Non-technical summary
Adverse environments during early life are linked with an increased risk of cardiovascular disease. There is an alarming increase in the prevalence of vitamin D (VitD) deficiency in women of reproductive age. We show that male and female rat offspring that were exposed to VitD deficiency in the womb and early life have high blood pressure. The arteries from VitD deficient offspring have an impaired ability to relax due to deficiencies in the production of two important factors, nitric oxide and endothelium-derived hyperpolarizing factor. VitD deficient female offspring have an additional impairment in the nitric oxide signalling pathway in the arterial muscle. The findings of this study are particularly relevant for women intending to become pregnant. Ensuring VitD sufficiency before and during pregnancy in women will reduce the burden of cardiovascular disease risk in their offspring.
Abstract
Abstract
Increasing evidence links vitamin D deficiency and cardiovascular dysfunction in human adults. There is a worldwide increase in the prevalence of vitamin D deficiency in women of reproductive age, particularly dark-skinned and/or veiled women and their infants. We used a rat model to determine the functional impact of vitamin D deficiency during intra uterine and early life on resistance artery reactivity and blood pressure in the offspring as young adults. Rat dams were maintained on vitamin D deficient or replete chow before and during pregnancy and lactation. The offspring were maintained on the same chow until studied at 7–8 weeks of age. Conscious blood pressure was measured. Endothelial and smooth muscle function were tested in mesenteric arteries on a pressure myograph. Vitamin D deficient male and female offspring had a 10-fold lower serum 25-hydroxyvitamin D (P < 0.0001) and markedly elevated blood pressures (11–20 mmHg, P < 0.001) and heart rates (21–40 beats min−1, P < 0.02) than control fed offspring. Serum calcium was unchanged. Mesenteric artery myogenic tone was doubled in vitamin D deficiency. Endothelium-derived nitric oxide-evoked dilation was halved in arteries from vitamin D deficient males and dioestrous females. Dilation attributed to endothelium-derived hyperpolarizing factor was all but abolished in vitamin D deficient oestrous females. Nitroprusside-evoked dilation was unaltered in arteries from males, but was markedly reduced in vessels of vitamin D deplete females. In conclusion, early life vitamin D deficiency is associated with endothelial vasodilator dysfunction, and this is likely to contribute to the accompanying elevation in blood pressure and an increased cardiovascular disease risk.
Introduction
The prevalence of vitamin D (VitD) deficiency is increasing in Western societies and is re-emerging as a public health issue (Holick, 2007; Lips, 2010). VitD insufficiency is thought to occur in approximately 50% of individuals in Western societies (Lips, 2010). Globally, around 1 billion people are estimated to be VitD insufficient or VitD deficient (Holick, 2011). Although, VitD deficiency has been associated with disorders of calcium/phosphorous handling and bone metabolism (e.g. osteomalacia and rickets), it is becoming increasingly evident that VitD has myriad effects on physiological function due, in large part, to the presence of VitD receptors in most tissues. VitD deficiency has been linked with autoimmune (Ponsonby et al. 2002), neurological (McGrath, 2001; Eyles et al. 2003), and cardiovascular diseases (Zittermann, 2006; Nemerovski et al. 2009; Valdivielso et al. 2009). There is an apparent inverse relationship between cardiovascular disease and UV exposure in adult humans (Rostand, 1997; Krause et al. 1998). There is an increased incidence of high blood pressures in dark-skinned individuals living at high latitudes (Boucher, 1998; Shaw & Pal, 2002), with increasing latitude (Rostand, 1997), in those who cover their skin when outdoors (Holick, 2007), or in winter months (Sherman et al. 1990; Carnevale et al. 2001; McGrath et al. 2001). The inverse association between VitD levels and blood pressure in humans (Kristal-Boneh et al. 1997; Pfeifer et al. 2001) appears to be independent of serum Ca2+ or parathyroid hormone (Kristal-Boneh et al. 1997). In the Framingham cohort, the incidence of a cardiovascular event in a 6 year period was doubled in individuals with low VitD levels (Wang et al. 2008). VitD supplementation increases flow-mediated dilation (Sugden et al. 2008; Harris et al. 2011) and reduces blood pressure in diabetic individuals (Sugden et al. 2008).
VitD deficiency in pregnant women is increasing in prevalence. VitD deficiency and even frank rickets in infants and young children is seen worldwide (Grover & Morley, 2001; Nozza & Rodda, 2001; Prentice, 2008). Offspring of women who are VitD deficient during gestation have lower levels of VitD at birth (Brooke et al. 1980), and dark skinned babies are at increased risk of VitD deficiency postnatally, since breast milk is a poor provider of VitD (Basile et al. 2006). A wealth of evidence from epidemiological and experimental studies shows that suboptimal conditions during early life can result in an increased risk of cardiovascular disease in adulthood (Gluckman et al. 2008). Offspring of VitD deficient rats are hypertensive, have enhanced aortic constriction (Weishaar & Simpson, 1987), cardiac hypertrophy and increased nephron number (Maka et al. 2008; Gezmish et al. 2010).
Blood pressure is elevated in mice lacking the gene encoding the VitD receptor (VDR−/−) (Li et al. 2002). In spontaneously hypertensive rats (SHR), VitD supplementation lowers blood pressure (Lucas et al. 1986; Borges et al. 1999). Mesenteric arteries of adult rats made VitD deficient had enhanced contractile responses (Bian et al. 1996), and exposure of aortic rings from SHR to VitD in vitro reduced the amplitude of the contractile response to some agents (Wong et al. 2008). Endothelium-dependent vasodilator function provides a significant counterbalance to vasoconstrictor influences in order to maintain normal blood pressure and tissue blood flow. Endothelial dysfunction is a prominent feature of cardiovascular disease (Félétou & Vanhoutte, 2006). Vascular endothelial function is vulnerable to the effects of early life environments (Poston, 2007), and the effect of early life VitD insufficiency on endothelial function has never been investigated. The aim of the present study was to test the hypothesis that early life VitD insufficiency leads to endothelial dysfunction which facilitates constriction in resistance blood vessels and contributes to elevated blood pressure. We used a rat model of VitD insufficiency in utero and early life, in view of the high prevalence of deficiency in pregnant women and their children. We studied vascular function and blood pressure in young adult male and female rats. The VitD levels achieved in this model approach those seen in dark-skinned pregnant women (Grover & Morley, 2001) and in children with rickets (Nozza & Rodda, 2001).
Methods
Ethical approval
All procedures were approved by the Physiology Animal Ethics Committee, Monash University and this study was conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. The experiments comply with the policies and regulations of The Journal of Physiology given by Drummond (2009).
Animals
Four-week-old female Sprague–Dawley rats received either a standard semi-purified chow (containing 1000 IU VitD, cholecalciferol; 4.5 g Ca2+ kg−1, AIN93G) or chow with no added VitD (Specialty Feeds, Glen Forrest, WA, Australia). Six weeks later, all were mated with VitD replete males. The females (14 per group) were maintained on VitD replete or deplete diets throughout pregnancy and lactation. Four days after birth each litter was reduced to 10 pups. Pups were weaned at 4 weeks of age. Offspring were maintained on the same chow as their mothers until experimentation (7–8 weeks of age). All rats were housed under incandescent light, which is free of UV irradiation in the VitD action spectrum (290–315 nm), with a 12 h light–dark cycle. For females, a vaginal smear was examined, the uterus was weighed, and the presence of follicles or corpora lutea was established to determine stage of the oestrous cycle.
Arterial pressure was measured in freely moving, conscious rats via a catheter inserted into the ventral tail artery under isofluorane anaesthesia (a 10 min procedure) (Parkington et al. 2004). The blood pressure values recorded during the final 30 min, 2–3 h after recovery from anaesthetic, were analysed. At the end of recording, blood was collected via the recording catheter and the animal anaesthetized with isoflurane and decapitated. Serum was stored at −70°C for determination of 25-hydroxy-VitD in accredited laboratories at the Royal Children's Hospital, Melbourne, using a radioimmunoassay kit (Immunodiagnostic Systems, Boldon, UK). The coefficient of variation for this laboratory is 10.2 and 10.1% at 30 and 100 nmol l−1, respectively. Serum Ca2+ levels were determined in the same laboratory.
Experimental protocols
The mesenteric arterial tree was isolated and placed in physiological saline solution (PSS) containing (mm): NaCl 120; KCl 5; NaHCO3 25; glucose 11; KH2PO4 1; MgSO4 1.2; CaCl2 2.5; gassed with 5% CO2 and 95% O2. A segment of a third order branch (∼250 μm outside diameter at 57 mmHg) was mounted on a pressure myograph (Living Systems Instrumentation, Burlington, VT, USA) as previously described (Parkington et al. 2004). Pressure within the segment was set at 57 ± 3 mmHg with no lumenal flow, and the outside was constantly superfused (Parkington et al. 2004). Diameter was recorded via Diamtrak software (Diamtrak, Flinders University, SA, Australia). Data were stored digitally for analysis using Axoscope software (Axon Instruments, Union City, CA, USA).
At the beginning of each experiment, smooth muscle constriction was tested with 10 μm phenylephrine. At the end of the experiment the arteries were exposed to PSS in which 100 mm NaCl had been replaced with KCl (HiK PSS). To study vasorelaxation, the segments were submaximally constricted (to 60–70% of the constriction evoked by HiK PSS) with arginine vasopressin (AVP; 1 pm to 5 nm) (Fig. 6B). If the level of myogenic tone was altered in the presence of endothelial blockers, the concentration of AVP used was adjusted accordingly to achieve the same level of preconstriction as outlined above. Then, endothelial vasodilator function was tested using a 2 min application of acetylcholine (ACh) to stimulate the endothelium. Increasing concentrations of ACh were applied discretely, with complete recovery of diameter to pre-dilation levels before the next concentration was applied. NO production was blocked using Nω-nitro-l-arginine methylester (l-NAME, 100 μm, 30 min) and prostanoid synthesis was blocked using indomethacin (1 μm, 30 min). Finally, the segment was again constricted and endothelium-independent relaxation was tested using sodium nitroprusside (SNP, 10 μm).
Figure 6. Influence of VitD deficiency on endothelium-independent vasodilation and vasoconstriction in mesenteric arteries.
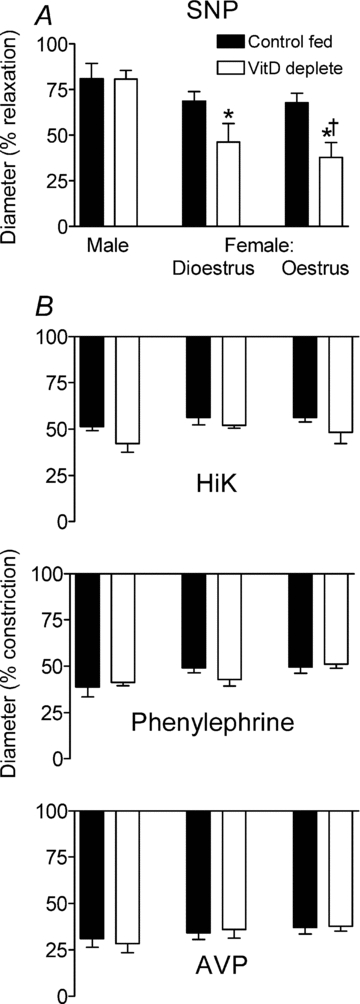
A, dilation evoked by sodium nitroprusside (SNP 10−5m) in PSS containing l-NAME plus indomethacin (males, n = 10; dioestrous females, n = 6; oestrous females n = 8). *Difference from control fed; †difference between females and males. B, diameter changes evoked by 100 mm K+ (HiK); and by 10 μm phenylephrine (PE). n = 5 in each group. Level of preconstriction in the presence of arginine vasopressin (AVP) (n = 4–6 per group).
Data analysis
Comparison between control and deficient groups was by Student's t test. Relaxations elicited by ACh (a) in control PSS, (b) in l-NAME and (c) in l-NAME plus indomethacin were integrated (area-under-curve) to take into account both the amplitude and duration of the responses (Tare et al. 2000), as the responses were shorter in arteries from VitD deplete rats. Sigmoid concentration–relaxation curves were constructed using Prism software (GraphPad Software Inc., La Jolla, CA, USA). Integrated relaxations were subtracted to estimate the component of the response attributable to NO (a–b), prostanoid (b–c), and EDHF (c alone) within each segment and results from all rats in each group were pooled (Tare et al. 2000). Constrictions to HiK and phenylephrine, basal tone and relaxation evoked by SNP were normalized to maximum diameter, obtained by exposure for 30 min at the end of the experiment in PSS containing zero Ca2+ and 3 mm EGTA. Two-way ANOVA was used to test the effects of VitD depletion in male and females, with post hoc Bonferroni testing as appropriate. Results were expressed as means ± SEM, except where indicated, and n = the number of offspring from separate mothers. A probability of P < 0.05 was accepted as statistically significant.
Results
VitD deprivation
Serum levels
Serum 25-hydroxy VitD levels were markedly lower in rats fed VitD deficient chow, while serum Ca2+ levels were not different (Table 1).
Table 1.
Effects of VitD deprivation in utero and into young adulthood on serum 25-hydroxyvitamin D and Ca2+, body mass, mean arterial pressure (MAP) and heart rate in 7- to 8-week-old rats
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Control fed | Vit D deplete | P | Control fed | Vit D deplete | P | |
| Serum 25OH-D (nmol l-1) | 120 ± 7 | 13 ± 1 | <0.001 | 138 ± 14 | 10 ± 1 | <0.001 |
| Serum Ca2+ (mmol l−1) | 2.29 ± 0.06 | 2.19 ± 0.10 | 0.7 | 2.36 ± 0.05 | 2.29 ± 0.07 | 0.3 |
| MAP (mmHg) | 104 ± 1 | 115 ± 2 | 0.001 | 102 ± 2 | 122 ± 2 | <0.001 |
| Heart rate (beats min−1) | 378 ± 6 | 418 ± 8 | 0.002 | 394 ± 5 | 415 ± 5 | 0.02 |
For males, n = 13 for control fed and n = 12 for VitD deplete; for females, n = 8 and 13 for control fed and VitD deplete, respectively. Values are given as means ± SEM. Vit D, vitamin D; MAP, mean arterial pressure; 25OH-D, 25-hydroxyvitamin D.
Body weights
Body weight at day 1 was not different between VitD deficient and control-fed pups (Fig. 1A and B). Growth rates of the offspring were equivalent before weaning. After weaning the VitD deficient groups tended to have lower body weights and this was significant in the males (P = 0.003).
Figure 1. Growth of male and female offspring.
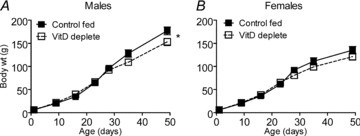
Growth of Control and VitD deplete male (n = 14/group) and female (n = 14/group) offspring. *Difference from control fed.
Mean blood pressure and heart rate
In both males and females, mean arterial blood pressure and heart rate were significantly higher in VitD deplete compared with control-fed rats (Table 1).
Vascular function
Spontaneous tone
Following pressurization of the vessels, spontaneous tone development (myogenic tone) was greater in arteries of VitD deplete males and dioestrous females (22% of maximal diameter) compared with vessels from control fed rats (8–10%) (P < 0.0001) (Fig. 2). Arteries of oestrous females developed greater tone (33%) (P < 0.0001), and this was significantly increased to 60% in vessels from VitD deplete animals (Fig. 2).
Figure 2. VitD insufficiency on myogenic tone.
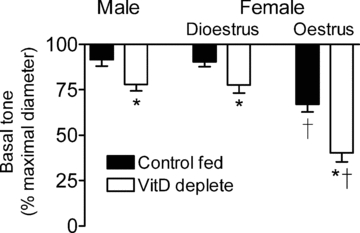
Effects of VitD and sex on basal tone development in isolated small mesenteric arteries of males (n = 7–12) and of females in dioestrus (n = 7) and in oestrus (n = 6). *Difference from control fed; †difference from dioestrous females and males.
Endothelium-dependent vasodilation
Arteries were preconstricted to between 60 and 70% of the HiK evoked constriction using AVP (Fig. 6B). Stimulation of the endothelium with ACh evoked concentration-dependent dilation of segments pre-constricted with AVP. Despite the fact that each application of ACh was for 2 min, the duration of endothelium-dependent vasodilation was strikingly shorter in arteries from VitD deplete rats (Fig. 3).
Figure 3. Influence of VitD insufficiency on vasodilation.
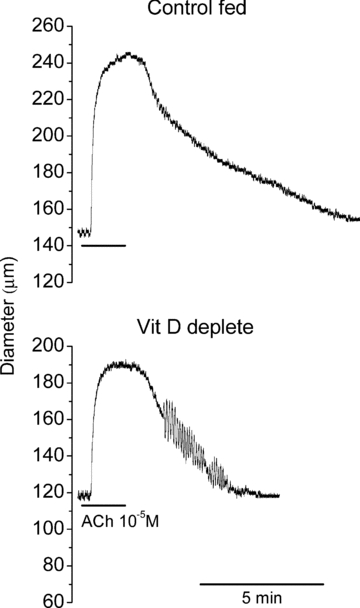
Examples of raw traces showing vasodilation evoked by endothelial stimulation with ACh 10 μm in mesenteric arteries from Control (upper trace) and VitD deplete (lower trace) rats.
In control PSS, maximum integrated endothelium-dependent vasodilations were significantly reduced in arteries from VitD deplete males and dioestrous females compared with vessels from their control fed counterparts (P < 0.0001, ANOVA) (Fig. 4). In arteries from oestrous females, maximum relaxation was smaller than in the other two groups (P < 0.0001) and was similar between control-fed and VitD deplete females (P = 0.18) (Fig. 4).
Figure 4. Effect of VitD deficiency on endothelium-dependent vasodilation.
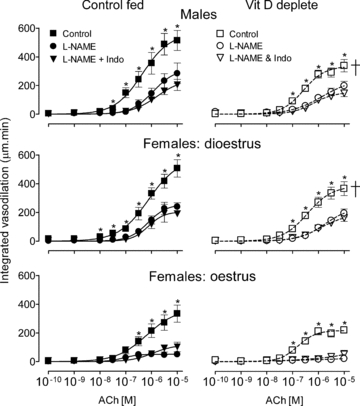
Integrated endothelium-dependent vasodilation in arteries from control fed (left panels) and VitD deplete rats (right panels), in control solution and in the presence of NO synthase blockade (l-NAME), and additional block of prostanoid synthesis (l-NAME + indomethacin, Indo). Number of animals: n = 6–7 for each group of control fed and n = 8–11 for VitD deplete rats. †Significant difference between VitD replete and VitD deplete in control PSS (ANOVA). *Individual point differences in control PSS versus in l-NAME.
Maximum integrated dilation in all tissues was reduced by l-NAME (P < 0.0001) (Fig. 4), with little additional effect of indomethacin. In tissues from VitD deplete male and dioestrous female rats, ACh-induced vasodilations were reduced by l-NAME. In arteries from oestrous females l-NAME all but abolished relaxation (Fig. 4).
Contributions of NO, prostanoid and EDHF to endothelium-dependent vasodilation
VitD depletion halved the NO component of endothelium-dependent dilation in mesenteric arteries of males and dioestrous females (P < 0.0001) (Fig. 5). The EDHF contribution was preserved in arteries from VitD deplete males (P = 0.2) and dioestrous females (P = 0.8). In contrast, in oestrous females the EDHF component was all but abolished in arteries of VitD deplete animals (P < 0.0001), with preservation of the NO component (P = 0.5) (Fig. 5). The small dilator prostanoid response was reduced in arteries from VitD deficient male rats (Fig. 5).
Figure 5. Contribution of NO, EDHF and prostanoids to endothelium-dependent vasodilation.
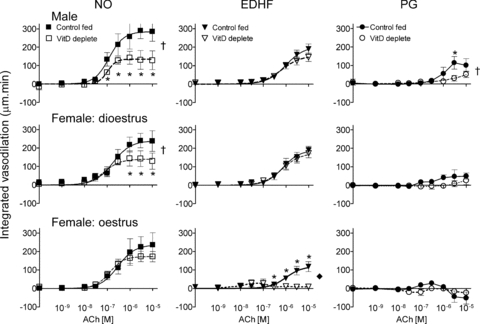
Responses in individual tissues were subtracted to reveal the absolute contribution of NO, EDHF and prostanoids to endothelium-dependent responses. Dilation, positive values; constriction, negative values. †Significance difference between VitD replete and VitD deplete (ANOVA; *individual point differences, same n as for Fig. 4).
Smooth muscle responsiveness
Endothelium-independent dilation
SNP (10 μm) evoked equivalent dilations in arteries of all control fed rats, irrespective of sex (P = 0.03 by ANOVA) (Fig. 6A). VitD depletion had no effect on SNP-evoked vasodilation in arteries from males (n = 10). However, vasodilations in vessels of VitD deplete dioestrous (n = 6) and oestrous (n = 8) females were significantly reduced compared with responses in arteries of VitD deplete males (P = 0.004 and 0.0002, respectively) (Fig. 6A). Vasodilations in arteries of VitD deplete oestrous females were approximately halved compared with their control fed counterparts (P = 0.02).
Constriction
Exposure for 1 min to HiK PSS (that is in which 100 mm Na+ had been replaced by K+) or to normal PSS containing 10 μm phenylephrine reduced external diameter to an equivalent extent (Fig. 6B, n = 5 in all groups) and was similar irrespective of sex or VitD status.
Rate of constriction/relaxation
The 10–90% rise times for relaxations to SNP (+VitD 11 ± 2 s, n = 9 and −VitD 20 ± 5 s, n = 11, P = 0.1) and for contractions to HiK PSS (+VitD 4 ± 1 s, n = 6 and −VitD 8 ± 2 s, n = 8, P = 0.1) were not different in arteries from VitD replete versus VitD deplete rats. This suggested that there was no physical impediment to contraction or relaxation as a result of VitD status.
Vessel diameter
Maximum passive diameter (in Ca2+ free, EGTA-containing PSS) was not affected by VitD deprivation (P = 0.3).
Discussion
The present study provides the first report of the deleterious effects of early life VitD insufficiency on endothelium-dependent vasodilator function in resistance arteries. This was associated with an elevation in blood pressure. Several other novel observations to emerge from our study include the development of significantly greater basal tone in mesenteric arteries of VitD deplete rats and a blunted responsiveness to SNP in arteries from females. There was a striking influence of sex and/or endogenous sex steroids on the endothelium-derived vasodilator targeted by VitD insufficiency. Thus, while NO-mediated vasodilation was preferentially reduced in arteries from males and dioestrous females, the EDHF component of vasodilation was all but abolished in tissues from females in oestrus.
These results add to the growing body of evidence implicating VitD deficiency in adverse cardiovascular outcomes in humans (Kristal-Boneh et al. 1997; Krause et al. 1998; Valdivielso et al. 2009) and the ability of VitD to ameliorate the high blood pressure in SHR rats (Borges et al. 1999) and increase flow-mediated vasodilation, an indicator of endothelial vasodilator function, in adult humans (Harris et al. 2011; Jablonski et al. 2011). Blood pressure was elevated by 11 (males) and 20 mmHg (females) in our study, similar to the 20 mmHg reported previously for VitD deplete male rats (Weishaar & Simpson, 1987) and VitD receptor knockout mice (Li et al. 2002). In humans, for each 10–20 mmHg increase in blood pressure there is a 2-fold risk of cardiovascular disease (Chobanian et al. 2003).
The effects of VitD deficiency we report cannot be explained by low serum Ca2+, since levels were similar in deficient and control groups. Our observation that both blood pressure and heart rate were elevated in VitD insufficiency suggests the possibility that VitD may also have an effect on the cardiovascular control centres in the brain. Studies in rats demonstrate that maternal VitD insufficiency can adversely affect brain development in the fetus (Brown et al. 2003; Eyles et al. 2003). Effects of VitD insufficiency on cardiovascular centres in the brain have not been studied.
Blood pressure is elevated in mice lacking the VitD receptor, by a mechanism implicating renin, and is independent of Ca2+ or parathyroid hormone (Li et al. 2002). Renin increases angiotensin II levels, predisposing to the development of hypertension and oxidative stress (Ortiz et al. 2001), and has action on baroreceptor pathways (Lohmeier et al. 2002) and structure in blood vessels. In the present study, arterial diameter was not affected by VitD insufficiency and histological examination of 10 μm sections of vessels failed to disclose detectable morphological differences in mesenteric resistance arteries between treatment groups (data not shown). Furthermore, an earlier study by Weishaar and colleagues (1990) found that plasma renin activity was unaltered in VitD deficient, normocalcaemic rats.
The endothelium achieves vasorelaxation by a variety of mechanisms, with NO an important endothelium-dependent vasodilator, especially in larger vessels, and the contribution of EDHF becomes increasingly important as vessel size decreases (Félétou & Vanhoutte, 2006). Early life insults increase cardiovascular disease risk and this may be mediated in part by endothelial vasodilator dysfunction (Lamireau et al. 2002; Payne et al. 2003; Taylor et al. 2004; Franco et al. 2006; Poston, 2007; Torrens et al. 2009). NO and EDHF contributed approximately equally to overall endothelium-dependent vasodilation in pressurized small mesenteric arteries from our males, similar to previous observations (McCulloch & Randall, 1998), and dioestrous females. Reduction in EDHF contributes, at least in part, to the endothelial dysfunction in mesenteric arteries in SHR, due to alterations in the nature or density of the K+ channels involved in the hyperpolarization. VitD supplementation of SHR normalizes blood pressure and restores K+ channels in these animals (Borges et al. 1999). It is interesting that Borges and colleagues used female rats in their study of the effects of VitD in SHR, although the stage of the oestrous cycle was not documented. In the present study, EDHF-mediated vasodilation was preserved in mesenteric arteries of males and dioestrous females deprived of VitD since early life but was all but abolished in vessels of females in oestrus. VitD deficiency resulted in a halving of the contribution of NO to endothelium-dependent vasorelaxation, leaving EDHF intact in our males and dioestrous females. Endothelial NOS is upregulated by oestrogen (Chambliss & Shaul, 2002) and it may be that this circumvents, in part, the reduction in the NO component of endothelium-dependent vasodilation in VitD deficient oestrous females. The contribution of EDHF to endothelium-dependent vasodilation is approximately halved in mesenteric arteries of oestrous females compared with dioestrous females or males, suggesting that the elevated oestrogen, and thus NO production, may have a suppressive effect on EDHF (Bauersachs et al. 1996). The virtual absence of EDHF responses in tissues from VitD deficient oestrous females could reflect a convergence of the effects of oestrogen and VitD deficiency, perhaps at the level of genome transcription, a major site of action of both VitD and oestrogen. Sex differences in endothelium-dependent dilation in mesenteric artery have been reported in normotensive rats (McCulloch & Randall, 1998). Additionally, adverse prenatal conditions, e.g. maternal hypertension or dietary fat, have been found to have disparate effects on cardiovascular function based on sex (Denton et al. 2003; Khan et al. 2003). In our study, blood pressure (BP) was significantly elevated in both males (by 11 mmHg) and to an even greater extent in females (20 mmHg), and these rats were only 7–8 weeks of age. Interestingly, although numbers are small following separation of the female BP data according to the stage of the oestrous cycle (oestrus versus dioestrus), VitD deficiency appeared to have a greater effect during oestrus than in dioestrus.
Endothelium-independent relaxation to SNP was reduced in VitD deplete female rats and may explain, in part, the reduction in the NO component of endothelium-dependent vasorelaxation in these animals. Offspring of calorific or protein restricted dams may also exhibit similar reductions in responsiveness to SNP (Lamireau et al. 2002; Brawley et al. 2003). Of these studies, one was associated with a reduction in smooth muscle guanylyl cyclase but no change in endothelial NO synthase (Lamireau et al. 2002). The impaired endothelium-dependent vasodilation in vessels with intact SNP responsiveness in males indicates a reduction in NO production or bioavailability, consistent with increased oxidative stress (see above). In arteries from oestrous females, although SNP responsiveness was decreased, endothelium-dependent relaxation attributed to NO was unchanged reflecting the possibility of an increase in NO production in these vessels, perhaps reflecting the stimulatory effect of oestrogen on eNOS (see above).
The mesenteric vessels studied here developed modest myogenic tone, confirming previous observations in this tissue (Davis & Hill, 1999). Although not extensively studied, it appears that early life environments may alter myogenic tone development in a sex- and region-dependent manner (Hemmings et al. 2005; Xiao et al. 2009). Tone was significantly increased in tissues obtained from all VitD deplete rats (Fig. 1). Basal release of NO from the endothelium can influence vascular tone (Davis & Hill, 1999), and blockade of NO production with l-NAME increased tone by approximately 15% in arteries from males and dioestrous females and by around 30% in oestrous vessels (data not shown). However, the extent of tone development following blockade of NOS was equivalent in vessels from VitD replete and deplete males and females, suggesting that the deleterious effect of VitD deficiency on tone is unlikely to be via basal NO production. In SHR, the enhanced myogenic tone in mesenteric arteries is ameliorated with VitD supplementation (Borges et al. 1999), a factor which may contribute to the normalisation of BP in these treated rats.
There was no evidence for an effect of VitD insufficiency on the ability of the smooth muscle of the mesenteric artery to contract, in response to either HiK PSS depolarization or receptor activation with phenylephrine or AVP. In a previous study of VitD depletion in utero, sensitivity of aortic rings to noradrenaline was increased. These rat offspring were hypocalcaemic and the vascular hyper-responsiveness was reversed by normalization of serum Ca2+ (Weishaar & Simpson, 1987). In our rats, serum Ca2+ levels were preserved.
This study provides a clear demonstration that VitD insufficiency in early life has serious repercussions on vascular function. These findings are particularly relevant for pregnant women and their children. Serum levels of 25-hydroxy VitD in our VitD deplete rat dams and pups are similar to those found in dark-skinned and veiled pregnant women and in children with rickets. There is evidence that potentially modifiable factors operating around the time of conception, during pregnancy or early childhood can ‘programme’ later health (Nathanielsz et al. 2007; Poston, 2007; Gluckman et al. 2008; Nijland et al. 2008). VitD may be an important factor in this regard (McGrath, 2001), since its active form has many critical physiological functions, including regulating genomic stability (Chatterjee, 2001; Sutton & MacDonald, 2003). VitD supplementation is widely available, cheap and safe and provides an alternative to sunshine exposure when lifestyle choices preclude adequately increased skin exposure to sunlight.
Acknowledgments
The authors thank Donna West for preparing the animals, Michelle Kett for assistance with recording conscious BP, and Peter Verras and Ronda Greaves for assay of VitD and serum Ca2+ determinations. This work was supported by the Ramaciotti Foundation (Perpetual Trustees) (H.C.P.), NHMRC (H.C.P., M.T., H.A.C., D.W.E. and C.S.) and VicHealth (Victorian Health Promotion Foundation) (R.M.).
Glossary
Abbreviations
- AVP
arginine vasopressin
- BP
blood pressure
- EDHF
endothelium-derived hyperpolarizing factor
- NO
nitric oxide
- NOS
nitric oxide synthase
- SHR
spontaneously hypertensive rats
- SNP
sodium nitroprusside
- VitD
vitamin D
- VDR
vitamin D receptor; PSS, physiological saline solution; HiK PSS, PSS with isosmolar replacement of Na+ with K+
Author contributions
M.T., S.J.E., H.C.P. experimental procedures. C.S. research assistance, H.A.C. analysis, D.W.E. provision of animals, R.M. clinical input, M.T., H.C.P., H.A.C., R.M. study design. All authors approved earlier versions of the manuscript. Functional studies done in Dept Physiology, Monash University, VitD & calcium assayed at Royal Chikdren's Hospital, Melbourne.
References
- Basile LA, Taylor SN, Wagner CL, Horst RL, Hollis BW. The effect of high-dose vitamin D supplementation on serum vitamin D levels and milk calcium concentration in lactating women and their infants. Breastfeed Med. 2006;1:27–35. doi: 10.1089/bfm.2006.1.27. [DOI] [PubMed] [Google Scholar]
- Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation. 1996;94:3341–3347. doi: 10.1161/01.cir.94.12.3341. [DOI] [PubMed] [Google Scholar]
- Bian K, Ishibashi K, Bukoski RD. 1,25(OH)2D3 modulates intracellular Ca2+ and force generation in resistance arteries. Am J Physiol Heart Circ Physiol. 1996;270:H230–H237. doi: 10.1152/ajpheart.1996.270.1.H230. [DOI] [PubMed] [Google Scholar]
- Borges ACR, Feres T, Vianna LM, Paiva TB. Effect of cholecalciferol treatment on the relaxant responses of spontaneously hypertensive rat arteries to acetylcholine. Hypertension. 1999;34:897–901. doi: 10.1161/01.hyp.34.4.897. [DOI] [PubMed] [Google Scholar]
- Boucher BJ. Inadequate vitamin D status: does it contribute to the disorders comprising syndrome ‘X’? Br J Nutr. 1998;79:315–327. doi: 10.1079/bjn19980055. [DOI] [PubMed] [Google Scholar]
- Brawley L, Itoh S, Torrens C, Barker A, Bertram C, Poston L, Hanson M. Dietary protein restriction in pregnancy induces hypertension and vascular defects in rat male offspring. Pediatr Res. 2003;54:83–90. doi: 10.1203/01.PDR.0000065731.00639.02. [DOI] [PubMed] [Google Scholar]
- Brooke OG, Brown IR, Bone CD, Carter ND, Cleeve HJ, Maxwell JD, Robinson VP, Winder SM. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. Br Med J. 1980;280:751–754. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Bianco JI, McGrath JJ, Eyles DW. 1,25-Dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett. 2003;343:139–143. doi: 10.1016/s0304-3940(03)00303-3. [DOI] [PubMed] [Google Scholar]
- Carnevale V, Modoni S, Pileri M, Di Giorgio A, Chiodini I, Minisola S, Vieth R, Scillitani A. Longitudinal evaluation of vitamin D status in healthy subjects from southern Italy: seasonal and gender differences. Osteoporos Int. 2001;12:1026–1030. doi: 10.1007/s001980170012. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev. 2002;23:665–686. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- Chatterjee M. Vitamin D and genomic stability. Mutat Res. 2001;475:69–87. doi: 10.1016/s0027-5107(01)00080-x. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- Denton KM, Flower RL, Stevenson KM, Anderson WP. Adult rabbit offspring of mothers with secondary hypertension have increased blood pressure. Hypertension. 2003;41:634–639. doi: 10.1161/01.HYP.0000052949.85257.8E. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience. 2003;118:641–653. doi: 10.1016/s0306-4522(03)00040-x. [DOI] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder. Am J Physiol Heart Circ Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- Franco MC, Christofalo DM, Sawaya AL, Ajzen SA, Sesso R. Effects of low birth weight in 8- to 13-year-old children: implications in endothelial function and uric acid levels. Hypertension. 2006;48:45–50. doi: 10.1161/01.HYP.0000223446.49596.3a. [DOI] [PubMed] [Google Scholar]
- Gezmish O, Tare M, Parkington HC, Morley R, Porrello ER, Bubb KJ, Black MJ. Maternal vitamin D deficiency leads to cardiac hypertrophy in rat offspring. Reprod Sci. 2010;17:168–176. doi: 10.1177/1933719109349536. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover SR, Morley R. Vitamin D deficiency in veiled or dark-skinned pregnant women. Med J Aust. 2001;175:251–252. doi: 10.5694/j.1326-5377.2001.tb143558.x. [DOI] [PubMed] [Google Scholar]
- Harris RA, Pedersen-White J, Guo D-H, Stallmann-Jorgensen IS, Keeton D, Huang Y, Shah Y, Zhu H, Dong Y. Vitamin D3 supplementation for 16 weeks improves flow-mediated dilation in overweight African-American adults. Am J Hypertens. 2011;24:557–562. doi: 10.1038/ajh.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings DG, Williams SJ, Davidge ST. Increased myogenic tone in 7-month-old adult male but not female offspring from rat dams exposed to hypoxia during pregnancy. Am J Physiol Heart Circ Physiol. 2005;289:H674–682. doi: 10.1152/ajpheart.00191.2005. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12:4–18. doi: 10.2174/138945011793591635. [DOI] [PubMed] [Google Scholar]
- Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57:63–69. doi: 10.1161/HYPERTENSIONAHA.110.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension. 2003;41:168–175. doi: 10.1161/01.hyp.0000047511.97879.fc. [DOI] [PubMed] [Google Scholar]
- Krause R, Buhring M, Hopfenmuller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998;352:709–710. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- Kristal-Boneh E, Froom P, Harari G, Ribak J. Association of calcitriol and blood pressure in normotensive men. Hypertension. 1997;30:1289–1294. doi: 10.1161/01.hyp.30.5.1289. [DOI] [PubMed] [Google Scholar]
- Lamireau D, Nuyt AM, Hou X, Bernier S, Beauchamp M, Gobeil F, Jr, Lahaie I, Varma DR, Chemtob S. Altered vascular function in fetal programming of hypertension. Stroke. 2002;33:2992–2998. doi: 10.1161/01.str.0000039340.62995.f2. [DOI] [PubMed] [Google Scholar]
- Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips P. Worldwide status of vitamin D nutrition. J Steroid Biochem Mol Biol. 2010;121:297–300. doi: 10.1016/j.jsbmb.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Lohmeier TE, Lohmeier JR, Warren S, May PJ, Cunningham JT. Sustained activation of the central baroreceptor pathway in angiotensin hypertension. Hypertension. 2002;39:550–556. doi: 10.1161/hy0202.103003. [DOI] [PubMed] [Google Scholar]
- Lucas PA, Brown RC, Drüeke T, Lacour B, Metz JA, McCarron DA. Abnormal vitamin D metabolism, intestinal calcium transport, and bone calcium status in the spontaneously hypertensive rat compared with its genetic control. J Clin Invest. 1986;78:221–227. doi: 10.1172/JCI112555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maka N, Makrakis J, Parkington HC, Tare M, Morley R, Black MJ. Vitamin D deficiency during pregnancy and lactation stimulates nephrogenesis in rat offspring. Pediatr Nephrol. 2008;23:55–61. doi: 10.1007/s00467-007-0641-9. [DOI] [PubMed] [Google Scholar]
- McCulloch AI, Randall MD. Sex differences in the relative contributions of nitric oxide and EDHF to agonist-stimulated endothelium-dependent relaxations in the rat isolated mesenteric arterial bed. Br J Pharmacol. 1998;123:1700–1706. doi: 10.1038/sj.bjp.0701781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. Does ‘imprinting’ with low prenatal vitamin D contribute to the risk of various adult disorders? Med Hypotheses. 2001;56:367–371. doi: 10.1054/mehy.2000.1226. [DOI] [PubMed] [Google Scholar]
- McGrath JJ, Kimlin MG, Saha S, Eyles DW, Parisi AV. Vitamin D insufficiency in south-east Queensland. Med J Aust. 2001;174:150–151. doi: 10.5694/j.1326-5377.2001.tb143195.x. [DOI] [PubMed] [Google Scholar]
- Nathanielsz PW, Poston L, Taylor PD. In utero exposure to maternal obesity and diabetes: animal models that identify and characterize implications for future health. Clin Perinatol. 2007;34:515–526. doi: 10.1016/j.clp.2007.09.005. v. [DOI] [PubMed] [Google Scholar]
- Nemerovski CW, Dorsch MP, Simpson RU, Bone HG, Aaronson KD, Bleske BE. Vitamin D and cardiovascular disease. Pharmacotherapy. 2009;29:691–708. doi: 10.1592/phco.29.6.691. [DOI] [PubMed] [Google Scholar]
- Nijland MJ, Ford SP, Nathanielsz PW. Prenatal origins of adult disease. Curr Opin Obstet Gynecol. 2008;20:132–138. doi: 10.1097/GCO.0b013e3282f76753. [DOI] [PubMed] [Google Scholar]
- Nozza JM, Rodda CP. Vitamin D deficiency in mothers of infants with rickets. Med J Aust. 2001;175:253–255. doi: 10.5694/j.1326-5377.2001.tb143559.x. [DOI] [PubMed] [Google Scholar]
- Ortiz MC, Manriquez MC, Romero JC, Juncos LA. Antioxidants block angiotensin II-induced increases in blood pressure and endothelin. Hypertension. 2001;38:655–659. doi: 10.1161/01.hyp.38.3.655. [DOI] [PubMed] [Google Scholar]
- Parkington HC, Dodd J, Luff SE, Worthy K, Coleman HA, Tare M, Anderson WP, Edgley AJ. Selective increase in renal arcuate innervation density and neurogenic constriction in chronic angiotensin II-infused rats. Hypertension. 2004;43:643–648. doi: 10.1161/01.HYP.0000117140.52220.85. [DOI] [PubMed] [Google Scholar]
- Payne JA, Alexander BT, Khalil RA. Reduced endothelial vascular relaxation in growth-restricted offspring of pregnant rats with reduced uterine perfusion. Hypertension. 2003;42:768–774. doi: 10.1161/01.HYP.0000084990.88147.0C. [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D3 and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–1637. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- Ponsonby AL, McMichael A, van der Mei I. Ultraviolet radiation and autoimmune disease: insights from epidemiological research. Toxicol. 2002;181–182:71–78. doi: 10.1016/s0300-483x(02)00257-3. [DOI] [PubMed] [Google Scholar]
- Poston L. Influences of maternal nutritional status on vascular function in the offspring. Curr Drug Targets. 2007;8:914–922. doi: 10.2174/138945007781386910. [DOI] [PubMed] [Google Scholar]
- Prentice A. Vitamin D deficiency: a global perspective. Nutr Rev. 2008;66:S153–164. doi: 10.1111/j.1753-4887.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30:150–156. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- Shaw NJ, Pal BR. Vitamin D deficiency in UK Asian families: activating a new concern. Arch Dis Child. 2002;86:147–149. doi: 10.1136/adc.86.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SS, Hollis BW, Tobin JD. Vitamin D status and related parameters in a healthy population: the effects of age, sex, and season. J Clin Endocrinol Metab. 1990;71:405–413. doi: 10.1210/jcem-71-2-405. [DOI] [PubMed] [Google Scholar]
- Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–325. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- Sutton AL, MacDonald PN. Vitamin D: more than a “bone-a-fide” hormone. Mol Endocrinol. 2003;17:777–791. doi: 10.1210/me.2002-0363. [DOI] [PubMed] [Google Scholar]
- Tare M, Parkington HC, Coleman HA. EDHF, NO and a prostanoid: hyperpolarization-dependent and -independent relaxation in guinea-pig arteries. Br J Pharmacol. 2000;130:605–618. doi: 10.1038/sj.bjp.0703332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PD, Khan IY, Hanson MA, Poston L. Impaired EDHF-mediated vasodilatation in adult offspring of rats exposed to a fat-rich diet in pregnancy. J Physiol. 2004;558:943–951. doi: 10.1113/jphysiol.2002.018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrens C, Kelsall CJ, Hopkins LA, Anthony FW, Curzen NP, Hanson MA. Atorvastatin restores endothelial function in offspring of protein-restricted rats in a cholesterol-independent manner. Hypertension. 2009;53:661–667. doi: 10.1161/HYPERTENSIONAHA.108.122820. [DOI] [PubMed] [Google Scholar]
- Valdivielso JM, Coll B, Fernandez E. Vitamin D and the vasculature: can we teach an old drug new tricks? Expert Opin Ther Targets. 2009;13:29–38. doi: 10.1517/14728220802564390. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishaar RE, Simpson RU. Involvement of vitamin D3 with cardiovascular function. II. Direct and indirect effects. Am J Physiol Endocrinol Metab. 1987;253:E675–683. doi: 10.1152/ajpendo.1987.253.6.E675. [DOI] [PubMed] [Google Scholar]
- Weishaar RE, Kim S-N, Saunders DE, Simpson RU. Involvement of vitamin D3 with cardiovascular function. III. Effects of physical and morphological properties. Am J Physiol Endocrinol Metab. 1990;258:E134–E142. doi: 10.1152/ajpendo.1990.258.1.E134. [DOI] [PubMed] [Google Scholar]
- Wong MS, Delansorne R, Man RY, Vanhoutte PM. Vitamin D derivatives acutely reduce endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2008;295:H289–296. doi: 10.1152/ajpheart.00116.2008. [DOI] [PubMed] [Google Scholar]
- Xiao D, Yang S, Zhang L. Prenatal cocaine exposure causes sex-dependent impairment in the myogenic reactivity of coronary arteries in adult offspring. Hypertension. 2009;54:1123–1128. doi: 10.1161/HYPERTENSIONAHA.109.138024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92:39–48. doi: 10.1016/j.pbiomolbio.2006.02.001. [DOI] [PubMed] [Google Scholar]


