Abstract
Recently, the Centers for Disease Control and Prevention reported an accurate, sensitive, specific, reproducible, and quantitative enzyme-linked immunosorbent assay (ELISA) for immunoglobulin G (IgG) antibodies to Bacillus anthracis protective antigen (PA) in human serum (C. P. Quinn, V. A. Semenova, C. M. Elie et al., Emerg. Infect. Dis. 8:1103-1110, 2002). The ELISA had a minimum detectable concentration (MDC) of 0.06 μg/ml, which, when dilution adjusted, yielded a whole-serum MDC of 3.0 μg of anti-PA IgG per ml. The reliable detection limit (RDL) was 0.09 μg/ml, while the dynamic range was 0.06 to 1.7 μg/ml. The diagnostic sensitivity of the assay was 97.6% and the diagnostic specificity was 94.2% for clinically verified cases of anthrax. A competitive inhibition anti-PA IgG ELISA was also developed to enhance the diagnostic specificity to 100%. We report a newly developed fluorescence covalent microbead immunosorbent assay (FCMIA) for B. anthracis PA which was Luminex xMap technology. The FCMIA MDC was 0.006 μg of anti-PA IgG per ml, the RDL was 0.016 μg/ml, and the whole-serum equivalent MDC was 1.5 μg/ml. The dynamic range was 0.006 to 6.8 μg/ml. Using this system, we analyzed 20 serum samples for anti-PA IgG and compared our results to those measured by ELISA in a double-masked analysis. The two methods had a high positive correlation (r2 = 0.852; P < 0.001). The FCMIA appears to have benefits over the ELISA for the measurement of anti-PA IgG, including greater sensitivity and speed, enhanced dynamic range and reagent stability, the use of smaller sample volumes, and the ability to be multiplexed (measurement of more than one analyte simultaneously), as evidenced by the multiplexed measurement in the present report of anti-PA and anti-lethal factor IgG in serum from a confirmed clinical anthrax infection.
In response to the anthrax terrorist attacks of 2001, the Centers for Disease Control and Prevention (CDC) undertook accelerated development for a quantitative enzyme-linked immunosorbent assay (ELISA) for detection of anti-protective antigen (PA)-specific immunoglobulin G (IgG) in human serum and the development of a competitive inhibition assay to enhance diagnostic specificity (15). This assay was shown to have a diagnostic sensitivity of 97.6% and a diagnostic specificity of 94.2%. Preadsorption of sera with PA enhanced the diagnostic specificity to 100%. A potential limitation of ELISA is that it is a monoplex technology. Only one analyte can be measured per assay; measurement of numerous analytes necessitates either simultaneous or sequential assays. When the number of analytes becomes large, resource and manpower limitations can occur. An alternative to the ELISA is an assay that can multiplex analytes, i.e., measure numerous analytes simultaneously. Fluorescent covalent microsphere immunoassay (FCMIA) is a technology that can accomplish this by using uniquely dually stained microspheres for the measurement of up to 100 analytes simultaneously (18). In the present report we describe a newly developed FCMIA and compare it to a specific, sensitive, and quantitative ELISA for anti-PA IgG and also present multiplexed data for measuring anti-PA and anti-lethal factor (LF) IgG in serum from a confirmed case of human clinical anthrax.
MATERIALS AND METHODS
Serum samples.
Twenty-two serum samples (3 quality control standards, 1 negative control standard, and 16 unknown samples, a sample from a case of clinically confirmed anthrax [AVR733], and a human anti-anthrax vaccine standard reference serum [15]) were used as a standardized reagent set for this study. The anti-AVA (Anthrax Vaccine Adsorbed, BioThrax; BioPort Corp., Lansing, Mich.) standard human reference serum, AVR414 (170.1 μg of anti-B. anthracis PA IgG per ml), was prepared by plasmapheresis of healthy adult CDC volunteers who had received at least four subcutaneous injections of AVA under the licensed regimen (0, 2, and 4 weeks; 6, 12, and 18 months; and yearly boosters). Serum AVR733 contained 65 μg of anti-B. anthracis LF per ml and 198 μg of anti-PA IgG per ml (the anti-PA IgG value for AVR733 was obtained from AVR414 standardization by ELISA). A subset of the reagent set, composed of 20 samples ranging from below the minimal detectable concentration (MDC) of the ELISA to 340 μg of anti-PA IgG per ml, was selected and coded by the Microbial Pathogenesis and Immune Response Laboratory Data Analysis Team proctor for the comparison. The samples were coded and supplied to the analysts in a masked fashion. After the data had been acquired, the codes were broken by the data proctor. Sera were stored frozen at −20°C until used and were coded and masked for all assays. The use of all human samples was approved by the CDC Human Subjects Review Board.
Antigens.
For the ELISA, recombinant anthrax toxin PA with an amino acid sequence concurring with that from the B. anthracis V770-NP1-R anthrax vaccine strain was obtained from the National Institute of Craniofacial and Dental Research, National Institutes of Health, Bethesda, Md. Antigen was produced and purified as described previously (9, 12) and was stored frozen at −80°C in small aliquots (10 to 100 μl, 4.75 mg/ml) in 5 mM HEPES (pH 7.3).
The antigens used in the FCMIA were recombinant PA and LF obtained from List Biological Laboratories, Inc., (Campbell, Calif.). Both antigens migrated as single major bands with apparent molecular masses of 83,000 Da (PA) and 90,000 Da (LF) on 10% polyacrylamide gels in the presence of sodium dodecyl sulfate. The PA and LF were reconstituted in distilled water and stored as aliquots at −20°C. Before use, individual aliquots were thawed, mixed thoroughly, and used immediately.
ELISA procedure.
The ELISA procedure has been described in detail previously (15). Serum standards and sera for testing were prepared at the appropriate dilutions in phosphate-buffered saline (PBS) containing 5% skim milk and 0.5% Tween 20 (pH 7.4). The human standard reference serum and test sera were serially diluted twofold in the same buffer solution in the plate. The minimum dilution of test serum was 1:50. The final volume in all wells was 100 μl. Bound anti-PA IgG was detected by using horseradish peroxidase-conjugated mouse anti-human IgG Fc PAN monoclonal HP6043 and 2,2′-azino-di(3-ethyl-benzthiazoline-6-sulfonate) (ABTS)-H2O2 substrate (100 μl/well). Color development was carried out over 30 min (±5 min) and was stopped by addition of 100 μl of peroxidase stop solution (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) to all wells of the test plates. Optical densities values were read within 30 min of addition of the stop solution with an MRX Revelation microtiter plate reader (Thermo Labsystems, Franklin, Mass.) at a wavelength of 410 nm with a 610-nm reference filter. Data were analyzed by using a four-parameter (4-PL) logistic-log curve fitting model with ELISA for Windows software (15). A calibration factor for the standard reference serum was used to determine the concentration of anti-PA IgG in micrograms per milliliter of serum. Comparison sera were diluted 1:50.
Preparation of PA and LF coupled microspheres.
Briefly, after being washed twice with 80 μl of activation buffer (0.1M NaH2PO4 [pH 6.2]), two sets of spectrally differentiable carboxylated microspheres (2.5 × 106; Luminex Corp., Austin, Tex.) were pelleted (5,000 × g for 2 min) in 1.5-ml centrifuge tubes in a microcentrifuge (Eppendorf, Hamburg, Germany). The microspheres were resuspended by sonication (mini sonicator; Cole Parmer, Vernon Hills, Ill.) and gentle vortexing (VWR International, West Chester, Pa.) in 80 μl of activation buffer to which 10 μl of activation buffer containing 50 mg of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC; Pierce Chemical Co., Rockford, Ill.) per ml and 10 μl of activation buffer containing 50 mg of N-hydroxysulfosuccinimide, sodium salt (sulfo-NHS; Pierce Chemical Co.) per ml were added. The mixture was allowed to incubate for 20 min at room temperature. The microspheres were then washed twice in 500 μl of coupling buffer [0.05 M 2-(N-morpholino)ethanesulfonic acid (MES) (Sigma Chemical Co.); pH 5.0], a solution of PA or LF (40 μg/ml) in 500 μl of coupling buffer was added, and the mixture was incubated for 2 h at room temperature. The 40-μg/ml concentration was used for coupling because preliminary experiments indicated that this coupling concentration yielded high mean fluorescence intensities (MFIs) and good inhibition (data not shown). The coupled microspheres were then washed twice in 1 ml of wash buffer (PBS containing 0.05% Tween 20 [Sigma no. 3563]) and stored in 0.5 ml of storage buffer (PBS, 1% bovine serum albumin [Sigma no. 3688], 0.05% sodium azide [EM Sciences, Cherry Hill, N.J.] [pH 7.4]). Microsphere concentrations were determined using a hemacytometer (Bright Line; VWR International).
Monoplex and multiplexed measurements obtained using FCMIA.
For comparison with the anti-PA IgG ELISA, a monoplex anti-PA IgG FCMIA format was used with pooled sera obtained from anthrax vaccinees (AVR414). Serum AVR414, however, contains only low concentrations of anti-LF IgG, making it unsuitable for use as a standard reagent for determining the concentrations of that antibody. For this reason, serum AVR733, which was obtained from a patient with a confirmed case of human anthrax and which contains both anti-PA and anti-LF IgG, was used in a multiplexed FCMIA format employing a duplex mixture of microspheres. Briefly, suspensions of microspheres (100 microspheres/μl) were incubated for 1 h at 37°C in blocking buffer (PBS [Sigma no. 3813], 5% dried skim milk [Difco. Sparks, Md.], 0.05% sodium azide [pH 7.4]) to minimize non-specific binding during subsequent steps. An aliquot (50 μl) of this suspension (approximately 5,000 microspheres) was added to the wells of a 1.2-μm-pore-size filter membrane microtiter plate (no. MABVN1250; Millipore Corp., Bedford, Mass.), and the liquid was aspirated by use of a vacuum manifold filtration system (Millipore no. MAVM09601). The microspheres were then washed three times with 200 μl of wash buffer, and each wash followed by vacuum aspiration. To run an experiment, 50-μl portions (in duplicate) of diluted comparison sera (1:250 in dilution buffer consisting of PBS, 5% dried skim milk, 0.05% sodium azide, and 1% Tween 20 [pH 7.4]), diluted standards prepared from AVR 414, dilutions of serum AVR733, or reagent blank were added to the microspheres in the wells of the filter membrane microtiter plate and incubated for 30 min at 37°C with shaking. The liquid was vacuum aspirated, and the wells were again washed three times with 200 μl of wash buffer. An aliquot (50 μl) of a 2-μg/ml solution of detection antibody (mouse anti-human IgG-red algae-phycoerythrin, clone HP6043 [Biotrend Chemicals, Inc., Destin, Fla.]) in dilution buffer was added to the wells of the plate and incubated for 15 min at 37°C. The wells were again washed three times with 200 μl of wash buffer and resuspended in 100 μl of wash buffer. The plate was shaken vigorously for approximately 1 min to disperse the microspheres and was placed into the autosampler platform of the Luminex 100 instrument (Luminex 100 flow analyzer, coupled with a 96-well plate autosampler, Luminex XYP [Luminex]) as previously described (3), using software, calibration microspheres, and sheath fluid supplied by the manufacturer. The instrument was programmed to collect data from 100 microspheres (classified by their internal fluorescence ratio) and acquire the MFI of the microsphere-PA-IgG(-LF)-anti-IgG-R-phycoerythrin complex.
Competitive inhibition FCMIA.
To determine the specificity of measurements performed by FCMIA, all sera which were used for the comparison with ELISA were preincubated with PA. For the multiplex analyses, serum AVR733 was preincubated with PA and LF (competitive inhibition). To perform an inhibition experiment, sera (final volume, 130 μl; final dilution, 1:250) were treated with either 80 μg of PA (AVR414) or PA and LF per ml singly or in combination (80 μg/ml each) or treated with dilution buffer and incubated overnight at 4°C. The sera were then centrifuged, and the supernatants were analyzed as outlined above. Inhibition was calculated by the following expression:
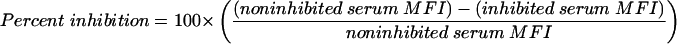 |
Cross-reactivity between PA and LF was assessed by comparing AVR733 (diluted 1:250) PA plus LF multiplexed MFIs when added individually or as a mixture at 100 μg/ml each.
Data analysis.
Standard curves were constructed from 4-PL logistic log fits of MFI versus the concentration (in micrograms per milliliter) of anti-PA IgG (AVR414) or anti-PA IgG and anti-LF (AVR733) IgG (11, 14) (SigmaPlot; SPSS, Chicago, Ill.). Duplicate results from individual samples were averaged, and concentrations were interpolated from the standard curve. The FCMIA was run three separate times, and the ELISA was run two or three separate times. Assessment of the “goodness of fit” and the dynamic range of the standards data to the 4-PL model was performed by standards recovery (10), evaluated by diluting AVR414 or AVR733 (in dilution buffer) from 1:25 to 1:1,000,000 (AVR414) or 1:25 to 1:100,000 (AVR733) and determining the resultant plot of measured concentration versus expected concentration by regression analyses of interpolated results from the 4-PL fit for linearity by regression. Recovery was calculated by the following equation:
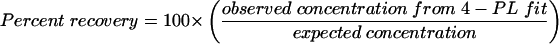 |
Analyses of recoveries at each dilution were investigated by a Kruskal-Wallis one-way analysis of variance Anova (SigmaStat). The stability of the PA-coupled microspheres on storage was investigated by comparing 4-PL fits from a single batch of microspheres which had been stored for 9 months at 4°C in storage buffer. The entire anti-PA IgG 4-PL curves were compared for equality by using a F-ratio method (1). The anti-PA IgG MDC was calculated from the intersection of the asymptote of the regression's 95% confidence interval (CI) with the regression line, while the reliable detection limit (RDL) was evaluated from the interpolated intersection of the upper 95% CI asymptote with the lower 95% CI of the standards data as previously described (11, 15). An averaging method (5) was used to minimize bias in cases where sera had results below the limit of detection of the assay. P values of ≤0.05 were considered statistically significant.
RESULTS
Anti-PA IgG ELISA versus FCMIA.
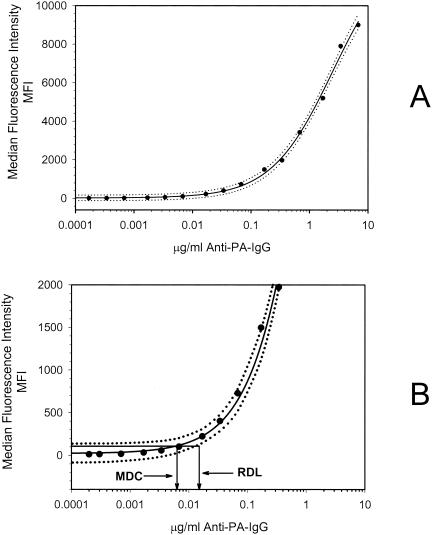
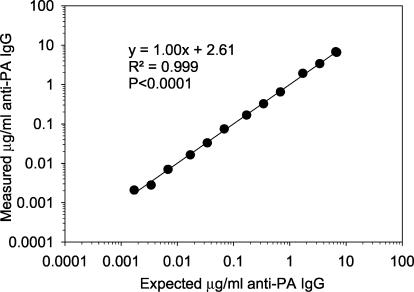
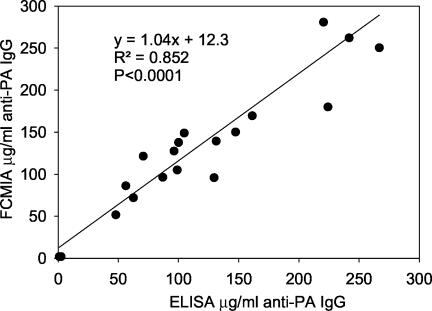
The accuracy, precision, limits of quantification, goodness of fit, limits of detection, etc., have been reported previously (15) for the anti-PA ELISA. The FCMIA anti-PA assay yielded an excellent fit for the 4-PL model (Fig. 1A) (r2 = 0.999; P < 0.0001). The method MDC (not adjusted for serum dilution) was 0.006 μg of anti-PA IgG per ml, while the RDL was 0.016 μg of anti-PA IgG per ml (Fig. 1B). When the MDC was multiplied by the 1:250 dilution used to measure sample sera (a dilution selected to allow all sera to be measured at one dilution and still be in the dynamic range of the assay), the whole-serum equivalent MDC was 1.5 μg of anti-PA IgG per ml. The FCMIA assay yielded intra- and interassay coefficients of variation of 5.7 and 13.1% (n = 3 independent assays, 20 sera, assayed in duplicate), respectively. Investigation of the linearity of dilution for serum AVR414, using dilutions of 1:25, 1:100, 1:250, 1:500, 1:1,000, 1:2,500, 1:5,000 and 1:10,000, 1:25,000, 1:50,000, 1:100,000, 1:250,000, 1:500,000, and 1:1,000,000, yielded a highly significant linear relationship (mean r2 = 0.999; P < 0.0001 [Fig. 2]), with a dynamic range of 0.006 to 6.8 μg of anti-PA IgG per ml. Investigation of the stability of the PA-coupled microspheres after storage yielded no significant differences in 4-PL anti-PA IgG standard curves (P = 0.894) when the microspheres were stored at 4°C for 9 months. The mean recovery of measured anti-PA IgG, compared to the expected anti-PA IgG concentrations, was 100.8% ± 13.0% (mean ± standard deviation; range, 82 to 120%). The recovery at each dilution was not significantly different (P = 0.91) from the calculated concentration obtained from the dilution of standard serum AVR414. Preadsorption of the serum samples with PA yielded a mean inhibition of the measured anti-PA IgG concentrations of 97.9% ± 1.9% for 18 of the 20 samples. One sample, with an anti-PA IgG concentration of 2.4 μg/ml, yielded an inhibition of 5.1%, while the negative control sample gave results below the MDC of the FCMIA. Evaluation of the association of anti-PA IgG concentrations measured by FCMIA and ELISA (Fig. 3) yielded a highly significant correlation (r2 = 0.852; P < 0.0001). The relationship between the assays was described by the following: FCMIA = 1.04 × ELISA + 12.3. Evaluation of the regression showed that the slope was not different from 1 (P = 0.73; 95% CI, 0.82 to 1.25) and the intercept was not different from 0 (P = 0.54; 95% CI, −18.90 to 43.51), indicating a line of identity and equivalence of the anti-PA IgG concentrations measured by each method. The interassay (FCMIA versus ELISA) coefficient of variation (CV) for the 18 sera with values above the MDC for both methods was 15.7 (calculated from the variance of the mean values for each sample measured by both methods).
FIG. 1.
(A) Representative FCMIA 4-PL (solid line) of the anti-PA IgG concentration versus MFI. The dotted lines represent the upper and lower 95% CI of the regression. The regression coefficient was r2 = 0.999 (P < 0.001). Data points are the mean of duplicate determinations. (B) Magnified view of panel A, showing a graphic representation of the MDC and RDL. The MDC is the concentration of anti-PA IgG corresponding to the interpolated intersection of the lower asymptote of the upper 95% confidence limit with the 4-PL logistic log fit of the standard curve data. The RDL is the concentration of anti-PA antibody corresponding to the interpolated intersection of the lower asymptote of the upper 95% confidence limit with the lower 95% confidence limit of the standard's data.
FIG. 2.
Linear regression of the results of diluting standard sera AVR414 from 1:50 to 1:100,000 and evaluating the resultant plot of measured concentration versus expected concentration for linearity. Data points are the mean of duplicate determinations.
FIG. 3.
Linear regression of anti-PA IgG concentrations measured by FCMIA and ELISA from 20 double-masked sera samples. Data points are the mean of two or three determinations performed in duplicate.
Multiplexed anti-PA and anti-LF IgG FMCIA.
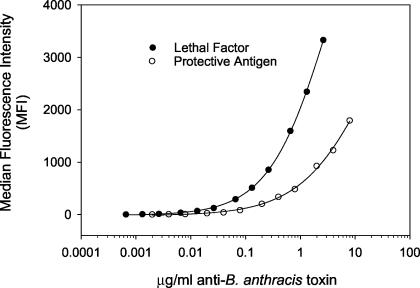
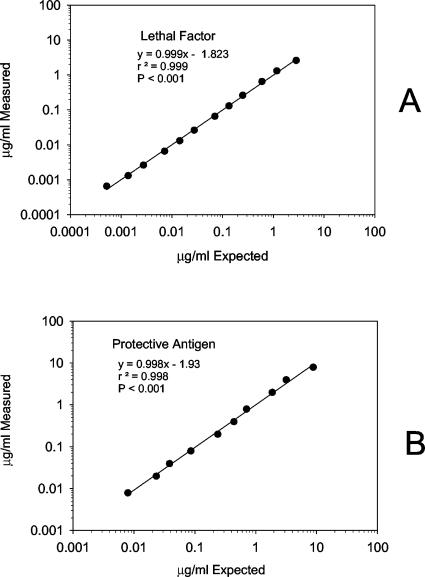
The 4-PL fit (Fig. 4) for the multiplexed anti-PA and anti-LF IgG standard curves was high (r2 = 0.999; P < 0.0001, both analytes). The MDC for anti-LF IgG was 0.001 μg/ml, while the RDL was 0.003 μg/ml, calculated from serum AVR733. The multiplexed FCMIA yielded intra- and interassay CVs of 11.1 and 9.7% and of 7.6 and 10.4% for PA and LF, respectively (n = 2 independent multiplexed assays). The MDC for anti-PA IgG was 0.01 μg/ml, while the RDL was 0.02 μg/ml, calculated from serum AVR733. Investigation of the linearity of dilution for serum AVR733 for anti-PA and anti-LF, measured in a multiplex assay using dilutions of 1:25 to 1:100,000, yielded highly significant (P < 0.001) linear relationships (r2 = 0.998 and 0.999), respectively (Fig. 5). The mean recoveries of measured anti-PA and anti-LF compared to the expected anti-PA and anti-LF concentrations were 100.7% ± 13.0% (range, 80 to 120%) and 100.4% ± 9.6% (range, 79 to 110%). The recoveries at each dilution were not significantly different (P = 0.91 and 1.00) from the calculated concentrations obtained from the dilution of serum AVR733 for PA and LF. Inhibition studies showed 94.8 and 85.3% inhibition from preadsorption values with LF and PA, respectively, of their nonpreadsorbed IgG values. There was no cross-reactivity between PA and LF. There was no significant difference (P = 0.770 by the t test) in the MDCs for anti-PA IgG when compared in the monoplex and multiplex formats.
FIG. 4.
Representative multiplexed FCMIA 4-PL (solid line) of the concentration of anti-PA IgG and anti-LF IgG versus MFI. The goodness of fit, as described by the regression coefficients, was r2 = 0.999 (P < 0.0001) for both curves. Data points are the mean of duplicate determinations.
FIG. 5.
Linear regression of the results of diluting clinical anthrax serum AVR733 from 1:25 to 1:100,000 and evaluating the resultant plot of measured concentration versus expected concentration for LF (A) and PA (B). Data points are the mean of duplicate determinations.
DISCUSSION
Numerous ELISAs have been described for measuring anti-PA antibodies (6, 8, 15, 17). The most recent of these (15), was developed in response to the bioterrorism-associated human anthrax epidemic in the fall of 2001 and has been shown to be sensitive, reproducible, and quantitative for the measurement of anti-PA IgG antibodies. That test, which was used for comparison with our anti-PA IgG FCMIA, had a minimum detection limit of 0.06 μg of anti-PA IgG/ml, a reliable lower limit of detection of 0.09 μg/ml, and a lower limit of quantification in undiluted serum specimens of 3.0 μg/ml. The intra- and interassay CVs were 8.5 and 17.0%, respectively. The diagnostic sensitivity of the assay was 97.6%, and the diagnostic specificity was 94.2%. Preadsorption of sera with PA was shown to enhance the diagnostic specificity to 100% (15).
The anti-PA FCMIA reported in the present study has intra- and interassay coefficients of variation of 5.7 and 13.1%, respectively, well within the <10 and <20% intra- and inter-assay guidelines for this type of assay (7), suggesting extremely good precision (15). The FCMIA also yielded a highly significant linear relationship on dilution and yielded a mean recovery of 101.7% ± 5.3% (SD) over the concentration range of 0.002 to 6.8 μg of anti-PA IgG per ml, suggesting that an accurate measurement of anti-PA IgG with an excellent dynamic range was performed by this method. The method MDC was 0.006 μg/ml, the RDL was 0.020 μg/ml, and the whole-serum equivalent MDC was 1.5 μg/ml, indicating greater sensitivity than that of ELISA. Investigation of the stability of the PA-coupled microspheres after storage yielded no significant differences in 4-PL anti-PA IgG standard curves (P > 0.10) after storage at 4°C for 9 months. Reagent stability for extended periods is desirable to be able to quickly respond to urgent requests for diagnostic testing. Preadsorption of the serum samples with PA yielded a mean inhibition of measured anti-PA IgG concentrations of 97.9% ± 1.9%, indicating immunochemical specificity. Anti-PA IgG concentrations calculated for the standard reagent set by FCMIA had a high positive correlation with those generated by ELISA (r2 = 0.852; P < 0.0001), and the interassay CV was 15.7% when the results from both assays for the masked sera samples (N = 20) were compared. Finally, analyses of the concentrations of anti-PA IgG found for identical samples measured by ELISA and FCMIA gave a linear regression line which was indistinguishable from a line of identity, indicating the equivalence of the methods. These data strongly suggest that FCMIA and ELISA are equivalent for the measurement of anti-PA IgG. They also indicate that FCMIA for anti-PA IgG, similar to ELISA, is accurate, reproducible, sensitive, stable, and precise. Discrepancies between FCMIA and ELISA became apparent at the lower limits of analyte detection (MDC and RDL). One sample, with an anti-PA IgG concentration by FCMIA of 2.4 μg/ml was above the MDC of the FCMIA (1.5 μg/ml) but below both the MDC and RDL of ELISA (3.0 and 4.5 μg/ml, respectively). This sample yielded an inhibition of 5.1% on preadsorption, which did not meet previously described criteria (15) for a “true”-positive sample; as such, it would be considered a false-positive result in FCMIA.
An inherent benefit of FCMIA over ELISA is its ability to be multiplexed (i.e., to measure numerous analytes simultaneously) (2, 4, 13). We demonstrated this by measuring anti-PA and anti-LF IgG levels simultaneously in a serum sample from a patient with a confirmed clinical anthrax infection. Both PA and LF, when measured as a multiplex, yielded highly linear responses on dilution and high mean percent recoveries (100.4 and 100.7%, respectively) and showed good inhibition from preadsorption with PA and LF (>85%). Anti-PA, -LF, and -endema factor IgG have been measured in sera from individuals with cutaneous and oral or oropharyngeal anthrax by three separate ELISAs (16); however, we believe this to be the first demonstration of a multiplexed measurement of anti-PA and anti-LF IgG.
According to the U.S. State Department and Department of Defense, the future deployment of biological agents by foreign militaries or terrorists is likely (19). Multiplexing becomes important in the postbioterrorism incident scenario, where multiple chemical and/or biological agents may be used simultaneously or sequentially. This scenario could potentially result in a need for numerous fast, accurate, and precise biological markers of infection by or exposure to many biological and chemical agents. Multiplexed FCMIA technology is unique in that both chemical and biological assays potentially could be performed simultaneously in one assay with the Luminex xMap technology reported on in the present work, an aspect we are presently investigating by using assays to detect immune responses from infection by or exposure to Francisella tularensis, Yersinia pestis, ricin, botulism toxin, staphylococcal enterotoxin B, and B. anthracis. Existing technologies (ELISA, gas-liquid chromatography/mass spectrometry, etc.) would have to be performed sequentially or simultaneously to measure numerous analytes. As the number of potentially exposed individuals and the number of potential analytes increases, use of these existing technologies could present significant resource and labor limitations. At present, numerous public health laboratories possess the necessary equipment and knowledge to perform ELISA testing for exposure to or infection by biological agents of terror. Incorporating multiplexed FCMIA technology into public health laboratories would involve the acquisition of specialized equipment and additional specific training; however, it may improve their capacity to respond to future, possibly more complex bioterrorism events.
In conclusion, we have described a FCMIA for anti-PA IgG in human serum which is accurate, precise, sensitive, and specific and agrees well with a specific, sensitive, and quantitative ELISA (15) when sera were analyzed in a double-masked analyses by the two methods. Compared with ELISA, FMCIA had improved sensitivity (ELISA MDC = 3.0 μg/ml; FCMIA MDC = 1.5 μg/ml) and a greater dynamic range (ELISA, 0.06 to 1.75 μg/ml; FCMIA, 0.006 to 6.8 μg/ml). The FCMIA is also faster, uses less sample, and has reagents which are stable for up to 9 months at 4°C. It has the ability to be multiplexed, as evidenced by the demonstration of the simultaneous measurement of anti-PA and anti-LF IgG in serum from patient with a confirmed anthrax case. The multiplexing capability of FCMIA is presently being exploited to detect immune responses from exposure to F. tularensis, Y. pestis, ricin, botulism toxin, and staphylococcal enterotoxin B as well as to measure antibody responses to B. anthracis.
Acknowledgments
This work was supported in part by an interagency agreement between NIOSH and NIEHS (Y02ES10189).
Mention of a product or company name does not constitute endorsement by CDC.
We thank Ed Kreig (NIOSH) for assistance in performing some of the statistical analyses and Brian D. Plikaytis and Thomas H. Taylor, Jr., of DBMD, NCID, for critical review of the manuscript.
REFERENCES
- 1.Bardsley, W. G., and P. B. McGinlay. 1987. The use of non-linear regression analysis and the F test for model discrimination with dose-response curves and ligand binding data. J. Theor. Biol. 126:183-201. [DOI] [PubMed] [Google Scholar]
- 2.Biagini, R. E., D. M. Murphy, D. L. Sammons, J. P. Smith, C. A. Striley, and B. A. MacKenzie. 2002. Development of multiplexed fluorescence microbead covalent assays (FMCAs) for pesticide biomonitoring. Bull. Environ. Contam. Toxicol. 68:470-477. [DOI] [PubMed] [Google Scholar]
- 3.Biagini, R. E., S. A. Schlottmann, D. L. Sammons, J. P. Smith, J. C. Snawder, C. A. Striley, B. A. MacKenzie, and D. N. Weissman. 2003. Method for simultaneous measurement of antibodies to 23 pneumococcal capsular polysaccharides. Clin. Diagn. Lab. Immunol. 10:744-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Jager, W., H. Te Velthuis, B. J. Prakken, W. Kuis, and G. T. Rijkers. 2003. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin. Diagn. Lab. Immunol. 10:133-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hornung, R., and L. Reed. 1990. Estimate of average concentration in the presence of non-detectable values. Appl. Occup. Environ. Hyg. 5:46-51. [Google Scholar]
- 6.Iacono-Connors, L. C., J. Novak, C. Rossi, J. Mangiafico, and T. Ksiazek. 1994. Enzyme-linked immunosorbent assay using a recombinant baculovirus-expressed Bacillus anthracis protective antigen (PA): measurement of human anti-PA antibodies. Clin. Diagn. Lab. Immunol. 1:78-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson, R. H. 1998. Validation of serological assays for diagnosis of infectious diseases. Rev. Sci. Tech. 17:469-526. [DOI] [PubMed] [Google Scholar]
- 8.Johnson-Winegar, A. 1984. Comparison of enzyme-linked immunosorbent and indirect hemagglutination assays for determining anthrax antibodies. J. Clin. Microbiol. 20:357-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leppla, S. 1991. Purification and characterization of adenyl cyclase from Bacillus anthracis. Methods Enzymol. 195:153-168. [DOI] [PubMed] [Google Scholar]
- 10.Nix, B., and D. Wild. 2001. Calibration curve-fitting, p. 198-210. In D. Wild (ed.), The immunoassay handbook. Nature Publishing Group, New York, N.Y.
- 11.O'Connell, M., B. Belanger, and P. Haaland. 1993. Calibration and assay development using the four-parameter logistic model. Chemometrics Intell. Lab. Systems 20:97-114. [Google Scholar]
- 12.Park, S., and S. Leppla. 2000. Optimized production and purification of Bacillus anthracis lethal factor. Protein Expression Purif. 18:293-302. [DOI] [PubMed] [Google Scholar]
- 13.Pickering, J. W., T. B. Martins, R. W. Greer, M. C. Schroder, M. E. Astill, C. M. Litwin, S. W. Hildreth, and H. R. Hill. 2002. A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am. J. Clin. Pathol. 117:589-596. [DOI] [PubMed] [Google Scholar]
- 14.Plikaytis, B. D., S. H. Turner, L. L. Gheesling, and G. M. Carlone. 1991. Comparisons of standard curve-fitting methods to quantitate Neisseria meningitidis group A polysaccharide antibody levels by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 29:1439-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn, C. P., V. A. Semenova, C. M. Elie, S. Romero-Steiner, C. Greene, H. Li, K. Stamey, E. Steward-Clark, D. S. Schmidt, E. Mothershed, J. Pruckler, S. Schwartz, R. F. Benson, L. O. Helsel, P. F. Holder, S. E. Johnson, M. Kellum, T. Messmer, W. L. Thacker, L. Besser, B. D. Plikaytis, T. H. Taylor, Jr., A. E. Freeman, K. J. Wallace, P. Dull, J. Sejvar, E. Bruce, R. Moreno, A. Schuchat, J. R. Lingappa, S. K. Martin, J. Walls, M. Bronsdon, G. M. Carlone, M. Bajani-Ari, D. A. Ashford, D. S. Stephens, and B. A. Perkins. 2002. Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax toxin protective antigen. Emerg. Infect. Dis. 8:1103-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sirisanthana, T., K. E. Nelson, J. W. Ezzell, and T. G. Abshire. 1988. Serological studies of patients with cutaneous and oral-oropharyngeal anthrax from northern Thailand. Am. J. Trop. Med. Hyg. 39:575-581. [DOI] [PubMed] [Google Scholar]
- 17.Turnbull, P. C., S. H. Leppla, M. G. Broster, C. P. Quinn, and J. Melling. 1988. Antibodies to anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Med. Microbiol. Immunol. 177:293-303. [DOI] [PubMed] [Google Scholar]
- 18.Vignali, D. A. 2000. Multiplexed particle-based flow cytometric assays. J. Immunol. Methods 243:243-255. [DOI] [PubMed] [Google Scholar]
- 19.Wasserman, G. M., J. D. Grabenstein, P. R. Pittman, M. V. Rubertone, P. P. Gibbs, L. Z. Wang, and L. G. Golder. 2003. Analysis of adverse events after anthrax immunization in US Army medical personnel. J. Occup. Environ. Med. 45:222-233. [DOI] [PubMed] [Google Scholar]