Abstract
The title compound, C4H10NO+·C5H8NOS2 −, is built up of a morpholinium cation and a dithiocarbamate anion. In the crystal, two structurally independent formula units are linked via N—H⋯S hydrogen bonds, forming an inversion dimer, with graph-set motif R 4 4(12).
Related literature
For the crystal structures of similar compounds, see: Wahlberg (1979 ▶, 1980 ▶, 1981 ▶); Mafud & Gambardella (2011a
▶,b
▶). For graph-set analysis, see: Bernstein et al. (1995 ▶). For puckering parameters, see: Cremer & Pople (1975 ▶).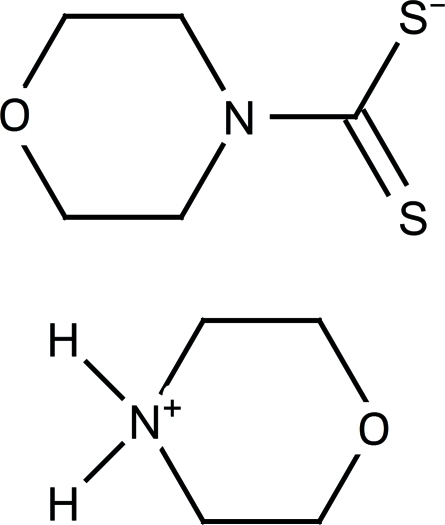
Experimental
Crystal data
C4H10NO+·C5H8NOS2 −
M r = 250.37
Monoclinic,
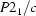
a = 7.938 (5) Å
b = 18.3232 (15) Å
c = 8.8260 (5) Å
β = 110.021 (5)°
V = 1206.2 (8) Å3
Z = 4
Mo Kα radiation
μ = 0.43 mm−1
T = 290 K
0.3 × 0.15 × 0.15 mm
Data collection
Enraf–Nonius TurboCAD-4 diffractometer
Absorption correction: ψ scan (North et al., 1968 ▶) T min = 0.795, T max = 0.902
3705 measured reflections
3487 independent reflections
2021 reflections with I > 2σ(I)
R int = 0.041
3 standard reflections every 120 min intensity decay: 5%
Refinement
R[F 2 > 2σ(F 2)] = 0.050
wR(F 2) = 0.145
S = 1.00
3487 reflections
190 parameters
All H-atom parameters refined
Δρmax = 0.56 e Å−3
Δρmin = −0.39 e Å−3
Data collection: CAD-4 EXPRESS (Enraf–Nonius, 1989) ▶; cell refinement: CAD-4 EXPRESS ▶; data reduction: XCAD4 (Harms & Wocadlo, 1995 ▶); program(s) used to solve structure: SIR92 (Altomare et al., 1994 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶) and Mercury (Macrae et al., 2006 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶), PLATON (Spek, 2009 ▶) and publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811026286/su2285sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811026286/su2285Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811026286/su2285Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H1N⋯S1 | 0.86 (4) | 2.47 (4) | 3.284 (3) | 158 (3) |
| N2—H2N⋯S1i | 0.91 (4) | 2.75 (4) | 3.453 (2) | 135 (3) |
| N2—H2N⋯S2i | 0.91 (4) | 2.39 (3) | 3.221 (2) | 151 (3) |
Symmetry code: (i) 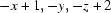 .
.
Acknowledgments
We are grateful to the Instituto de Química de São Carlos and the Universidade de São Paulo for supporting this study.
supplementary crystallographic information
Comment
The first thiocarbamic acid-ammonium salt, pyrrolidinedithiocarbamic acid-pyrrolidineammonium salt, was reported on previously by (Wahlberg, 1979; 1980; 1981). Our group have recently described the synthesis and crystal structures of ammonium piperidine-1-carbodithioate and sodium piperidine-1-carbodithioate dihydrate (Mafud & Gambardella, 2011a,b). Continuing our research on this subject, we report herein on the synthesis and crystal structure of the title salt, 1-Morpholinedithiocarbamic Acid-morpholineammonium Salt.
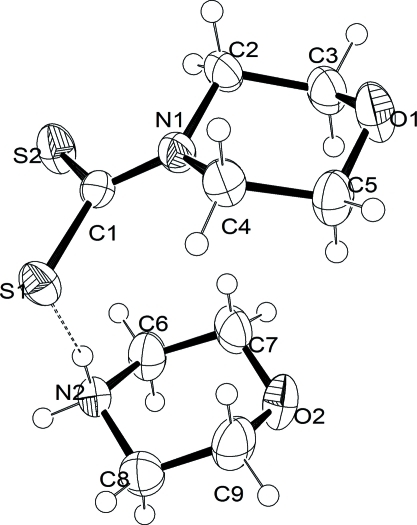
In the molecular structure of the title compound (Fig. 1) there is an intramolecular hydrogen bond involving the cation, via the nitrogen atom from amine group, and the anion, via the sulfur atom of dithiocarbamate (Table 1). The six membered rings have chair conformations, with puckering parameters are Q=0.554 (3) Å, θ = 177.4 (3)°, φ2 = 168 (6)° for the anion and Q = 0.566 (3) Å, θ = 1.4 (4)°, φ2 = 60 (14)° for the cation (Cremer & Pople, 1975).
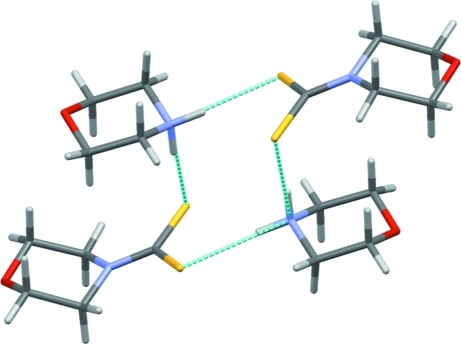
In the crystal two structurally independent formula units are linked via N—H···S hydrogen bonds (Fig. 2, Table 1), to form a dimer arrangement centered about an inversion center, with graph-set R44(12) [Bernstein et al., 1995].
Experimental
The RNH2+ salt of the morpholinedithiocarbamate was prepared by slow addition of 0.1 mol of CS2 to a cold solution (ice bath) containing 0.2 mol of the morpholien amine dissolved in 30 ml of ethanol-water 1:1 (v/v) medium. The obtained solid was recrystallized from ethanol-water 1:1 (v/v) and dried in a vacuum oven at 323 K for 8 h. Colourless single crystals, suitable for X-ray diffraction analysis, were obtained. On heating they sublimed and decomposed.
Refinement
All H-atom positions were located in a difference Fourier map and were freely refined.
Figures
Fig. 1.
Perspective view of the molecular structure of the title salt, with numering scheme and displacement ellipsoids drawn at the 50% probability level.
Fig. 2.
Perspective view of the N-H···S hydrogen bonded (dashed cyan lines) dimer in the title salt, with graph-set R44(12).
Crystal data
| C4H10NO+·C5H8NOS2− | F(000) = 536 |
| Mr = 250.37 | Dx = 1.379 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 15 reflections |
| a = 7.938 (5) Å | θ = 5.5–15.9° |
| b = 18.3232 (15) Å | µ = 0.43 mm−1 |
| c = 8.8260 (5) Å | T = 290 K |
| β = 110.021 (5)° | Prism, colourless |
| V = 1206.2 (8) Å3 | 0.3 × 0.15 × 0.15 mm |
| Z = 4 |
Data collection
| Enraf–Nonius TurboCAD-4 diffractometer | Rint = 0.041 |
| graphite | θmax = 30.0°, θmin = 2.7° |
| non–profiled ω/2θ scans | h = 0→11 |
| Absorption correction: ψ scan (North et al., 1968) | k = 0→25 |
| Tmin = 0.795, Tmax = 0.902 | l = −12→11 |
| 3705 measured reflections | 3 standard reflections every 120 min |
| 3487 independent reflections | intensity decay: 5% |
| 2021 reflections with I > 2σ(I) |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.050 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.145 | All H-atom parameters refined |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.0759P)2] where P = (Fo2 + 2Fc2)/3 |
| 3487 reflections | (Δ/σ)max = 0.004 |
| 190 parameters | Δρmax = 0.56 e Å−3 |
| 0 restraints | Δρmin = −0.39 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.20657 (9) | 0.07843 (4) | 0.84253 (7) | 0.03827 (19) | |
| S2 | 0.34115 (10) | −0.02991 (4) | 0.66044 (8) | 0.0448 (2) | |
| O2 | 0.7186 (3) | 0.20279 (12) | 0.8050 (3) | 0.0558 (5) | |
| O1 | 0.1390 (3) | 0.19488 (11) | 0.2852 (2) | 0.0547 (6) | |
| N1 | 0.1685 (3) | 0.09147 (11) | 0.5323 (2) | 0.0344 (5) | |
| N2 | 0.6455 (3) | 0.09075 (12) | 0.9933 (3) | 0.0352 (5) | |
| C1 | 0.2314 (3) | 0.04987 (13) | 0.6648 (3) | 0.0301 (5) | |
| C2 | 0.1739 (4) | 0.06872 (15) | 0.3746 (3) | 0.0425 (6) | |
| C3 | 0.2422 (4) | 0.13083 (16) | 0.2981 (4) | 0.0458 (7) | |
| C4 | 0.0698 (4) | 0.15994 (15) | 0.5227 (3) | 0.0390 (6) | |
| C5 | 0.1441 (5) | 0.21801 (15) | 0.4412 (4) | 0.0472 (7) | |
| C6 | 0.7585 (5) | 0.07751 (17) | 0.8933 (4) | 0.0478 (7) | |
| C7 | 0.7033 (5) | 0.12905 (18) | 0.7531 (4) | 0.0506 (7) | |
| C8 | 0.6555 (5) | 0.16802 (17) | 1.0443 (4) | 0.0515 (7) | |
| C9 | 0.6074 (5) | 0.21637 (17) | 0.8988 (4) | 0.0556 (8) | |
| H1N | 0.537 (5) | 0.079 (2) | 0.936 (4) | 0.067* | |
| H2N | 0.681 (4) | 0.063 (2) | 1.085 (4) | 0.067* | |
| H2A | 0.050 (4) | 0.053 (2) | 0.304 (4) | 0.067* | |
| H2B | 0.248 (4) | 0.027 (2) | 0.394 (4) | 0.067* | |
| H3A | 0.368 (4) | 0.1441 (19) | 0.369 (4) | 0.067* | |
| H3B | 0.229 (4) | 0.1177 (19) | 0.187 (4) | 0.067* | |
| H4A | −0.053 (5) | 0.1527 (19) | 0.457 (4) | 0.067* | |
| H4B | 0.088 (5) | 0.1751 (18) | 0.628 (4) | 0.067* | |
| H5A | 0.274 (5) | 0.2265 (19) | 0.512 (4) | 0.067* | |
| H5B | 0.073 (4) | 0.261 (2) | 0.419 (4) | 0.067* | |
| H6A | 0.887 (5) | 0.0884 (19) | 0.966 (4) | 0.067* | |
| H6B | 0.748 (4) | 0.031 (2) | 0.863 (4) | 0.067* | |
| H7A | 0.582 (5) | 0.1189 (19) | 0.685 (4) | 0.067* | |
| H7B | 0.786 (4) | 0.125 (2) | 0.697 (4) | 0.067* | |
| H8A | 0.791 (5) | 0.1709 (19) | 1.118 (4) | 0.067* | |
| H8B | 0.590 (5) | 0.1737 (19) | 1.108 (4) | 0.067* | |
| H9A | 0.481 (5) | 0.2048 (19) | 0.826 (4) | 0.067* | |
| H9B | 0.628 (5) | 0.265 (2) | 0.933 (4) | 0.067* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0437 (4) | 0.0446 (4) | 0.0318 (3) | 0.0029 (3) | 0.0197 (3) | 0.0001 (3) |
| S2 | 0.0620 (5) | 0.0378 (4) | 0.0438 (4) | 0.0134 (3) | 0.0300 (3) | 0.0079 (3) |
| O2 | 0.0626 (14) | 0.0449 (12) | 0.0676 (13) | −0.0052 (10) | 0.0324 (11) | 0.0156 (10) |
| O1 | 0.0774 (15) | 0.0474 (12) | 0.0479 (11) | 0.0132 (10) | 0.0324 (10) | 0.0173 (9) |
| N1 | 0.0462 (13) | 0.0290 (10) | 0.0307 (10) | 0.0011 (8) | 0.0165 (9) | 0.0002 (8) |
| N2 | 0.0395 (12) | 0.0350 (11) | 0.0336 (10) | −0.0027 (9) | 0.0157 (9) | 0.0043 (8) |
| C1 | 0.0302 (11) | 0.0313 (11) | 0.0309 (11) | −0.0057 (9) | 0.0131 (9) | −0.0006 (9) |
| C2 | 0.0670 (19) | 0.0366 (14) | 0.0269 (12) | −0.0020 (13) | 0.0199 (12) | −0.0014 (10) |
| C3 | 0.0606 (19) | 0.0446 (16) | 0.0388 (14) | 0.0013 (14) | 0.0254 (13) | 0.0046 (12) |
| C4 | 0.0445 (16) | 0.0373 (14) | 0.0382 (13) | 0.0062 (11) | 0.0180 (12) | 0.0031 (11) |
| C5 | 0.0602 (19) | 0.0332 (14) | 0.0537 (17) | 0.0073 (13) | 0.0267 (14) | 0.0094 (12) |
| C6 | 0.0612 (19) | 0.0389 (15) | 0.0571 (17) | 0.0089 (14) | 0.0379 (15) | 0.0054 (13) |
| C7 | 0.0625 (19) | 0.0549 (18) | 0.0460 (16) | −0.0029 (15) | 0.0336 (15) | 0.0057 (13) |
| C8 | 0.071 (2) | 0.0428 (16) | 0.0476 (16) | 0.0035 (14) | 0.0285 (15) | −0.0029 (12) |
| C9 | 0.071 (2) | 0.0342 (15) | 0.069 (2) | 0.0078 (15) | 0.0329 (17) | 0.0062 (14) |
Geometric parameters (Å, °)
| S1—C1 | 1.728 (2) | C3—H3B | 0.98 (4) |
| S2—C1 | 1.709 (2) | C4—C5 | 1.512 (4) |
| O2—C7 | 1.418 (4) | C4—H4A | 0.96 (3) |
| O2—C9 | 1.423 (4) | C4—H4B | 0.93 (3) |
| O1—C3 | 1.414 (3) | C5—H5A | 1.02 (3) |
| O1—C5 | 1.428 (3) | C5—H5B | 0.94 (4) |
| N1—C1 | 1.341 (3) | C6—C7 | 1.498 (4) |
| N1—C4 | 1.466 (3) | C6—H6A | 1.02 (3) |
| N1—C2 | 1.468 (3) | C6—H6B | 0.89 (4) |
| N2—C6 | 1.478 (3) | C7—H7A | 0.96 (3) |
| N2—C8 | 1.480 (4) | C7—H7B | 0.95 (4) |
| N2—H1N | 0.86 (4) | C8—C9 | 1.498 (4) |
| N2—H2N | 0.91 (4) | C8—H8A | 1.05 (3) |
| C2—C3 | 1.515 (4) | C8—H8B | 0.90 (3) |
| C2—H2A | 1.01 (3) | C9—H9A | 1.01 (3) |
| C2—H2B | 0.94 (4) | C9—H9B | 0.94 (4) |
| C3—H3A | 1.01 (3) | ||
| C7—O2—C9 | 110.7 (2) | H4A—C4—H4B | 115 (3) |
| C3—O1—C5 | 110.1 (2) | O1—C5—C4 | 111.3 (2) |
| C1—N1—C4 | 124.7 (2) | O1—C5—H5A | 108.8 (19) |
| C1—N1—C2 | 122.8 (2) | C4—C5—H5A | 107.2 (19) |
| C4—N1—C2 | 112.2 (2) | O1—C5—H5B | 103 (2) |
| C6—N2—C8 | 111.0 (2) | C4—C5—H5B | 112 (2) |
| C6—N2—H1N | 107 (2) | H5A—C5—H5B | 114 (3) |
| C8—N2—H1N | 111 (2) | N2—C6—C7 | 108.9 (2) |
| C6—N2—H2N | 112 (2) | N2—C6—H6A | 106.0 (19) |
| C8—N2—H2N | 107 (2) | C7—C6—H6A | 109.9 (19) |
| H1N—N2—H2N | 109 (3) | N2—C6—H6B | 109 (2) |
| N1—C1—S2 | 120.49 (17) | C7—C6—H6B | 113 (2) |
| N1—C1—S1 | 119.70 (18) | H6A—C6—H6B | 110 (3) |
| S2—C1—S1 | 119.79 (13) | O2—C7—C6 | 111.4 (3) |
| N1—C2—C3 | 109.9 (2) | O2—C7—H7A | 110 (2) |
| N1—C2—H2A | 109.1 (19) | C6—C7—H7A | 110 (2) |
| C3—C2—H2A | 111 (2) | O2—C7—H7B | 104 (2) |
| N1—C2—H2B | 106 (2) | C6—C7—H7B | 109 (2) |
| C3—C2—H2B | 113 (2) | H7A—C7—H7B | 112 (3) |
| H2A—C2—H2B | 108 (3) | N2—C8—C9 | 109.5 (2) |
| O1—C3—C2 | 111.9 (2) | N2—C8—H8A | 100.0 (19) |
| O1—C3—H3A | 106 (2) | C9—C8—H8A | 114.1 (19) |
| C2—C3—H3A | 109.6 (19) | N2—C8—H8B | 109 (2) |
| O1—C3—H3B | 105 (2) | C9—C8—H8B | 116 (2) |
| C2—C3—H3B | 109 (2) | H8A—C8—H8B | 107 (3) |
| H3A—C3—H3B | 115 (3) | O2—C9—C8 | 111.6 (3) |
| N1—C4—C5 | 110.0 (2) | O2—C9—H9A | 105.6 (19) |
| N1—C4—H4A | 109 (2) | C8—C9—H9A | 109.2 (19) |
| C5—C4—H4A | 107 (2) | O2—C9—H9B | 106 (2) |
| N1—C4—H4B | 107 (2) | C8—C9—H9B | 109 (2) |
| C5—C4—H4B | 108 (2) | H9A—C9—H9B | 116 (3) |
| C4—N1—C1—S2 | 178.8 (2) | C2—N1—C4—C5 | −53.1 (3) |
| C2—N1—C1—S2 | 5.9 (3) | C3—O1—C5—C4 | −60.0 (3) |
| C4—N1—C1—S1 | −3.0 (3) | N1—C4—C5—O1 | 56.4 (3) |
| C2—N1—C1—S1 | −175.9 (2) | C8—N2—C6—C7 | −55.7 (4) |
| C1—N1—C2—C3 | −133.9 (3) | C9—O2—C7—C6 | −60.0 (4) |
| C4—N1—C2—C3 | 52.4 (3) | N2—C6—C7—O2 | 58.1 (4) |
| C5—O1—C3—C2 | 59.7 (3) | C6—N2—C8—C9 | 54.9 (4) |
| N1—C2—C3—O1 | −55.7 (3) | C7—O2—C9—C8 | 59.0 (4) |
| C1—N1—C4—C5 | 133.4 (3) | N2—C8—C9—O2 | −56.2 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H1N···S1 | 0.86 (4) | 2.47 (4) | 3.284 (3) | 158 (3) |
| N2—H2N···S1i | 0.91 (4) | 2.75 (4) | 3.453 (2) | 135 (3) |
| N2—H2N···S2i | 0.91 (4) | 2.39 (3) | 3.221 (2) | 151 (3) |
Symmetry codes: (i) −x+1, −y, −z+2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SU2285).
References
- Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C., Polidori, G. & Camalli, M. (1994). J. Appl. Cryst. 27, 435.
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- Enraf–Nonius (1989). CAD-4 EXPRESS Enraf–Nonius, Delft, The Netherlands.
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst. 32, 837–838.
- Harms, K. & Wocadlo, S. (1995). XCAD4 University of Marburg, Germany.
- Macrae, C. F., Edgington, P. R., McCabe, P., Pidcock, E., Shields, G. P., Taylor, R., Towler, M. & van de Streek, J. (2006). J. Appl. Cryst. 39, 453–457.
- Mafud, A. C. & Gambardella, M. T. P. (2011a). Acta Cryst. E67, o879. [DOI] [PMC free article] [PubMed]
- Mafud, A. C. & Gambardella, M. T. P. (2011b). Acta Cryst. E67, m942. [DOI] [PMC free article] [PubMed]
- North, A. C. T., Phillips, D. C. & Mathews, F. S. (1968). Acta Cryst. A24, 351–359.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst D65, 148–155. [DOI] [PMC free article] [PubMed]
- Wahlberg, A. (1979). Acta Cryst. B35, 485–487.
- Wahlberg, A. (1980). Acta Cryst. B36, 2099–2103.
- Wahlberg, A. (1981). Acta Cryst. B37, 1240–1244.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811026286/su2285sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811026286/su2285Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811026286/su2285Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report