tachibana. 2000. Entamoeba histolytica: bacterial expression of a human monoclonal antibody which inhibits in vitro adherence of trophozoites. Exp. Parasitol. 96:52-56. [DOI] [PubMed] [Google Scholar]
- 9.Diamond, L. S., and C. G. Clark. 1993. A redescription of Entamoeba histolytica Schaudinn, 1903 (Emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. J. Eukaryot. Microbiol. 40:340-344. [DOI] [PubMed] [Google Scholar]
- 10.Diamond, L. S., D. R. Harlow, and C. C. Cunnick. 1978. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc Trop. Med. Hyg. 72:431-432. [DOI] [PubMed] [Google Scholar]
- 11.Haque, R., K. Kress, S. Wood, T. F. Jackson, D. Lyerly, T. Wilkins, and W. A. Petri, Jr. 1993. Diagnosis of pathogenic Entamoeba histolytica infection using a stool ELISA based on monoclonal antibodies to the galactose-specific adhesin. J. Infect. Dis. 167:247-249. [DOI] [PubMed] [Google Scholar]
- 12.Haque, R., N. U. Mollah, I. K. M. Ali, K. Alam, A. Eubanks, D. Lyerly, and W. A. Petri, Jr. 2000. Diagnosis of amebic liver abscess and intestinal infection with the TechLab Entamoeba histolytica II antigen detection and antibody tests. J. Clin. Microbiol. 38:3235-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mousli, M., M. Goyffon, and P. Billiald. 1998. Production and characterization of a bivalent single chain Fv/alkaline phosphatase conjugate specific for the hemocyanin of the scorpion Androctonus australis. Biochim. Biophys. Acta 1425:348-360. [DOI] [PubMed] [Google Scholar]
- 14.Petri, W. A., Jr., T. F. H. G. Jackson, V. Gathiram, K. Kress, L. D. Saffer, T. L. Snodgrass, M. D. Chapman, Z. Keren, and D. Mirelman. 1990. Pathogenic and nonpathogenic strains of Entamoeba histolytica can be differentiated by monoclonal antibodies to the galactose-specific adherence lectin. Infect. Immun. 58:1802-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skerra, A., and A. Pluckthun. 1988. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science 240:1038-1041. [DOI] [PubMed] [Google Scholar]
- 16.Strachan, W. D., P. L. Chiodini, W. M. Spice, A. H. Moody, and J. P. Ackers. 1988. Immunological differentiation of pathogenic and non-pathogenic isolates of Entamoeba histolytica. Lancet i:561-563. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki, C., H. Ueda, E. Suzuki, and T. Nagamune. 1997. Construction, bacterial expression, and characterization of hapten-specific single-chain Fv and alkaline phosphatase fusion protein. J. Biochem. (Tokyo) 122:322-329. [DOI] [PubMed] [Google Scholar]
- 18.Tachibana, H., X.-J. Cheng, K. Watanabe, M. Takekoshi, F. Maeda, S. Aotsuka, Y. Kaneda, T. Takeuchi, and S. Ihara. 1999. Preparation of recombinant human monoclonal antibody Fab fragments specific for Entamoeba histolytica. Clin. Diagn. Lab. Immunol. 6:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tachibana, H., S. Kobayashi, X.-J. Cheng, and E. Hiwatashi. 1997. Differentiation of Entamoeba histolytica from E. dispar facilitated by monoclonal antibodies against a 150-kDa surface antigen. Parasitol. Res. 83:435-439. [DOI] [PubMed] [Google Scholar]
- 20.Tachibana, H., S. Kobayashi, Y. Kaneda, T. Takeuchi, and T. Fujiwara. 1997. Preparation of a monoclonal antibody specific for Entamoeba dispar and its ability to distinguish E. dispar from E. histolytica. Clin. Diagn. Lab. Immunol. 4:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tachibana, H., S. Kobayashi, Y. Kato, K. Nagakura, Y. Kaneda, and T. Takeuchi. 1990. Identification of a pathogenic isolate-specific 30,000-Mr antigen of Entamoeba histolytica by using a monoclonal antibody. Infect. Immun. 58:955-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tachibana, H., M. Takekoshi, X.-J. Cheng, F. Maeda, S. Aotsuka, and S. Ihara. 1999. Bacterial expression of a neutralizing mouse monoclonal antibody Fab fragment to a 150-kilodalton surface antigen of Entamoeba histolytica. Am. J. Trop. Med. Hyg. 60:35-40. [DOI] [PubMed] [Google Scholar]
- 23.Tachibana, H., K. Watanabe, X.-J. Cheng, H. Tsukamoto, Y. Kaneda, T. Takeuchi, S. Ihara, and W. A. Petri, Jr. 2003. VH3 gene usage in neutralizing human antibodies specific for the Entamoeba histolytica Gal/GalNAc lectin heavy subunit. Infect. Immun. 71:4313-4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takekoshi, M., F. Maeda, H. Tachibana, H. Inoko, S. Kato, I. Takakura, T. Kenjyo, S. Hiraga, Y. Ogawa, T. Horiki, and S. Ihara. 1998. Human monoclonal anti-HCMV neutralizing antibody from phage display libraries. J. Virol. Methods 74:89-98. [DOI] [PubMed] [Google Scholar]
- 25.Weiss, E., and G. Orfanoudakis. 1994. Application of an alkaline phosphatase fusion protein system suitable for efficient screening and production of Fab-enzyme conjugates in Escherichia coli. J. Biotechnol. 33:43-53. [DOI] [PubMed] [Google Scholar]