Abstract
To assess the validity of the reference values for hematologic and immunologic indices currently used in Africa, we evaluated blood samples from 3,311 human immunodeficiency virus (HIV)-negative Ugandans aged 1 week to 92 years. Erythrocyte, hemoglobin, and hematocrit levels and mean corpuscular volume all significantly increased with age (P < 0.001) and were independent of gender until the age of 13 years, after which the levels were higher in males than in females (P < 0.001). White blood cell, neutrophil, lymphocyte, basophil, and monocyte counts significantly declined with age until the age of 13 years (P < 0.001), with no differences by gender, while platelet counts declined with age (P < 0.001) and showed differences by gender only among adults older than age 24 years. CD4+- and CD8+-cell counts declined with age until the age of 18 years; thereafter, females had higher counts than males. The absolute values for many of these parameters differed from those reported for populations outside Africa, suggesting that it may be necessary to develop tables of reference values for hematologic and immunologic indices specific for the African population. This may be particularly important with regard to CD4+-cell counts among children because significant differences in absolute and percent CD4+-cell counts exist between the values for Western populations and the values for the population evaluated in our study. These differences could influence the decision to initiate antiretroviral therapy among children infected with HIV.
The reference values of hematologic and immunologic indices currently used in Africa are derived from data collected for populations living in industrialized countries (27). The few small studies with African populations that have been reported indicate differences in normal values compared with those for populations in industrialized countries (2, 3, 5, 8, 10, 23, 25, 26). Ethnic origin, genetics, gender, altitude, and environmental factors, especially pathogens, may influence some values of hematologic and immunologic indices, suggesting that the development of reference values for the African population may be beneficial for improved quality of health care.
Reference values of hematologic and immunologic indices are necessary for the assessment of health and illness. Aside from their use in routine assessments for anemia, infection, and blood disorders, they are important surrogate markers for assessments of immune status, disease progression, and response to antiretroviral treatment in individuals infected with human immunodeficiency virus (HIV) (16, 18-20). The CD4+-cell count is an essential tool for clinical monitoring of antiretroviral treatment. CD4+-cell counts change dramatically with age, and specific recommendations for the initiation and monitoring of therapy among HIV-positive children have been made on the basis of data from North America and Europe (17). At present, 29.4 million people in sub-Saharan Africa are infected with HIV, and 3 million (10%) of these are children under the age of 15 years (12). As antiretroviral therapy becomes more widely available, it is important that normal reference values for CD4+-cell counts as well as other immunologic and hematologic markers be developed for the African population in order to appropriately manage individuals infected with HIV.
To assess the validity of current reference values of hematologic and immunologic indices for an African population, we collected blood samples from more than 3,000 HIV-negative Ugandans aged 1 week to 92 years.
MATERIALS AND METHODS
Subjects.
Between January and September 2002 we conducted a population-based cross-sectional survey to investigate risk factors for human herpesvirus 8 infection in a rural parish in eastern Uganda. We administered a household census to all homes and enrolled all willing adults and children resident in the study area during the previous 6 months. Moribund, mentally ill, and institutionalized persons were not requested to participate. The demographic characteristics of all participants were recorded.
Consent.
Informed consent was obtained in Luganda, the language commonly spoken locally. Adult participants in the study provided consent for minors younger than age 17 years, in addition to individual assent for participants aged 13 to 17 years. Permission for HIV testing was requested from all participants, and counseling was offered to all consenting participants before the HIV test results were given.
Laboratory.
Blood was collected between 9:00 a.m. and 4:00 p.m. by venipuncture, placed in 3-ml Vacutainer tubes (Becton Dickinson, Franklin Lakes, N.J.) containing EDTA, and transported at ambient temperature to the Uganda Virus Research Institute laboratory at Entebbe. Serological tests for HIV were performed by using a standard HIV testing algorithm of two enzyme-linked immunosorbent assays in parallel with Western blotting, as required; reverse transcription-PCR was carried out to confirm the HIV infection status of children younger than age 18 months who were positive by enzyme-linked immunosorbent assay.
A complete blood cell count (CBC) and differential were performed within 12 h of the blood draw by using the Act 5Diff instrument (Beckman Coulter). For determination of CD4+ and CD8+ cell counts, TriTEST reagents (CD3, fluorescein isothiocyanate/CD4, phycoerythrin/CD45, peridinin chlorophyll protein and CD3, fluorescein isothiocyanate/CD8, phycoerythrin/CD45, peridinin chlorophyll protein) were used to stain peripheral blood mononuclear cells according to the protocol of the manufacturer. Blood was stained within 12 h of collection, and the results were analyzed within 24 h. Flow cytometry was performed with a FACScan instrument and MultiSET software (Becton Dickinson) modified to accept manual entry of the total white cell count from the CBC and the percentage of lymphocytes derived from Attractors software (Becton Dickinson), which reports a three-part differential based on cell surface markers and side scatter. By use of this dual-platform approach, the MultiSET software reported the absolute CD3+ CD4+ and CD3+ CD8+ cell counts for the specimen.
Ethics.
Study protocols were approved by the Science and Ethics Committee at the Uganda Virus Research Institute, the Uganda National Council for Science and Technology, and the Institutional Review Board at the Centers for Disease Control and Prevention (Atlanta, Ga.).
Statistics.
Data were entered into Epi-Info software (version 6; Centers for Disease Control and Prevention) and analyzed by using SAS software (version 9; SAS Institute, Cary, N.C.). The normal distribution was tested by the Kolmogorov-Smirnov test. To investigate the normal distribution of the dependent variables, we plotted the cumulative quantiles for each variable versus the known quantiles of the normal distribution plot (normal Q-Q plots). The sample population was grouped according to the age and gender distribution, and the median was used as a measure of central tendency. The 5th and 95th percentile distributions of the dependent variables were used to specify the 90% reference interval. Kruskal-Wallis nonparametric tests were used to test for differences by age group, and Mann-Whitney U tests were used to test for differences by gender. In the absence of significant differences by gender, the data for all subjects were combined and analyzed as one group. An error probability (P value) of <0.05 was considered significant. Box and whisker plots for lymphocyte subsets were plotted to compare the distribution of this variable across age groups.
RESULTS
A total of 3,421 individuals, 94% of the total population of the community, participated in the study; data for 3,048 individuals were included in the analyses. Of those individuals whose data were excluded, 110 (29%) were HIV positive and data for age, gender, or CBCs were missing for 263 (71%). Testing for CD4+- and CD8+-cell counts was conducted for 1,365 randomly selected samples. Fifty percent of the participants were female, and 65% were children younger than 18 years of age.
Hematologic indices.
The medians and the 5th and 95th percentile reference intervals for the hemoglobin (Hb) level, red blood cell (RBC) count, hematocrit (Hct) level, and mean corpuscular volume (MCV) are presented in Table 1. There were significant differences in hematologic parameters by age and gender; Hb levels, RBC counts, Hct levels, and MCVs increased with age until age 13 years (P < 0.001). Absolute values for Hb levels, RBC counts, Hct levels, and MCVs were significantly higher among male adults than female adults older than age 13 years (P < 0.001). Significant differences by gender were not detected for any of the indices for children younger than 12 years of age.
TABLE 1.
Medians and 90% reference intervals for Hb levels, RBC counts, Hct levels, MCVs, and platelet counts for a Ugandan population, by age group and sex
| Age (yr) and sexa | Hb concn (g/dl) | RBC count (1012/liter) | % Hct | MCV (Fl) | Platelet count (109/liter) |
|---|---|---|---|---|---|
| <1 (373) | 10 (6.8-14.7)b | 4.2 (3.0-5.4) | 29.4 (20.4-42.6) | 70.8 (54.9-88.3) | 230 (123-487) |
| 1-5 (518) | 10.8 (8.8-12.5) | 4.4 (3.5-5.2) | 31.8 (25.9-36.3) | 72.9 (60.7-82.8) | 237 (126-376) |
| 6-12 (731) | 11.8 (10.0-13.7) | 4.6 (3.8-5.4) | 34.4 (29.2-39.4) | 75.8 (63.3-83.9) | 233 (134-355) |
| 13-18 | |||||
| M (182) | 13.1 (11.2-15.9) | 4.9 (4.1-5.8) | 38 (32.3-45.5) | 80 (65.0-89.5) | 218 (110-327) |
| F (164) | 12.5 (9.9-14.5) | 4.6 (3.5-5.4) | 36 (28.1-42.4) | 79.1 (67.4-89.9) | 217 (124-353) |
| 19-24 | |||||
| M (102) | 14.4 (11.5-17.1) | 5.1 (4.3-6.1) | 41.8 (33.7-48.7) | 80.4 (67.2-91.8) | 189 (98-306) |
| F (133) | 12.4 (9.9-13.7) | 4.4 (3.6-5.4) | 35.4 (28.9-40.0) | 79.9 (64.2-91.6) | 199 (95-368) |
| >24 | |||||
| M (410) | 14.1 (11.1-16.8) | 4.9 (3.8-6.0) | 40.7 (32.2-47.8) | 84 (69.9-95.2) | 171 (80-288) |
| F (435) | 12.5 (10.1-14.3) | 4.5 (3.7-5.3) | 36.2 (29.6-41.4) | 81.4 (67.7-92.6) | 198 (100-297) |
Values in parentheses are numbers of subjects. M, male; F, female.
The 90% reference intervals are provided in parentheses.
Platelet counts.
Platelet counts declined steadily with age up to the age of 18 years (P < 0.001) (Table 1). The values were similar for both males and females for those under 24 years of age and significantly higher among adult (age, >18 years) female participants than adult male participants (P < 0.001).
WBC counts.
Table 2 shows the medians and the 5th and 95th percentile reference intervals for the total white blood cell (WBC), neutrophil, lymphocyte, basophil, and monocyte counts; the counts of all of these cells declined with age. Significant differences among age groups were detected among children younger than age 12 years (P < 0.001). Eosinophil levels markedly increased until 13 years of age and declined thereafter. None of the values for the leukocyte subset showed any differences by gender.
TABLE 2.
Medians and 90% reference intervals for WBC and WBC subset counts for a Ugandan population, by age group
| Age (yr) (no. of subjects) | Cell count (109/liter [90% reference interval])
|
|||||
|---|---|---|---|---|---|---|
| WBCs | Neutrophils | Lymphocytes | Monocytes | Eosinophils | Basophils | |
| <1 (373) | 8.8 (4.1-15.8) | 2.1 (0.9-4.4) | 5.1 (1.9-10.3) | 0.68 (0.22-1.83) | 0.38 (0.07-1.85) | 0.09 (0.02-0.30) |
| 1-5 (518 | 7.8 (4.9-13.6) | 2.1 (1.0-3.9) | 4.4 (2.4-8.4) | 0.48 (0.26-1.04) | 0.58 (0.14-2.03) | 0.07 (0.03-0.17) |
| 6-12 (731) | 7.1 (4.4-11.5) | 1.8 (0.9-3.6) | 3.6 (2.2-5.9) | 0.42 (0.24-0.75) | 0.91 (0.20-3.14) | 0.05 (0.02-0.12) |
| 13-18 (346) | 6.2 (4.1-10.7) | 1.8 (0.9-3.5) | 2.9 (1.7-4.7) | 0.38 (0.21-0.73) | 0.91 (0.26-2.77) | 0.04 (0.02-0.10) |
| 19-24 (235) | 5.8 (3.7-9.7) | 1.8 (1.0-3.5) | 2.6 (1.3-4.1) | 0.34 (0.18-0.62) | 0.82 (0.19-2.42) | 0.04 (0.02-0.08) |
| >24 (845) | 5.3 (3.4-8.7) | 1.8 (0.84-3.37) | 2.4 (1.4-4.2) | 0.32 (0.17-0.59) | 0.58 (0.13-2.11) | 0.03 (0.01-0.07) |
CD4+- and CD8+-cell counts.
The medians and the 5th and 95th percentile reference intervals for absolute lymphocyte, CD4+-cell, and CD8+-cell counts displayed dramatic declines from birth through 18 years of age (P < 0.001) (Table 3). The CD4+- and CD8+-cell percentages increased progressively from birth to adulthood. The distributions of the lymphocyte subset were statistically different by age and gender; among adults, females had higher CD4+- and CD8+-cell counts and percentages than males, and children under 18 had higher CD4+- and CD8+-cell counts than adults. No gender-specific differences were seen for children younger than age 18 years.
TABLE 3.
Medians and 90% reference intervals for CD4+- and CD8+-cell counts, percentages, and ratios for a Ugandan population, by age group
| Age (yr) and sexa | Cell count (103/mm3)
|
%
|
CD4/CD8 ratio | ||
|---|---|---|---|---|---|
| CD4 | CD8 | CD4 | CD8 | ||
| <1 (174) | 1,693 (700-3,514) | 877 (339-2,180) | 31.9 (18.7-44.4) | 17.1 (8.4-28.5) | 1.9 (0.9-3.5) |
| 1-5 (243) | 1,517 (733-2,943) | 870 (423-1,940) | 33.5 (18.8-45.8) | 18.8 (11-30.7) | 1.8 (0.8-2.9) |
| 6-12 (346) | 1,315 (704-2,304) | 740 (356-1,366) | 36.6 (25.2-47.2) | 20.7 (12.2-31.7) | 1.8 (0.9-3.1) |
| 13-18 (143) | 1,093 (548-1,564) | 580 (282-1,262) | 35.3 (25.4-46.2) | 19.9 (13.0-33.1) | 1.8 (1.0-2.9) |
| 19-24 | |||||
| M (33) | 803 (504-1,334) | 522 (286-1,579) | 28.7 (18.5-42.2) | 21 (12.7-41.7) | 1.5 (0.6-2.5) |
| F (53) | 908 (560-1,961) | 494 (151-1,226) | 38.1 (27.4-53) | 20.1 (9.2-29.5) | 1.9 (1.3-4.6) |
| >24 | |||||
| M (174) | 754 (362-1,376) | 455 (204-1,174) | 33.3 (16.5-45.3) | 20.6 (8.4-36.3) | 1.6 (0.7-3.5) |
| F (199) | 894 (454-1,485) | 492 (196-1,133) | 36.5 (22.5-48.3) | 20.6 (10.2-33.7) | 1.9 (1.0-3.1) |
Values in parentheses are numbers of subjects. M, male; F, female.
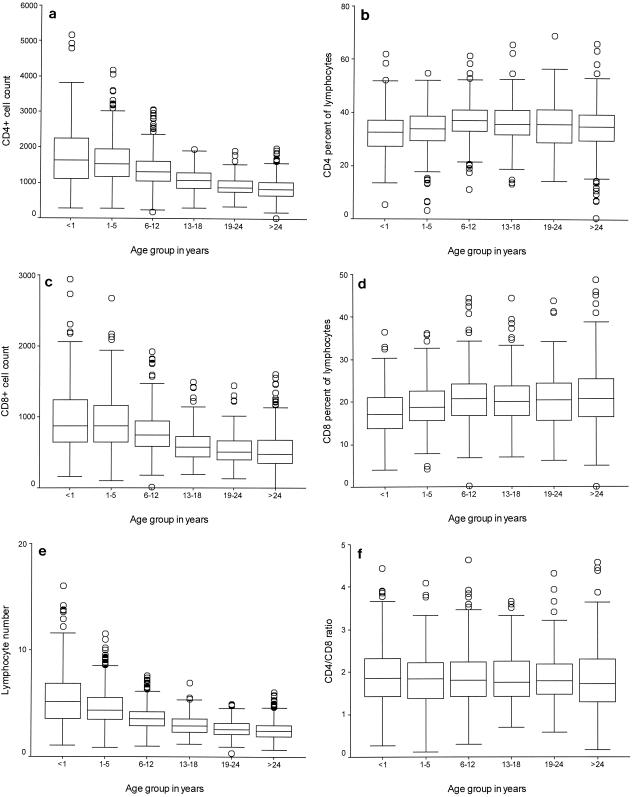
Box and whisker plots for WBC, CD4+-cell, and CD8+-cell counts and ratios by age are shown in Fig. 1. Individual plots of absolute WBC, CD4+-cell, and CD8+-cell counts showed remarkable declines with age up to the age of 19 years, when values typical of those for adults were attained. The percentages of CD4+ and CD8+ cells increased with age and leveled off at the age of 6 years, while the CD4+-cell/CD8+-cell ratio was fairly constant among all age groups. There were wider variations in WBC, CD4+-cell, and CD8+-cell counts among the younger age groups; but the variation was less among the adult age groups.
FIG. 1.
Box and whisker plot showing the median, interquartile, and range CD4+- and CD8+-cell counts (103 cells per milliliter), lymphocyte counts (109 cells per liter), and proportions by age group. The heights of the boxes in the box plots indicate the interquartile lengths. The central bar in each box is the median. Whiskers extend to the most extreme values to 1.5 box lengths from the edge of the box. Circles indicate outliers beyond 1.5 box lengths from the edge of the box. (a) CD4+-cell count by age group; (b) CD4+-cell proportion by age group; (c) CD8+-cell count by age group; (d) CD8+-cell proportion by age group; (e) WBC count by age group; (f) CD4/CD8 ratio by age group.
The means and the 5th and 95th reference intervals for the Ugandan population evaluated in the present study and those collected for Western populations are summarized in Table 4 (27). In Uganda, Hb levels, RBC counts, Hct levels, MCVs, platelet counts, WBC counts, and neutrophil counts are lower than standard reference values. WBC, eosinophil, and monocyte counts are higher. Inspection of the normal probability plots for all dependent variables for this population in Uganda revealed that they matched the test distribution and were reasonably normally distributed.
TABLE 4.
Means and 90% reference intervals for absolute units of WBC and RBC subsets for a Ugandan population compared with standard valuesa
| Group age [yr] | % Hct
|
Platelet count (109/liter)
|
Hb (g/dl)
|
RBCs
|
MCV (fl)
|
Eosinophils (109/liter)
|
Monocytes (109/liter)
|
Neutrophils (109/liter)
|
Lymphocytes (109/liter)
|
WBCs (109/liter)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | This study | Standard | This study | Standard | This study | Standard | This study | Standard | This study | Standard | This study | Standard | This study | Standard | This study | Standard | This study | Standard | This study | |
| Males (23-31) | 49 (44-54)b | 41 (34-49) | 280 (147-412) | 184 (82-290) | 15.5 (14.2-18.1) | 14.3 (11.4-17.1) | 5.2 (4.8-6.0) | 5.1 (4.1-6.0) | 90 (83-97) | 82 (69-93) | ||||||||||
| Females (18-49) | 41 (36)c | 36 (29-44) | NAd | 203 (103-311) | 14 (12)c | 12.3 (10.0-14.3) | 4.6 (4.0)a | 4.4 (3.6-5.3) | 90 (80)c | 80 (67-92) | ||||||||||
| Adults (>21) | 0.2 (NA) | 1 (0.2-3.4) | 0.3 (NA) | 0.4 (0.06-0.8) | 4.4 (1.8-7.7) | 2.2 (1.1-5.0) | 2.5 (1.0-4.8) | 2.7 (1.4-4.1) | 7.4 (4.5-11.0) | 6.4 (3.8-10.7) | ||||||||||
Standard values were obtained from Wintrobe's Clinical Hematology, 10th ed. (27).
Unless indicated otherwise, the values in parentheses are 90% reference intervals.
The values in parentheses are standard deviations.
NA, not available.
DISCUSSION
The values of several important immunologic and hematologic indices for more than 3,000 apparently healthy HIV-negative children and adults from Uganda were different from the standard reference values. Among adults, most values of hematologic indices were lower than the standard values (27). Similar differences have previously been reported for other African populations (1, 2, 5). However, most of the previous studies had less than 200 individuals and did not cover the entire age range of the population. Similar to studies of Western populations, Hb levels, Hct levels, and absolute RBC counts for children in Uganda are comparable from infancy through the age of 12 years, regardless of gender (9, 24). Thereafter, males tend to have higher Hb and Hct levels. The lower Hb levels detected in the Ugandan adult population compared with those detected in Western adult populations have been reported in other studies with African populations (1, 2, 5). This may be due to low levels of dietary iron intake, which cause an iron deficiency state, impaired hematopoiesis, chronic blood loss due to hookworm infestation (2), or chronic Plasmodium infection.
The low platelet counts and the differences in platelet counts by gender in this population are similar to those detected in other studies with African populations (1, 3, 10, 25). The cause of the low platelet counts among populations of African origin is unknown. While environmental factors and undetected illnesses have been suggested to explain the difference (1, 10), genetic factors may also play a role (3).
The WBC and neutrophil counts in this population are similar to those reported in studies of other African and Afro-Caribbean populations and are lower than standard values (3, 8, 13, 21, 23, 25). The etiology of these differences is unknown, although dietary, environmental, and genetic factors have been proposed (3, 8, 23).
The eosinophil counts found in this study are similar to those reported in other studies from Africa (2, 8, 21, 23). They are consistently higher than those found in Western subjects (27). However, the relative eosinophilia does not appear among African or Afro-Caribbean residents in Europe (3) or among black residents in Cape Peninsula, an urban suburb in South Africa (2), suggesting an environmental rather than a genetic etiology (8). This eosinophilia that was observed may result from a high prevalence of infestations with helminths, particularly schistosomes, in this community resident at the shores of Lake Victoria, predominantly engaged in fishing.
The progressive declines in the absolute values of the total WBC counts and the counts of the CD4+- and CD8+-cell subsets from infancy to adulthood and the higher values for women are similar to other findings (6, 7, 11, 15, 22). An important observation in our study is that the decline in CD4+- and CD8+-cell counts continues throughout childhood until the age of 18 years. European and American studies have shown that the counts for T-cell subsets for children younger than 10 years of age approximate the counts for adults (4, 14, 28, 29). However, a study from neighboring Kenya (6) also found that CD4+- and CD8+-cell counts declined with age until age 10 years, the oldest age group assessed, and that at that age children had not attained the counts detected in adults. Similar to previous reports, women had higher numbers of CD4+ cells and percentages than men in this study. Overall, the population evaluated in this study and that from Kenya (6) tend to have higher CD4+-cell counts and CD4+-cell/CD8+-cell proportions than populations in Europe, North America, and Asia (4, 7, 11), suggesting an ethnic or environmental etiology. The normal range of CD4+-cell counts for the population evaluated in this study is higher than the range of standard Western population-based reference values, which complicates patient management decisions, especially when clinicians must determine the CD4+-cell count or percentage that should be used for HIV-positive children and adults for the initiation of antiretroviral therapy.
The study had several limitations. Despite the large population-based sample, the health status of the individuals was not assessed and no medical or laboratory examinations were performed. In this community, all consenting apparently health individuals found at home at the time of the survey were recruited. The only specific exclusion criterion used was HIV infection. Although none of the participants was moribund at the time of the study, a proportion could have had minor illnesses. This is unlikely to have affected the median values for the population but may have produced proportionately more extreme values. The influences of environmental factors, pregnancy, social habits like smoking, dietary components, and lifestyle were not taken into account, yet there is evidence to suggest that they may be responsible for variations in hematologic parameters (1, 2, 5, 8). Specimen collection was not conducted at exactly the same time of day for all subjects. The temperature and length of blood storage have been shown to cause alterations in the values for some hematologic parameters, and some hematologic parameters have been shown to vary diurnally and day to day, although these differences should have been randomly distributed throughout the study population. All blood samples were shipped at ambient temperature and were processed within 12 h, as recommended for hematologic and immunologic investigations. These findings have been drawn from a preliminary study of 3,048 individuals in a single rural community in Uganda. The very high acceptance rate (94%) for both enrollment in the study and HIV testing allowed the results to be generalizable to the community. However, it would be useful to conduct more expansive studies that include information regarding the time of blood collection and the presence of intercurrent illnesses.
In conclusion, there appear to be regional variations in reference values and ranges of hematologic and immunologic indices between the African population and the population in the industrialized West. These data provide region-specific reference values which can be used to guide patient management and interpretation of clinical research findings and which may potentially improve the quality of clinical care provided to patients.
Acknowledgments
This study was supported by the Centers for Disease Control and Prevention. Eric S. Lugada was supported by the Norwegian government through the University of Bergen, Bergen, Norway.
REFERENCES
- 1.Azikiwe, A. N. 1984. Platelet count values in healthy Nigeria medical students in Jos. East Afr. Med. J. 61:482-485. [PubMed] [Google Scholar]
- 2.Badenhorst, C. J., J. Fourie, K. Steyn, P. L. Jooste, C. J. Lombard, L. Bourne, and W. Slazus. 1995. The haematological profile of urban black Africans aged 15-64 years in the Cape Peninsula. East Afr. Med. J. 72:19-24. [PubMed] [Google Scholar]
- 3.Bain, B. J. 1996. Ethnic and sex differences in the total and differential white cell count and platelet count. J. Clin. Pathol. 49:664-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bofill, M., G. Janossy, C. A. Lee, D. MacDonald-Burns, A. N. Phillips, C. Sabin, A. Timms, M. A. Johnson, and P. B. Kernoff. 1992. Laboratory control values for CD4 and CD8 T lymphocytes. Implications for HIV-1 diagnosis. Clin. Exp. Immunol. 88:243-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coetzee, M. J., P. N. Badenhorst, J. I. de Wet, and G. Joubert. 1994. Haematological condition of the San (Bushmen) relocated from Namibia to South Africa. S. Afr. Med. J. 84:416-420. [PubMed] [Google Scholar]
- 6.Embree, J., J. Bwayo, N. Nagelkerke, S. Njenga, P. Nyange, J. Ndinya-Achola, H. Pamba, and F. Plummer. 2001. Lymphocyte subsets in human immunodeficiency virus type 1-infected and uninfected children in Nairobi. Pediatr. Infect. Dis. J. 20:397-403. [DOI] [PubMed] [Google Scholar]
- 7.Erkeller-Yuksel, F. M., V. Deneys, B. Yuksel, I. Hannet, F. Hulstaert, C. Hamilton, H. Mackinnon, L. T. Stokes, V. Munhyeshuli, F. Vanlangendonck, et al. 1992. Age-related changes in human blood lymphocyte subpopulations. J. Pediatr. 120:216-222. [DOI] [PubMed] [Google Scholar]
- 8.Ezeilo, G. C. 1972. Non-genetic neutropenia in Africans. Lancet ii:1003-1004. [DOI] [PubMed] [Google Scholar]
- 9.Flegar-Mestric, Z., A. Nazor, and N. Jagarinec. 1999. Reference intervals for haematological parameters in urban school children and adolescents. Clin. Lab. Haematol. 21:72-74. [PubMed] [Google Scholar]
- 10.Gill, G. V., A. England, and C. Marshal. 1979. Low platelet counts in Zambians. Trans. R. Soc. Trop. Med. Hyg. 73:111-112. [DOI] [PubMed] [Google Scholar]
- 11.Hannet, I., F. Erkeller-Yuksel, P. Lydyard, V. Deneys, and M. DeBruyere. 1992. Developmental and maturational changes in human blood lymphocyte subpopulations. Immunol. Today 13:215-218. [DOI] [PubMed] [Google Scholar]
- 12.The Joint United Nations Programme on HIV/AIDS. December 2002. AIDS epidemic update. The Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland.
- 13.Kasili, E. G., C. L. Cardwell, and J. R. Taylor. 1969. Leucocyte counts on blood donors in Nairobi. East Afr. Med. J. 46:676-679. [PubMed] [Google Scholar]
- 14.Kotylo, P. K., N. S. Fineberg, K. S. Freeman, N. L. Redmond, and C. Charland. 1993. Reference ranges for lymphocyte subsets in pediatric patients. Am. J. Clin. Pathol. 100:111-115. [DOI] [PubMed] [Google Scholar]
- 15.Lee, B. W., H. K. Yap, F. T. Chew, T. C. Quah, K. Prabhakaran, G. S. Chan, S. C. Wong, and C. C. Seah. 1996. Age- and sex-related changes in lymphocyte subpopulations of healthy Asian subjects: from birth to adulthood. Cytometry 26:8-15. [DOI] [PubMed] [Google Scholar]
- 16.Mellors, J. W., A. Munoz, J. V. Giorgi, J. B. Margolick, C. J. Tassoni, P. Gupta, L. A. Kingsley, J. A. Todd, A. J. Saah, R. Detels, J. P. Phair, and C. R. Rinaldo, Jr. 1997. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann. Intern. Med. 126:946-954. [DOI] [PubMed] [Google Scholar]
- 17.National Institutes of Health. 2001. Guidelines for the use of antiretroviral agents in pediatric HIV infection. December 14. [Online.] http://www.aidsinfo.nih.gov/guidelines/pediatric.
- 18.O'Brien, W. A., P. M. Hartigan, E. S. Daar, M. S. Simberkoff, J. D. Hamilton, et al. 1997. Changes in plasma HIV RNA levels and CD4+ lymphocyte counts predict both response to antiretroviral therapy and therapeutic failure. Ann. Intern. Med. 126:939-945. [DOI] [PubMed] [Google Scholar]
- 19.Phillips, A. N., C. A. Sabin, J. Elford, M. Bofill, G. Janossy, and C. A. Lee. 1994. Use of CD4 lymphocyte count to predict long-term survival free of AIDS after HIV infection. BMJ 309:309-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pomerantz, R. J. 2001. Initiating antiretroviral therapy during HIV infection: confusion and clarity. JAMA 286:2597-2599. [DOI] [PubMed] [Google Scholar]
- 21.Sahr, F., P. K. Hazra, and T. A. Grillo. 1995. White blood cell count in healthy Sierra Leoneans. West Afr. J. Med. 14:105-107. [PubMed] [Google Scholar]
- 22.Shahabuddin, S., I. Al-Ayed, M. O. Gad El-Rab, and M. I. Qureshi. 1998. Age-related changes in blood lymphocyte subsets of Saudi Arabian healthy children. Clin. Diagn. Lab. Immunol. 5:632-635. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Shaper, A. G., and P. Lewis. 1971. Genetic neutropenia in people of African origin. Lancet ii:1021-1023. [DOI] [PubMed] [Google Scholar]
- 24.Taylor, M. R., C. V. Holland, R. Spencer, J. F. Jackson, G. I. O'Connor, and J. R. O'Donnell. 1997. Haematological reference ranges for schoolchildren. Clin. Lab. Haematol. 19:1-15. [DOI] [PubMed] [Google Scholar]
- 25.Tsegaye, A., T. Messele, T. Tilahun, E. Hailu, T. Sahlu, R. Doorly, A. L. Fontanet, and T. F. Rinke de Wit. 1999. Immunohematological reference ranges for adult Ethiopians. Clin. Diagn. Lab. Immunol. 6:410-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tugume, S. B., E. M. Piwowar, T. Lutalo, P. N. Mugyenyi, R. M. Grant, F. W. Mangeni, K. Pattishall, and E. Katongole-Mbidde. 1995. Hematological reference ranges among healthy Ugandans. Clin. Diagn. Lab. Immunol. 2:233-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wintrobe, M. M., and G. R. Lee. 1999. Wintrobe's clinical hematology, 10th ed. The Williams & Wilkins Co., Baltimore, Md.
- 28.Yachie, A., T. Miyawaki, T. Nagaoki, T. Yokoi, M. Mukai, N. Uwadana, and N. Taniguchi. 1981. Regulation of B cell differentiation by T cell subsets defined with monoclonal OKT4 and OKT8 antibodies in human cord blood. J. Immunol. 127:1314-1317. [PubMed] [Google Scholar]
- 29.Yanase, Y., T. Tango, K. Okumura, T. Tada, and T. Kawasaki. 1986. Lymphocyte subsets identified by monoclonal antibodies in healthy children. Pediatr. Res. 20:1147-1151. [DOI] [PubMed] [Google Scholar]