Abstract
The gene encoding the major envelope glycoprotein (gp51) with its signal sequence, represented by an additional NH2-terminal 33-residue amino acid sequence of bovine leukemia virus (BLV), was inserted into a baculovirus transfer vector. A recombinant virus expressing a secreted gp51 protein in insect cells was isolated. The recombinant gp51 expressed was characterized by using an anti-BLV monoclonal antibody by both Western blotting analysis and enzyme-linked immunosorbent assay (ELISA). The secreted gp51 was used as an antigen, and an ELISA with recombinant gp51 (rgp51) was developed for the detection of BLV antibodies. This new procedure was compared with a previous ELISA method for the detection of BLV antibodies and an agar gel immunodiffusion test performed with an unpurified BLV antigen preparation. The comparative testing of field samples showed that the ELISA with rgp51 is more specific and also suitable for the testing of pooled sera.
Bovine leukemia virus (BLV), the etiological agent of enzootic bovine leukosis (EBL), is a C-type retrovirus of cattle with a worldwide distribution (5). The virus induces a persistent lymphocytosis and in some cases lymphoid tumors in domestic cattle.
The BLV envelope (Env) glycoprotein, which consists of the gp51 outer membrane glycoprotein and the gp30 transmembrane glycoprotein, is directly involved in infectivity events and, like the p24 major structural protein, can elicit a strong immune response in infected cattle (23). Serological diagnosis of EBL is mainly based on screening of field samples for gp51 antibodies. It follows that diagnostic procedures that use native gp51 constitute a prerequisite for the design of an efficient EBL eradication program. In recent decades different serological methods, such as the agar gel immunodiffusion test (AGID) and the enzyme-linked immunosorbent assay (ELISA), have been developed. ELISAs are based on the use of partially purified BLV gp51 and monoclonal antibodies (MAbs) against BLV gp51 epitopes (4, 17). These procedures are particularly useful for samples with low antibody titers, such as milk samples or pooled sera. A permanent fetal lamb kidney (FLK) cell line chronically infected with BLV (FLK-BLV) is used to produce gp51. Although highly productive BLV-infected cell lines are available, their production is rather laborious, expensive, and time-consuming.
In recent decades different investigators have described the expression of the BLV Env glycoproteins in heterologous expression systems such as Escherichia coli (2, 21, 22), Saccharomyces cerevisiae (11), and recombinant vaccinia virus (10, 18) systems and, recently, a baculovirus system (9, 19). However, the use of recombinant gp51 (rgp51) in an ELISA method has not yet been described. The aim of this paper is therefore to describe the production of secreted rgp51 by recombinant baculovirus in insect cells and its use in an ELISA for the detection of BLV antibodies.
MATERIALS AND METHODS
Cells and viruses.
BLV-infected and uninfected FLK cells (National Veterinary Laboratory, Copenhagen, Denmark) were cultured in Eagle's minimum essential medium containing 10% fetal bovine serum. Spodoptera frugiperda insect cells (Sf21 cells; Invitrogen, Inc., Carlsbad, Calif.) were cultured in Grace's insect medium (Biowhittaker, Walkersville, Md.) containing 10% fetal bovine serum and antibiotics (gentamicin, penicillin, and streptomycin). Sf21 insect cells were also cultured in HYQ CCM3 serum-free medium (HyClone Laboratories Inc., Logan, Utah) for the expression of the rgp51 protein. Recombinant baculovirus carrying the lacZ gene (AcLacZ; Invitrogen, Inc.) was used as a negative control in the study. BLV was purified from the FLK cell supernatant by the protocol described by Walker et al. (25) and was used as a positive control in the study.
Cloning of gp51 gene.
Genomic DNA was extracted from BLV-infected and uninfected FLK cells as described by Maniatis et al. (13). Two oligonucleotide primers (5′-GCTGCAGGCCTTCAAATGCCTAAA-3′, in which a PstI restriction site was introduced [the first underlined sequence], and 5′-CGACTTAACTACGTCTGACCC-3′, in which a TGA stop codon was introduced [underlined]) were designed according to the nucleotide sequence of the BLV genome (20) and were used to amplify the gp51 gene by PCR. The amplified DNA was subcloned into the pCR-Blunt vector (Zero Blunt PCR cloning kit; Invitrogen, Inc.) to construct plasmid pCRgp51. The inserted gene encoding gp51 was sequenced with appropriate primers by use of the ALFexpress II DNA analysis system (Amersham Pharmacia Biotech, Uppsala, Sweden) to exclude mutations introduced by PCR.
Generation of recombinant baculovirus.
The gp51 gene was recovered from pCRgp51 after digestion with PstI and was then ligated into the PstI site of baculovirus transfer vector pBAC 4 (Invitrogen, Inc.). The resultant recombinant transfer vector with the correct orientation to the promoter was designated pB4gp51. Sf21 cells were cotransfected with linear Bac-N Blue viral DNA (Invitrogen, Inc.) and recombinant transfer vector pB4gp51 by using Lipofectin reagent (Gibco BRL, Grand Island, N.Y.). After 3 days of incubation at 27°C, the culture supernatant containing recombinant baculovirus was harvested and the plaques were purified. Recombinant baculovirus, which expresses rgp51, was isolated and designated Bac-rgp51. A high-titer virus stock (>107 PFU/ml) was created by amplification of Bac-rgp51.
Expression and characterization of rgp51.
Sf21 cells were infected with Bac-rgp51 and AcLacZ. The culture media were harvested and centrifuged at 100,000 × g for 2 h to remove the baculovirus, and the supernatants were collected. The cells were washed twice with cold phosphate-buffered saline (PBS) and lysed in cold lysis buffer (50 mM Tris-HCl [pH 8], 150 mM NaCl, 0.02% sodium azide, 100 μg of phenylmethylsulfonyl fluoride per ml, 1 μg of aprotinin per ml, 1% Triton X-100). The cell lysates were centrifuged at 13,000 × g for 15 min at 4°C, and the supernatant fractions were collected. Both culture media and cell extracts were used to analyze the expression and characterization of recombinant gp51 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis-Western blotting and by the BLV ELISA.
Western blotting.
The rgp51 protein was tested for its reactivity to an anti-gp51 MAb by Western blotting, as described by Maniatis et al. (13). Antigen levels were determined by chemiluminescence by an enhanced chemiluminescence protocol (Amersham Pharmacia Biotech).
BLV ELISA.
The BLV ELISA, commonly used for routine serological diagnosis, was developed by using an unpurified BLV antigen preparation to detect antibodies against the gp51 Env glycoprotein and p24 structural protein of BLV in milk and serum samples (7). This procedure was used for the identification and characterization of rgp51.
Collection of sera.
A set of 1,230 individual serum samples was collected from cows from selected herds of cattle. All cows were more than 1 year old. Of these serum samples, 480 were from BLV-free herds and were considered true negative for BLV. This set of serum samples was tested by the AGID test, the BLV ELISA, and the improved ELISA with rgp51 described in this paper.
In order to evaluate whether the ELISA with rgp51 was suitable for determination of antibodies to BLV in pooled sera, 500 pools were made by pipetting 100 μl of each of 5,000 serum samples collected during an EBL eradication campaign. In addition, 10 pools of sera, each of which contained one weakly positive serum sample selected from our serum bank, were artificially prepared. The pooled sera were tested only by the ELISA with rgp51. Finally, international reference serum sample E4 (8) was used as the standard for determination of the sensitivity of the ELISA.
AGID test.
The AGID test was carried out as described in the technical annex of directive 84/643/CEE (6).
ELISA with rgp51.
The ELISA with rgp51 was performed as described previously (7), with some modification. Briefly, ELISA plates (MaxiSorp; Nalgene Nunc International) were coated with a predetermined optimal quantity of purified anti-BLV gp51 MAb at 4°C overnight. Then, the plates were washed three times with PBS containing 0.05% Tween 20 (PBS-Tween). The cell culture supernatant containing rgp51 (100 μl/well), which was previously diluted 1:10 in blocking solution (PBS-Tween plus 1% yeast extract), was added to each well. The plates were incubated for 1 h at 37°C and then washed three times with PBS-Tween. The single serum samples, including positive and negative controls, were diluted 1:50 in blocking solution; and 100 μl was added to each of duplicate wells. Pooled serum samples were diluted 1:5 in blocking solution, and 100 μl was added to each of triplicate wells (two wells with the recombinant antigen plus another well with blocking solution only as a blank). After a 1-h incubation period at 37°C, the plates were washed three times with PBS-Tween, and then the plates were inoculated with an anti-bovine immunoglobulin G1 (IgG1) and IgG2 MAb labeled with horseradish peroxidase. The plates were incubated for 1 h at 37°C and washed, and 100 μl of 3,3′,5,5′-tetramethylbenzidine chromogen substrate (Sigma Aldrich Inc., St. Louis, Mo.) was added to each well. After about 10 min of incubation, the reaction was stopped with 0.5 M H2SO4 and the optical density (OD) was measured at 450 nm. For each single serum sample tested, the result was expressed as the mean of the OD value for duplicate wells. For pooled serum samples, the result was expressed as the change in the OD (ΔOD) and was calculated by subtracting the OD for the well without antigen (blank) from the mean OD value for the corresponding wells with the recombinant antigen. This measure has become a necessity, since pooled serum samples are tested at a 1:5 dilution, resulting in a higher background compared to that for the single serum samples.
Statistical evaluation of interlaboratory reproducibility.
In order to provide evidence of the reproducibility of the method, the ELISA with rgp51, together with a set of 44 serum samples, was distributed to nine other laboratories located throughout Italy. The serum sample set contained 19 positive field serum samples and 10 positive serum samples with low antibody concentrations obtained by dilution of a positive reference serum sample to different levels. Each laboratory performed three blind tests with the set of serum samples described above.
RESULTS
Cloning of gp51 gene.
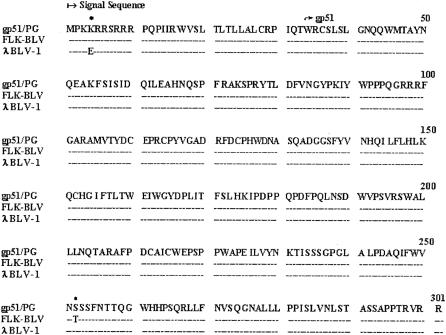
The gene encoding gp51 of BLV was amplified from the genomic DNA of FLK cells infected with BLV by PCR. The predicted fragment of 903 bp was amplified from BLV-infected FLK cells, but it was not amplified from uninfected FLK cells (data not shown). The PCR product was inserted into the pCR-Blunt vector and then sequenced. An open reading frame of 903 nucleotides was identified. The nucleotide sequence of the amplified gp51 gene was compared with previously published sequences (12, 20). The only variations between these sequences consisted of nucleotide substitutions, while no frameshift, insertion, deletion, or nonsense mutation was observed (data not shown). The variations in the amino acid sequence (gp51/PG) of the amplified gp51 gene consisted of 1 amino acid change with respect to the amino acid sequences of the gp51 glycoproteins of BLV variants FLK-BLV (12) and λBLV-1 (20) (Fig. 1).
FIG. 1.
Comparison of the amino acid sequences of the gp51 glycoproteins of BLV variants gp51/PG, FLK-BLV, and λBLV-1. Amino acid changes are indicated by asterisks.
Expression and characterization of rgp51.
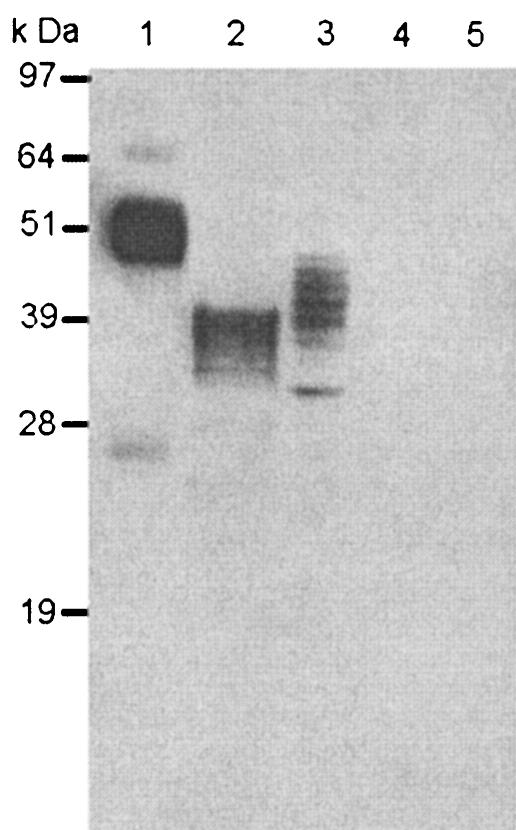
Sf21 cells were infected at 1 PFU/cell with a recombinant baculovirus carrying the gp51 gene (Bac-rgp51) and with a control recombinant baculovirus carrying the lacZ gene (AcLacZ). After incubation for 3 days, cell extracts and culture media were tested by Western blotting with an anti-gp51 MAb. Figure 2 shows that this MAb reacted to major bands with molecular masses of 40 to 42 and 48 to 50 kDa for the Bac-rgp51-infected Sf21 cell extract and supernatant, respectively (Fig. 2, lanes 2 and 3, respectively). The molecular mass of rgp51 was smaller than that of the native gp51 obtained from purified BLV (Fig. 2, lane 3). No band was detected for the AcLacZ-infected insect cell extract or its culture medium (Fig. 2, lanes 4 and 5, respectively).
FIG. 2.
Western blot of rgp51 glycoprotein expressed in insect cells with a mouse anti-gp51 MAB. Lane 1, purified BLV from supernatant of BLV-infected FLK cells; lane 2, Bac-rgp51-infected Sf21 cell extract; lane 3, Bac-rgp51-infected Sf21 cell culture medium; lane 4, AcLacZ-infected Sf21 cell extract; lane 5, AcLacZ-infected Sf21 cell culture medium.
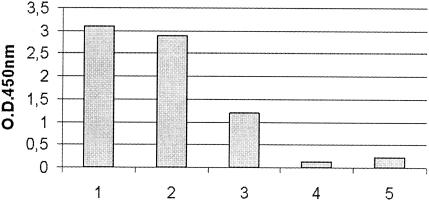
The BLV ELISA was used to determine the antigen structure of recombinant gp51 by replacing the usual antigen preparation with the cell extract and culture medium from Sf21 cells infected with Bac-rgp51. The cell extract and culture medium from Sf21 cells infected with AcLacZ were also included as negative controls (Fig. 3).
FIG. 3.
Values from the BLV ELISA with rgp51. Bar 1, supernatant of BLV-infected FLK cells (positive control); bar 2, Bac-rgp51-infected Sf21 cell culture medium; bar 3, Bac-rgp51-infected Sf21 cell extract; bar 4, AcLacZ-infected Sf21 cell culture medium (negative control); bar 5 AcLacZ-infected Sf21 cell extract (negative control).
A specific OD was observed for Bac-rgp51-infected insect cells, while the OD for AcLacZ recombinant baculovirus-infected Sf21 cells was not detected. The OD obtained for the culture medium containing rgp51 was about 2.5 times higher than that obtained for the cell extract. These results, like those of Western blotting analysis, indicated that rgp51 is secreted in the cell culture medium.
Determination of a cutoff value.
To discriminate between the presence and the absence of BLV antibody, the mean OD value for the individual negative serum samples and the mean ΔOD for the negative pooled sera with the respective standard deviation (SD) were calculated. The mean ± SD OD value for individual negative serum samples was 0.058 ± 0.064, and the mean ± SD ΔOD for the negative pooled serum samples was 0.043 ± 0.057.
The threshold for positivity was defined as the mean OD plus 2 SDs (for the individual serum samples) and the mean ΔOD plus 2 SDs (for the pooled serum samples). On the basis of these thresholds, values of 0.058 + 2 (0.064) = 0.186 and 0.043 + 2 (0.057) = 0.157, respectively, were found. From these calculations, the cutoff values for both individual and pooled samples were established to be 0.200.
Individual and pooled sera.
A total of 1,230 serum samples were analyzed by the BLV ELISA, the ELISA with gp51, and the AGID test. A total of 1,212 serum samples, including the 480 true-negative serum samples, were negative by all three tests. Eighteen samples reacted positively by the BLV ELISA, whereas 12 of them were positive by the ELISA with rgp51 and the AGID test. The buffy coats of the six samples from the cows that reacted in contradictory ways by the three tests were subsequently investigated by PCR and scored negative (data not shown). The sensitivity of each of the ELISAs compared with that of the AGID test was the same (100%), whereas the specificities were 99% for the BLV ELISA and 100% for the ELISA with rgp51. The E4 anti-BLV international reference serum sample was diluted in negative serum and tested by the ELISA with rgp51. The results indicated that serum could be diluted 1,600 times and still score positive.
Nine of the 500 pooled serum samples were found to be positive. All the single serum samples used to make these pools were processed individually, and one serum sample from each pool scored positive both by the AGID test and by the ELISA with rgp51. The 10 pools containing one weakly positive serum sample each were found to be clearly positive. Generally, one of every five pools of sera showed a background OD in the well without antigen (blank) that was quite high, with the OD being greater than the cutoff. However, we have observed that even in these cases positive sera could be clearly detected only if the OD for the corresponding blank was less than 0.700 (data not shown). In 11 cases, the well with blocking solution only showed a high background OD (OD ≥ 0.700). We therefore added a weakly positive serum sample to each of these 11 pools in order to verify if this background OD masked its presence. In four cases the pools with the weakly positive serum sample scored negative (ΔOD < 0.200), whereas in the remaining pools it still scored positive (ΔOD > 0.200).
Interlaboratory reproducibility.
Table 1 summarizes the results of the interlaboratory reproducibility tests. The first replicate for laboratory 9 was considered invalid because the OD for the negative control was >0.200. The results were classified according to the expected data and were considered correct.
TABLE 1.
Results of interlaboratory reproducibility trialsa
| Laboratory no. | Test 1
|
Test 2
|
Test 3
|
|||
|---|---|---|---|---|---|---|
| n | c | n | c | n | c | |
| 1 | 44 | 44 | 44 | 43 | 44 | 44 |
| 2 | 44 | 44 | 44 | 44 | 44 | 44 |
| 3 | 44 | 43 | 44 | 44 | 44 | 44 |
| 4 | 44 | 44 | 44 | 44 | 44 | 44 |
| 5 | 44 | 44 | 44 | 44 | 44 | 44 |
| 6 | 44 | 44 | 44 | 44 | 44 | 44 |
| 7 | 44 | 39 | 44 | 44 | 44 | 43 |
| 8 | 44 | 44 | 44 | 44 | 44 | 44 |
| 9 | 0 | 0 | 44 | 41 | 44 | 44 |
| Total | 352 | 346 | 396 | 392 | 396 | 395 |
n, number of samples analyzed; c, number of samples with correct results.
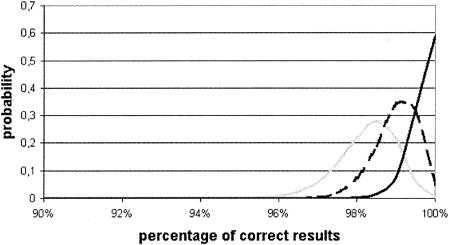
On this basis, the reliability of the ELISA with rgp51 was evaluated by determination of the beta distribution of the estimated probability of supplying different percentages of correct results. It was therefore calculated that the most probable values for the proportion of correct results were 98.5% for the first examination, 99.5% for the second examination, and 99.8% for the third examination. It is also evident that tests giving a proportion of correct results of <95% have a zero probability of occurring (Fig. 4).
FIG. 4.
Distribution of percentages of correct results. Gray line, test 1; dashed line, test 2; black line, test 3.
To obtain more information about the validation parameters of the ELISA with rgp51, reproducibility was calculated by Cochran's nonparametric Q test. By comparison of the data from the three repetitions, statistically significant differences were not found, as evidenced by the values obtained (Q = 1,556 and P > 0.05).
DISCUSSION
During virus infection of the host, the gp51 Env glycoprotein and the p24 major structural protein of BLV are the main targets of the immune response and are therefore logical targets for use as diagnostic reagents. The gp51 protein has the highest degree of antigenicity and the greatest potential as a diagnostic reagent for the detection of antibodies to BLV (14, 23). In the present study, we expressed the gp51 protein of BLV in insect cells using a recombinant baculovirus and evaluated its diagnostic potential by ELISA. The ATG start codon at position 4826 within the nucleotide sequence of BLV (20) was used to express the gp51 protein. This choice was based on the role of the signal sequence, represented by the NH2-terminal 33-residue amino acid sequence that precedes the amino acid sequence of gp51 (12, 20). The predicted amino acid sequence of gp51 shared the highest degree of homology with those of FLK-BLV and λBLV-1 compared with the degree of homology to the other strain tested (data not shown).
rgp51 was analyzed by Western blotting, by which several recombinant protein fractions from 32 kDa to 48 to 50 kDa were detected (Fig. 2). All the proteins that migrated faster than native gp51 could represent incomplete and/or incorrectly glycosylated molecules, as is frequently observed in insect cells (16, 19) and different mammalian cell lines (1, 26).
The antigenic structure of the rgp51 protein was examined by the BLV ELISA. Despite the incomplete glycosylation, the results indicated that the rgp51 protein showed the same antigenicity as native gp51. Moreover, these data also showed that a larger amount of gp51 was present in the supernatant than in the cellular extract of Bac-rgp51-infected insect cells (Fig. 3).
In our view, the presence of the signal peptide may explain the secretion of gp51. This hypothesis is supported by previous studies: (i) the expression of Env BLV glycoproteins without a signal sequence did not produce a secreted gp51 protein (11, 19), and (ii) expression systems in which Env BLV glycoproteins or gp51 are expressed with the signal sequence produced a secreted protein (14, 24).
Secreted gp51 offers several advantages over intracellular gp51 or gp51 produced by FLK-BLV mammalian cells. The preparation of secreted rgp51 is simple, its use overcomes the problems of contamination with proteins from insect cells or fetal calf serum when it is expressed in serum-free medium, and it can be produced in large amounts.
A BLV eradication program requires serological methods which are rapid, simple to perform, and sensitive enough to clearly distinguish between positive and negative reactors even when the test is applied to pooled sera. The AGID test is not able to detect BLV-infected cows when pooled sera is used and is not suitable for application in automated systems. Several commercial ELISAs fulfill these requirements, but often, a lack of specificity is found, mainly due to aspecific reactions with bovine viral diarrhea virus antibodies (data not shown), which is likely to occur when bovine serum samples are tested. The present study was initiated to solve these problems by investigating the possibility of developing the test by using a BLV rgp51 as the antigen. This resulted in an improved ELISA format, based on the principle of a preexisting ELISA which was in routine use in our laboratory. The results of tests with 1,230 individual serum samples yielded a specificity of the ELISA with rgp51 of 100%, whereas the specificity of the previous BLV ELISA was 99%. The sensitivities of both tests are difficult to determine since the reference test represented by the AGID test is not very sensitive. The use of the ELISA with rgp51 with pooled sera has many advantages. With good organization, all the problems connected with the sampling and the preparation of the pooled sera can easily be solved. When the ELISA with rgp51 is applied to pooled sera, it seems to be able to detect practically all the BLV-infected cattle with a minimum of false-negative results. This is probably the consequence of aspecific binding between components of some field sera and the murine IgG capture antibody, which could cause a high background OD in the control well, or could be due to the binding of nonspecific antibodies present in the tested samples to the walls of the wells (15). On the other hand, these cross-reactions have rarely been observed (0.8% of pooled sera). It should still be stressed that, because the anti-BLV gp51 antibodies appear earlier and their titers are consistently higher than the anti-BLV p24 titer (3), serological methods involving the gp51 antigen are considered the methods of choice for the early detection of BLV infection. The ELISA with rgp51 described here has proven to be sensitive and specific, to have a good interlaboratory reproducibility, to be easy to perform, and to be suitable for the monitoring of animals for BLV infection.
Acknowledgments
We thank Sabrina Monaco for the English language revisions.
REFERENCES
- 1.Altaner, C., M. Merza, V. Altenerova, and B. Morein. 1993. Envelope glycoprotein gp51 of bovine leukemia virus is differently glycosylated in cells of various species and organ origin. Vet. Immunol. Immunopathol. 36:163-177. [DOI] [PubMed] [Google Scholar]
- 2.Ban, J., S. Czene, C. Altaner, I. Callebaut, V. Krchnak, M. Merza, A. Burny, R. Kettmann, and D. Portetelle. 1992. Mapping of sequential epitopes recognized by monoclonal antibodies on the bovine leukemia virus external glycoprotein expressed in Escherichia coli by means of antipeptide antibodies. J. Gen. Virol. 73:2457-2461. [DOI] [PubMed] [Google Scholar]
- 3.Bex, F., C. Bruck, M. Mammerickx, D. Portetelle, J. Ghysdael, Y. Cleuter, M. Leclercq, D. Dekegel, and A. Burny. 1979. Humoral antibody response to bovine leukemia virus infection in cattle and sheep. Cancer Res. 79:1118-1123. [PubMed] [Google Scholar]
- 4.Bruck, C., D. Portetelle, M. Mammerickx, M. Mathot, and A. Burny. 1984. Epitopes of BLV glycoprotein gp51 recognized by sera of infected cattle and sheep. Leukemia Res. 8:315. [DOI] [PubMed] [Google Scholar]
- 5.Burny, A., Y. Cleuter, R. Kettman, M. Mammerickx, G. Marbaix, D. Portetelle, A. Van der Broeke, L. Willems, and R. Thomas. 1990. Bovine leukemia: facts and hypotheses derived from the study of an infectious cancer, p. 9-32. In R. C. Gallo and F. Wong-Staal (ed.), Retrovirus biology and human disease. Marcel Dekker Inc., New York, N.Y.
- 6.Council of the European Communities. 1984. Directive 84/643/CEE. 11 December 1984. Council of the European Communities, Brussels, Belgium.
- 7.De Mia, G. M., C. Marini, D. Rutili, and T. Frescura. 1991. Diagnosi sierologica della leucosi enzootica bovina mediante ELISA con impiego di anticorpi monoclonali. Atti Soc. Ital. Sci. Vet. 44:863-867. [Google Scholar]
- 8.Hoff-Jørgensen, R. 1989. An international comparison of different laboratory tests for the diagnosis of bovine leukosis: suggestions for international standardization. Vet. Immunol. Immunopathol. 22:293-297. [DOI] [PubMed] [Google Scholar]
- 9.Kabeya, H., K. Ohashi, K. Ohishi, C. Sugimoto, H. Amanuma, and M. Onuma. 1996. An effective peptide vaccine to eliminate bovine leukemia virus (BLV) infected cells in carrier sheep. Vaccine 14:1118-1122. [DOI] [PubMed] [Google Scholar]
- 10.Kumar, S., M. E. Andrew, D. B. Boyle, R. B. Brandon, M. F. Lavin, and R. C. W. Daniel. 1990. Expression of bovine leukemia virus envelope gene by recombinant vaccinia virus. Virus Res. 17:131-142. [DOI] [PubMed] [Google Scholar]
- 11.Legrain, M., D. Portetelle, J. Dumont, A. Burny, and F. Hilger. 1989. Biochemical and immunological characterization of the bovine leukemia virus (BLV) envelope glycoprotein (gp51) produced in Saccharomyces cerevisiae. Gene 79:227-237. [DOI] [PubMed] [Google Scholar]
- 12.Mamoun, R. Z., M. Morisson, N. Rebeyrotte, B. Busetta, D. Couez, R. Kettmann, M. Hospital, and B. Guillemain. 1990. Sequence variability of bovine leukemia virus env gene and its relevance to the structure and antigenicity of the glycoproteins. J. Virol. 64:4180-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 14.Merza, M., B. Sundquist, J. Söber, and B. Morein. 1991. Immunoaffinity purification of two major proteins of bovine leukemia virus (gp51 and p24) and their use for discrimination between vaccinated and infected animals. J. Virol. Methods 33:345-353. [DOI] [PubMed] [Google Scholar]
- 15.Molloy, J. B., P. J. Walker, F. C. Baldock, B. J. Rodwell, and J. A. Cowley. 1990. An enzyme-linked immunosorbent assay for bovine leukemia virus antibody. J. Virol. Methods 28:47-58. [DOI] [PubMed] [Google Scholar]
- 16.Noteborn, M. H. M., G. F. de Boer, A. Kant, G. Koch, J. L. Bos, A. Zantema, and A. J. Van der Eb. 1990. Expression of avian leukemia virus env-gp85 in Spodoptera frugiperda cells by use of baculovirus expression vector. J. Gen. Virol. 71:2641-2648. [DOI] [PubMed] [Google Scholar]
- 17.Portetelle, D., C. Bruck, M. Mammerickx, and A. Burny. 1983. Use of monoclonal antibody in an ELISA test for detection of antibodies to bovine leukemia virus. J. Virol. Methods 6:19-29. [DOI] [PubMed] [Google Scholar]
- 18.Portetelle, D., K. Limbach, A. Burny, M. Mammerickx, P. Desmettre, M. Riviere, J. Zavada, and E. Paoletti. 1991. Recombinant vaccinia virus expression of the bovine leukemia virus envelope gene and protection of immunized sheep against infection. Vaccine 9:194-200. [DOI] [PubMed] [Google Scholar]
- 19.Russo, S., Montermini, L., R. Berkovitz-Siman-Tov, W. Ponti, and G. Poli. 1998. Expression of bovine leukemia virus Env glycoprotein in insect cells by recombinant baculovirus. FEBS Lett. 436:11-16. [DOI] [PubMed] [Google Scholar]
- 20.Sagata, N., T. Yasunaga, J. Tsuzuku-Kawamura, K. Ohishi, Y. Ogawa, and Y. Ikawa. 1985. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retrovirus. Proc. Natl. Acad. Sci. USA 82:677-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siakkou, H., R. Ulrich, A. Uelze, R. Möhring, and S. Rosenthal. 1990. Immunological characterization of BLV proteins synthesized in Escherichia coli. Acta Virol. 34:256-262. [PubMed] [Google Scholar]
- 22.Ulrich, R., H. Siakkou, C. Platzer, H. Bossmann, R. Möhring, M. Wiedmann, S. Bähring, and S. Rosenthal. 1990. Synthesis of bovine leukemia virus antigen in Escherichia coli. Arch. Exp. Vet. Med. Leipzig 44:909-916. [PubMed] [Google Scholar]
- 23.Van der Maaten, M., and J. M. Miller. 1990. Bovine leukosis virus, p. 419-429. In Z. Dinter and B. Morein (ed.), Virus infections of ruminants. Elsevier Science Publishers B.V., Amsterdam, The Netherlands.
- 24.Vonèche, V., D. Portetelle, R. Kettmann, L. Willems, K. Limbach, E. Paoletti, J. M. Ruysschaert, A. Burny, and R. Brasseur. 1992. Fusogenic segment of bovine leukemia virus and simian immunodeficiency virus are interchangeable and mediate fusion by means of oblique insertion in the lipid bilayer of their target cells. Proc. Natl. Acad. Sci. USA 89:3810-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker, P. J., J. B. Molloy, and B. J. Rodwell. 1987. A protein immunoblot test for detection of bovine leukemia virus p24 in cattle and experimentally infected sheep. J. Virol. Methods 15:201-211. [DOI] [PubMed] [Google Scholar]
- 26.Zajac, V., K. Slavikova, and O. Babusikova. 1994. Expression of env gene of bovine leukemia virus in rodent cells. Arch. Virol. 135:201-207. [DOI] [PubMed] [Google Scholar]