Abstract
Parasitic plants in the Scrophulariaceae develop infective root structures called haustoria in response to chemical signals released from host-plant roots. This study used a simple in vitro assay to characterize natural and synthetic molecules that induce haustoria in the facultative parasite Triphysaria versicolor. Several phenolic acids, flavonoids, and the quinone 2,6-dimethoxy-p-benzoquinone induced haustoria in T. versicolor root tips within hours after treatment. The concentration at which different molecules were active varied widely, the most active being 2,6-dimethoxy-p-benzoquinone and the anthocyanidin peonidin. Maize (Zea mays) seeds are rich sources of molecules that induce T. versicolor haustoria in vitro, and chromatographic analyses indicated that the active molecules present in maize-seed rinses include anthocyanins, other flavonoids, and simple phenolics. The presence of different classes of inducing molecules in seed rinses was substantiated by the observation that maize kernels deficient in chalcone synthase, a key enzyme in flavonoid biosynthesis, released haustoria-inducing molecules, although at reduced levels compared with wild-type kernels. We discuss these results in light of existing models for host perception in the related parasitic plant Striga.
Plants naturally produce more than 8000 different phenolic compounds for functions as varied as cell wall biosynthesis, flower pigmentation, and host defense (Harborne and Moss, 1993). The prevalence of plant phenolic molecules and the broad spectrum of potential structures and electrochemical forms make these effective signaling molecules for mediating interactions between plants and other organisms in the soil (Sisqueira et al., 1991).
Flavonoids released by legume roots activate a set of genes in Rhizobium sp. whose products are responsible for the biosynthesis of nodulation factors (Pueppke, 1996). The NodD protein binds to specific promoter sequences in nod (nodulation) genes and, when NodD perceives the appropriate flavonoids, activates their transcription (Fisher and Long, 1992). Although a single nodD gene can respond to multiple flavonoids, different nodD genes are optimally responsive to specific structures. This can represent one level of host-range specificity in the Rhizobium-legume interaction (Spaink et al., 1987). Host-plant phenolics released from wounded plant cells induce virulence genes in the soil pathogen Agrobacterium tumefaciens (Hooykaas and Beijersbergen, 1994). The phenolics are perceived by a two-component system composed of the VirA sensor protein and the VirG transcriptional regulator. VirA responsiveness to phenolic signals is further refined by synergistic association of signaling phenolics with specific monosaccharides. Different virA gene products sense multiple phenolic compounds, but preferences for particular compounds can in some cases affect host range (Heath et al., 1997).
Phenolic compounds are also important signaling molecules for mediating parasitic plant-host plant interactions in the rhizosphere (Musselman, 1980; Press and Graves, 1995). Seeds of the parasitic weeds Striga and Orobanche remain dormant in the soil until they sense specific hydroquinones that are released from potential host roots (Chang and Lynn, 1986). These germination stimulants become inactive at increasing distances from the root, thereby allowing the parasite to judge the availability and distance to a potential host root (Fate and Lynn, 1996).
Parasitic plants in the Scrophulariaceae also use host-encoded phenolic derivatives to signal the transition from vegetative to parasitic growth. In response to these host factors, parasitic plants develop haustoria near their root tips (Riopel and Timko, 1995). Haustoria serve several functions for the parasite: they attach the parasite and host roots, they invade host tissues through a combination of enzymatic and physical processes, and they serve as the physiological conduit through which the parasite robs the host plant of water and nutritional resources (Kuijt, 1969). A diverse array of phenolic derivatives that induce haustoria in S. asiatica and Agalinis purpurea has been identified (MacQueen, 1984; Riopel and Timko, 1995; Smith et al., 1996). When parasite roots are exposed to these HIFs in vitro, haustorium development is rapid, and highly synchronous morphological changes can be observed within hours (Baird and Riopel, 1984).
We studied the genetic mechanisms by which phenolic signals are perceived and interpreted by parasitic plants. The genus Triphysaria (previously Orthocarpus) of the Scrophulariaceae is composed of five cross-hybridizing species within the subtribe Castillejinae (Chuang and Heckard, 1991). Triphysaria is a common herbaceous annual in coastal fields and bluffs, inland grasslands, and serpentine slopes distributed along the Pacific Coast from Baja to British Columbia (Hickman, 1993). It is a facultative root parasite that can be grown without a host, but will parasitize a broad spectrum of host plants, including Arabidopsis, tobacco, and maize (Zea mays) (Atsatt and Strong, 1970; Estabrook and Yoder, 1998). Triphysaria sp. are diploid and have perfect flowers amenable to classical genetic analysis (Chuang and Heckard, 1982; Yoder, 1998). The generation time of Triphysaria sp. is 3 to 4 months, with each flower producing about 100 seeds.
We used a simple bioassay to examine the ability of different phenolic compounds to induce haustoria in the self-incompatible species Triphysaria versicolor. Several phenolic molecules were active in haustoria induction, although the concentrations at which they were active varied widely. We show, for the first time to our knowledge, that anthocyanins induce haustoria, and we discuss these findings in light of existing models of quinone recognition. Chromatographic analyses were consistent with multiple molecules that induce T. versicolor haustoria in vitro being released into aqueous and methanol rinses of maize kernels. The study of maize mutants deficient in CHS further confirmed that a redundancy of signaling molecules is released from maize kernels.
MATERIALS AND METHODS
Materials
Triphysaria versicolor (Fischer & C. Meyer) seeds were collected from grassland stands near Napa, CA, and stored at 4°C. Maize seeds (Zea mays cv B73) were kindly provided by Pioneer Hi-Bred International (Johnston, IA). Maize seeds bearing the two CHS mutations c2 and whp1 (Coe et al., 1981) were generously supplied by the Maize Genetic Cooperative Stock Center (stock no. 224H, University of Illinois, Urbana). This stock contains seeds of two genotypes: C2/c2,whp1/whp1 and c2/c2,whp1/whp1. Expression of CHS in the aleurone layer of maize is encoded by the C2 locus, whereas expression in other plant parts is encoded by Whp (Dooner et al., 1991).
Phenolic compounds were obtained from Sigma, DMBQ from Pfalz and Bauer (Waterbury, CT), and flavonoids from Indofine Chemical (Belle Mead, NJ). Chemicals and HPLC fractions were dissolved in 50% methanol and frozen at −80°C. Anthocyanidin stock solutions were kept in the dark. Dilutions were prepared in distilled water just before use.
Bioassay for Haustorial Induction
T. versicolor seeds were surface-sterilized for 5 min in 70% ethanol followed by 30 min in 50% bleach plus 2% Triton X-100 before rinsing with sterile water. Germination was carried out at 16°C under high-output, cool-white fluorescent lights with a 12-h photoperiod in 0.25× Murashige and Skoog medium (0.75 mm CaCl2, 0.3 mm KH2PO4, 5 mm KNO3, 0.2 mm MgSO4, and 5 mm NH4NO3) supplemented with micro-nutrients (10 nm CoCl2, 500 nm CuSO4, 70 μm H3BO3, 14 μm MnCl2, 10 μm NaCl, 200 nm NaMoO4, and 1 μm ZnSO4), 0.75% Suc, and solidified with 0.6% Phytagar (GIBCO-BRL).
Five to seven 3-week-old seedlings were aseptically transferred to the surface of 0.25× Hoagland agar (1.25 mm Ca[NO3]2, 1.25 mm KNO3, 0.25 mm KH2PO4, 0.5 mm MgSO4) with micronutrients, 1% Suc, and 1% Phytagar in 90- × 90-mm dishes. The dishes were sealed with Micropore tape (3M), and placed for 1 week at a nearly vertical angle at 25°C with a 16-h photoperiod. Under these conditions T. versicolor roots grew along the surface of the agar.
To assay haustoria-inducing activity, the candidate inducer in 3 mL of water was applied to T. versicolor roots. After the liquid absorbed into the medium (1–3 h), the plates were returned to the 25°C growth chamber. After 24 h each root tip was scored for the localized swelling and hair proliferation typical of developing haustoria. Results are typically expressed as the proportion of root tips with haustoria.
Characterization of Maize-Seed Rinses
Maize kernels were swirled for 16 h at room temperature in either 50% methanol or water. Samples analyzed by HPLC were prepared by shaking 300 g of seeds for 4 h in 100% methanol (300 mL) at room temperature. The resulting seed rinse was concentrated under vacuum at 50°C, frozen as 30% methanol, and lyophilized to dryness. The dried material was dissolved in 50% acetonitrile, diluted with water to 5% acetonitrile, and injected into a HPLC system (Millipore) fitted with a RP-18 column (250 × 4.6 mm; Lichrosorb, Alltech Associates, Deerfield, IL). The column was rinsed for 2 min with 5% acetonitrile and then eluted with a 58-min linear gradient (5%–100% acetonitrile), followed by a 30-min rinse in 100% acetonitrile using a flow rate of 2 mL/min. Eluate was monitored (200–400 nm) with a photodiode array detector (model 996, Waters). In other tests, seed rinses were fractionated by open-column chromatography on preparative 125-Å C18 columns (Waters) using methanolic gradients.
RESULTS
Purified Phenolics Induce Haustoria in Vitro
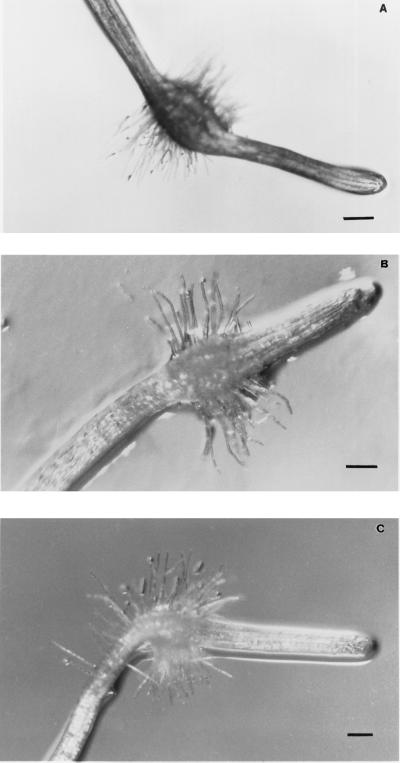
We used an in vitro system to bioassay various phenolic derivatives for their ability to induce haustoria in T. versicolor roots. Seedlings were grown in vertically oriented Petri dishes such that the roots grew along the surface of the agar. Candidate haustoria-inducing compounds were diluted in water and applied to the roots. With active HIFs, epidermal hairs began to proliferate and elongated near the root tip within 4 h after exposure. At about the same time, cortical cells underlying the proliferating hairs began to swell and divide, resulting in an observable swelling near the root tip (Estabrook and Yoder, 1998). The swelling and hair proliferation continued for approximately 24 h. Under these conditions only those cells that were near the root tip at the time of HIF treatment differentiated into haustorial cells. Within a few hours after exposure to HIFs, root-tip development reverted to its typical growth pattern, and normal roots grew out of the haustoria. This resulted in the globe-shaped haustoria being located proximal to the root tip (Fig. 1).
Figure 1.
Secondary haustoria on T. versicolor roots. Haustoria were induced by a fraction (the 30-min eluant from Fig. 7B) of the purified methanolic rinse of maize seeds (0.04 g seed equivalents/mL) (A), by 10 μm peonidin (B), or by 50 μm DMBQ (C). Photographs were taken approximately 24 h after treatment. Scale bars = 100 μm.
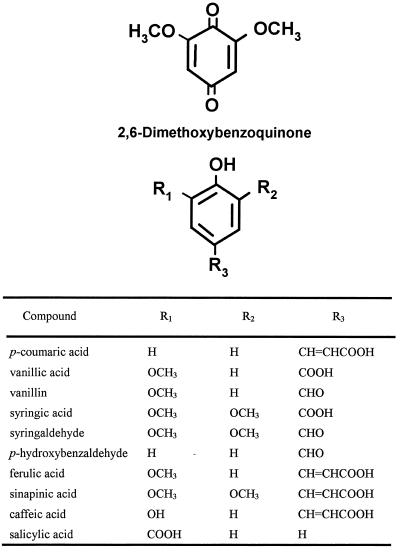
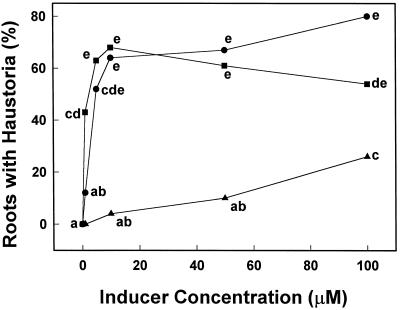
The ability of various phenolic derivatives to induce haustoria in T. versicolor was assayed (Figs. 2–4; Tables I and II). The most active HIFs were DMBQ and the anthocyanidin peonidin. Both DMBQ and peonidin were maximally active at a concentration near 10 μm (Table I; Fig. 5). At higher DMBQ concentrations some of the root tips became brown and necrotic, resulting in a smaller proportion of root tips with haustoria; this was not the case with peonidin.
Figure 2.
Phenolics, phenolic acids, and quinones assayed for haustoria-inducing activity in T. versicolor.
Figure 4.
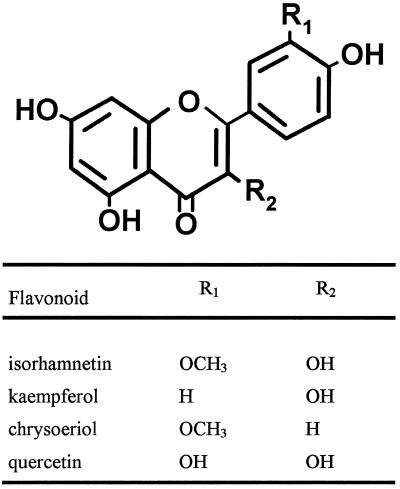
Flavonoids assayed for haustoria-inducing activity in T. versicolor.
Table I.
Induction of T. versicolor haustoria by simple phenolics and quinones
| Compound | Root Tips with Haustoria
|
||
|---|---|---|---|
| 1.0 μm | 10 μm | 50 μm | |
| proportion | |||
| DMBQ | 0.31 ± 0.11 | 0.73 ± 0.17 | 0.65 ± 0.19 |
| p-Coumaric acid | 0.23 ± 0.02 | 0.29 ± 0.14 | 0.35 ± 0.20 |
| Vanillic acid | 0.00 ± 0.00 | 0.35 ± 0.02 | 0.78 ± 0.16 |
| Vanillin | 0.01 ± 0.03 | 0.16 ± 0.08 | 0.74 ± 0.12 |
| Syringic acid | 0.03 ± 0.03 | 0.04 ± 0.04 | 0.48 ± 0.16 |
| Syringaldehyde | 0.05 ± 0.07 | 0.10 ± 0.05 | 0.38 ± 0.08 |
| p-Hydroxybenzaldehyde | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.16 ± 0.14 |
| Ferulic acid | 0.00 ± 0.00 | 0.03 ± 0.02 | 0.16 ± 0.19 |
| Caffeic acid | 0.05 ± 0.05 | 0.01 ± 0.02 | 0.02 ± 0.03 |
| Sinapinic acid | 0.00 ± 0.00 | 0.05 ± 0.03 | 0.00 ± 0.00 |
| Salicylic acid | 0.03 ± 0.04 | 0.00 ± 0.00 | 0.00 ± 0.00 |
Compounds were diluted in water to the concentrations shown. Values are the means ± sd of three plates with six T. versicolor plants each (50–90 total root tips per treatment).
Table II.
Induction of T. versicolor haustoria by flavonoids
| Compound | Root
Tips with Haustoria
|
||
|---|---|---|---|
| 1.0 μm | 10 μm | 100 μm | |
| proportion | |||
| Peonidin | 0.12 ± 0.15 | 0.83 ± 0.15 | 0.65 ± 0.16 |
| Pelargonidin | 0.00 ± 0.00 | 0.04 ± 0.04 | 0.26 ± 0.10 |
| Cyanidin | 0.03 ± 0.04 | 0.01 ± 0.02 | 0.11 ± 0.14 |
| Malvidin | 0.02 ± 0.03 | 0.04 ± 0.07 | 0.11 ± 0.11 |
| Quercetin | 0.03 ± 0.04 | 0.00 ± 0.00 | 0.04 ± 0.06 |
| Kaempferol | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.04 ± 0.06 |
| Delphinidin | 0.00 ± 0.00 | 0.03 ± 0.05 | 0.03 ± 0.05 |
| Isorhamnetin | 0.00 ± 0.00 | 0.04 ± 0.06 | 0.04 ± 0.04 |
| Chrysoeriol | 0.01 ± 0.02 | 0.02 ± 0.02 | 0.01 ± 0.02 |
Flavonoids were diluted in water to the concentrations shown. Values are the means ± sd of three plates with six T. versicolor plants each (50–90 total root tips per treatment).
Figure 5.
Dosage responses to haustoria-inducing anthocyanidins and phenolics. Haustoria-inducing activity in T. versicolor of two anthocyanidins, peonidin (•) and pelargonidin (▴), and DMBQ (▪). Mean values associated with the same letter were not significantly different (P ≤ 0.05).
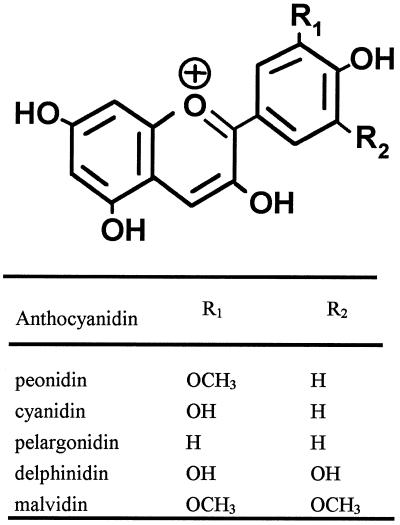
Many of the simple phenolics evaluated induced haustoria to some degree over background; only caffeic acid, sinapinic acid, and salicylic acid were inactive at all the concentrations examined. In addition, three anthocyanidins, cyanidin, pelargonidin, and delphinidin (but none of the flavones or flavonols), had haustoria-inducing activity. However, unlike DMBQ and peonidin, none of these molecules was maximally active at 10 μm. All of the HIFs induced morphologically similar haustoria in T. versicolor (Fig. 1).
Maize-Seed Rinses Contain HIFs
Based on observations that T. versicolor parasitizes maize and that haustoria develop in response to different anthocyanins, we decided to test whether HIFs could be identified in maize kernels, a rich and abundant source of anthocyanins.
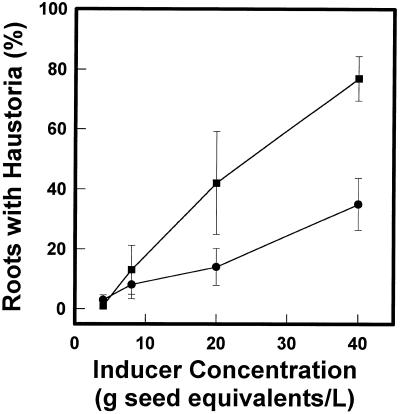
Maize kernels were rinsed with water or 50% methanol and the rinsates were assayed on T. versicolor roots. Both rinses induced haustoria in a concentration-dependent manner, but the activity of the methanol rinse was two to three times higher (Fig. 6). Under these conditions the water and methanol controls induced haustoria in less than 0.2% of the root tips.
Figure 6.
Haustoria-inducing activity of maize-seed rinses. Ten grams of B73 maize kernels was swirled overnight in 25 mL of water (•) or 50% methanol (▪). The seed rinse was diluted in water and 3 mL was applied to the roots of in vitro-grown T. versicolor. Data are averages ± sd of three experiments, with about 18 plants treated in each experiment.
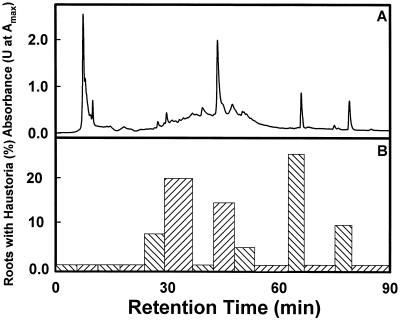
When maize-seed rinses were separated by HPLC (Fig. 7A), 6 of 14 fractions exhibited haustoria-inducing activity (Fig. 7B). Spectral characteristics of the active fractions were consistent with the presence of both flavonoids and simple phenolics. The color and photolability of three red bands separating on open C18 columns were consistent with the presence of anthocyanidins in the active fractions. The hydrophilicity of these fractions suggested that the molecules were present as anthocyanin glycosides (data not shown).
Figure 7.
HPLC characteristics and haustoria-inducing activity of maize-seed rinse. A, Amax (200–400 nm) of 100% methanolic maize-seed rinse fractionated on a C18 column and eluted by a linear acetonitrile gradient of 5% to 100% for 2 to 60 min, followed by 100% acetonitrile for 60 to 90 min. B, Haustoria-inducing activity in T. versicolor for different HPLC fractions assayed at 0.9 g seed equivalents/mL. In the same assay, 50 μm DMBQ induced haustoria in 57% of the roots, but no haustoria were induced in the methanol-treated control.
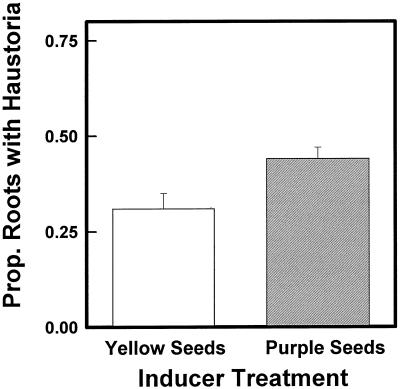
We then assayed haustoria-inducing activity in rinsates of maize seeds deficient in CHS, a key enzyme in flavonoid biosynthesis. The seeds segregated 1:1 for C2/c2,whp1/whp1 and c2/c2,whp1/whp1. Kernels could be distinguished because C2/c2 seeds are purple and c2/c2 seeds are yellow. An equal number of kernels with approximately the same weight of each genotype were rinsed overnight in 50% methanol, and the rinsate was diluted 1:10 in water and assayed in the roots of T. versicolor. Although less activity was recovered from c2/c2 kernels than from those with a C2/c2 genotype, rinses from both genotypes contained active HIFs (Fig. 8). This means that although some flavonoids were active HIFs, they were not the only HIFs released from maize kernels.
Figure 8.
Haustoria-inducing activity of CHS-deficient maize seeds. Rinses from yellow (c2/c2,whp1/whp1) or purple (C2/c2,whp1/whp1) maize seeds were assayed on roots of T. versicolor at 40 g seed equivalents/L. Each value represents the mean ± sd of three tests. Seed weights of the yellow and purple genotypes did not differ significantly.
DISCUSSION
Molecular phylogenic studies indicate that all of the parasitic Scrophulariaceae species, including those in the genera Triphysaria and Striga, share common evolutionary origins (DePamphilis et al., 1997). This suggests that the fundamental mechanisms used by the two genera may be similar. Both T. versicolor and S. asiatica induce root haustoria in response to several different phenolic compounds (Riopel and Timko, 1995; Smith et al., 1996). Redox potential is a critical characteristic of haustoria-inducing molecules in S. asiatica. Structurally diverse quinones that induce S. asiatica haustoria have redox potentials within a narrow window, and related quinones that fall outside of the redox window are inactive as inducers (Smith et al., 1996). This suggests that haustoria development is initiated when the appropriate quinone associates with a parasite oxidoreductase to complete a redox circuit. Redox control of developmental programs in many organisms, including phototropism and defense in plants, is well documented (Hammond-Kosack et al., 1996; Huala et al., 1997).
Differences in the S. asiatica response to quinone and phenolic HIFs suggest how a common redox mechanism can use both structures. The phenol-exposure time for S. asiatica seedlings is longer and the concentrations higher than for analogous quinones (Lynn and Chang, 1990). When syringic acid, a common phenolic component of plant cell walls, is incubated with S. asiatica roots, DMBQ accumulates with kinetics similar to those seen during haustorium development (Kim et al., 1998). When hydrogen peroxide is removed from the reactions by the addition of catalase, haustorial induction with syringic acid, but not the quinone, is inhibited. These observations led to the hypothesis that root peroxidases convert inactive phenolic molecules to active quinones (Kim et al., 1998).
Apoplastic peroxidases have been identified in S. asiatica that catalyze the oxidation of p-hydroxy acids to quinones with the same kinetics and pH dependence as haustoria induction in response to syringic acid (Kim et al., 1998). Because similar peroxidases were found in host roots, it was suggested that hydrogen peroxide is the limiting component in the system. In this model, S. asiatica roots supply the hydrogen peroxide oxidant required for the conversion of phenols to quinones. We are in the process of determining whether similar oxidation reactions are required for phenolic-acid induction of T. versicolor haustoria.
One novel aspect of this report is the observation that peonidin, an anthocyanidin that was apparently present in maize-seed rinses, induced haustoria in T. versicolor. Anthocyanidins are common in plants, and those released from legume seeds induce nodulation genes in Rhizobium bacteria (Hungria et al., 1991). A natural role for anthocyanins in the Rhizobium-bean system is supported by genetic and surgical variables showing that seed anthocyanins contribute to root-nodule formation at the top of the primary root (Hungria and Phillips, 1993). However, anthocyanins are not typically found in root exudates, and different nod-inducer molecules have been identified in seed effusates and root exudates (Schlaman et al., 1998). Thus, although anthocyanins induce haustoria in vitro, their role as natural signals for parasitic plants in the soil is not clear.
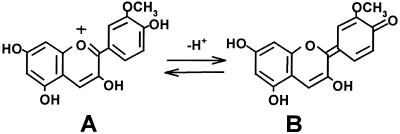
It is possible that the similarity in the activity of DMBQ and peonidin is related to the redox potential of different tautomeric forms of the anthocyanidin. Anthocyanidins exist in at least nine forms that change with pH and temperature (Cheminat and Brouillard, 1986). Several quinone forms of peonidin would likely be in equilibrium under our conditions, one of which would contain a quinone form of the methoxylated ring that could potentially satisfy the same electrochemical and/or structural requirements fulfilled by DMBQ (Fig. 9). One complicating factor for this model is that malvidin, the anthocyanidin that has a methoxylation pattern identical to that of DMBQ, showed essentially no inducing activity. Therefore, it seems reasonable to conclude that the remainder of the anthocyanidin molecule also contributes to biological activity, whether structurally (by changing equilibrium structures) or by changing redox states.
Figure 9.
Two structures of the anthocyanin peonidin. As the pH was increased, the flavylium cation (A) was converted into several quinone structures, including that shown in B (Cheminat and Brouillard, 1986).
In addition to the anthocyanidins described here, other flavonoids have previously been identified as HIFs. For example, one of the first two HIFs identified was xenognosin B, which belongs to the isoflavonoid subclass of flavonoids (Lynn et al., 1981). CHS is the key enzyme in flavonoid biosynthesis and is therefore necessary for the synthesis of these compounds. The ability of T. versicolor to develop haustoria in response to rinses of maize kernels lacking CHS indicates that, in addition to phenol-propanoid biosynthesis, the pathways encode HIFs. Similarly, although flavonoids stimulate growth of arbuscular mycorrhizal fungi (Chabot et al., 1992), roots of CHS and wild-type maize are colonized by arbuscular mycorrhizal fungi to the same degree (Becard et al., 1995). These experiments demonstrate that multiple biosynthetic pathways generate a spectrum of signaling molecules that are active in vitro.
Although most of the phenolics that promote haustorium development in T. versicolor also stimulate haustoria in its close relative A. purpurea, there are exceptions. For example, coumaric acid is active in T. versicolor but not in A. purpurea, whereas sinapinic acid is active in A. purpurea but not in T. versicolor (Riopel, 1979). This suggests that different parasitic species distinguish different haustoria-inducing molecules. The quinone-dependent oxidoreductase receptors in different parasitic species may have different substrate affinities or redox optima, or peroxidases from different genera might be selective for particular structures. The amenability of T. versicolor to genetic analyses should help to clarify these possibilities.
The haustoria produced by Triphysaria and Striga are commonly distinguished as being primary and secondary, respectively (Kuijt, 1969). In the continued presence of DBMQ, haustorium development in S. asiatica results in a terminal differentiation of the radicle, giving rise to a primary haustorium. However, if DMBQ is washed from S. asiatica seedlings a few hours after exposure, normal root growth resumes. This cyclic reversion to normal root growth is observed when S. asiatica seedlings are exposed to syringic acid (Kim et al., 1998). The authors propose that hydrogen peroxide production is reduced in the presence of quinones, so during haustorium development phenols are not oxidized and normal roots develop.
The response of T. versicolor roots to DMBQ is different. Haustorium development is transient. Only those cells near the root tip when DMBQ is applied develop into (secondary) haustoria. Normal T. versicolor root growth commences after a few hours in the continued presence of DMBQ, mimicking the periodic response of S. asiatica to syringic acid. It may be that the phenol oxidation and quinone recognition mechanisms are different between T. versicolor and S. asiatica. Such differences may reflect the need for S. asiatica to infect a host soon after germination, whereas T. versicolor, being facultative, can be more opportunistic in its pursuit of host resources. Alternatively, later stages in the haustoria-development pathway may be autoregulatory in T. versicolor but not in S. asiatica.
Many of the active haustoria-inducing molecules are common constituents of plant cells, where they function in lignin biosynthesis, host defense, and other specialized physiological processes. Their recognition as HIFs allows the parasites to form haustoria in response to a broad spectrum of host plants. Host specificity in obligate parasitic Scrophulariaceae species such as S. asiatica is not defined at the haustoria-initiation stage, but earlier, at seed germination, or later, at haustorium penetration (Parker and Riches, 1993; Hood et al., 1998).
Figure 3.
Anthocyanidins assayed for haustoria-inducing activity in T. versicolor.
ACKNOWLEDGMENTS
The authors thank Dr. D.G. Lynn for insightful discussions and Dr. L.R. Teuber for helpful advice on statistical analyses.
Abbreviations:
- CHS
chalcone synthase
- DMBQ
2,6-dimethoxy-p-benzoquinone
- HIF
haustoria-inducing factor
Footnotes
This work was supported by the National Science Foundation (Developmental Biology grant no. 94-07737) and by the Rockefeller Foundation.
LITERATURE CITED
- Atsatt P, Strong D. The population biology of annual grassland hemiparasites. I. The host environment. Evolution. 1970;24:278–291. doi: 10.1111/j.1558-5646.1970.tb01761.x. [DOI] [PubMed] [Google Scholar]
- Baird WV, Riopel JL. Experimental studies of haustorium initiation and early development in Agalinis purpurea (L.) Raf. (Scrophulariaceae) Am J Bot. 1984;71:803–814. [Google Scholar]
- Becard G, Taylor LP, Douds DD, Pfeffer PE, Doner LW. Flavonoids are not necessary plant signal compounds in arbuscular mycorrhizal symbioses. Mol Plant Microbe Interact. 1995;8:252–258. [Google Scholar]
- Chabot S, Bel-Rhlid R, Chenevert R, Piche Y. Hyphal growth promotion in vitro of the VA mycorrhizal fungus, Gigaspora margarita Becker & Hall, by the activity of structurally specific flavonoid compounds under CO2-enriched conditions. New Phytol. 1992;122:461–467. doi: 10.1111/j.1469-8137.1992.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Chang M, Lynn DG. The haustorium and host recognition in parasitic angiosperms. J Chem Ecol. 1986;12:561–579. doi: 10.1007/BF01020572. [DOI] [PubMed] [Google Scholar]
- Cheminat AR, Brouillard R. PMR investigation of 3-O-(β-d-glucosyl)malvidin structural transformations in aqueous solutions. Tetrahedron Lett. 1986;27:4457–4460. [Google Scholar]
- Chuang TI, Heckard LR. Chromosomal numbers of Orthocarpus and related monotypic genera (Scrophulariaceae:subtribe Castillejinae) Brittonia. 1982;34:89–101. [Google Scholar]
- Chuang TI, Heckard LR. Generic realignment and synopsis of subtribe Castillejinae (Scrophulariaceae: tribe Pediculareae) Syst Bot. 1991;16:644–666. [Google Scholar]
- Coe EH, McCormick SM, Modena SA. White pollen in maize. J Hered. 1981;72:318–320. [Google Scholar]
- DePamphilis CW, Young ND, Wolfe AD. Evolution of plastid gene rps2 in a lineage of hemiparasitic and holoparasitic plants: many losses of photosynthesis and complex patterns of rate variation. Proc Natl Acad Sci USA. 1997;94:7367–7372. doi: 10.1073/pnas.94.14.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooner HG, Robbins TP, Jorgensen RA. Genetic and developmental control of anthocyanin biosynthesis. Annu Rev Genet. 1991;25:173–199. doi: 10.1146/annurev.ge.25.120191.001133. [DOI] [PubMed] [Google Scholar]
- Estabrook EM, Yoder JI. Plant-plant communications: rhizosphere signaling between parasitic angiosperms and their hosts. Plant Physiol. 1998;116:1–7. [Google Scholar]
- Fate GD, Lynn DG. Xenognosin methylation is critical in defining the chemical potential gradient that regulates the spatial distribution in Striga pathogenesis. J Am Chem Soc. 1996;118:11369–11376. [Google Scholar]
- Fisher RF, Long SR. Rhizobium-plant signal exchange. Nature. 1992;357:655–660. doi: 10.1038/357655a0. [DOI] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Jones JDG. Resistance gene-dependent plant defense responses. Plant Cell. 1996;8:1773–1791. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne JB, Moss GP (1993) Phytochemical Dictionary: A Handbook of Bioactive Compounds from Plants. Taylor and Francis, Washington, DC
- Heath JD, Boulton MI, Raineri DM, Doty SL, Mushegian AR, Charles TC, Davies JW, Nester EW. Discrete regions of the sensor protein VirA determine the strain-specific ability of Agrobacterium to agroinfect maize. Mol Plant Microbe Interact. 1997;10:221–227. doi: 10.1094/MPMI.1997.10.2.221. [DOI] [PubMed] [Google Scholar]
- Hickman JC. The Jepson Manual: Higher Plants of California. Berkeley: University of California Press; 1993. [Google Scholar]
- Hood ME, Condon JM, Timko MP, Riopel JL. Primary haustorial development of Striga asiatica on host and nonhost species. Phytopathology. 1998;88:70–75. doi: 10.1094/PHYTO.1998.88.1.70. [DOI] [PubMed] [Google Scholar]
- Hooykaas PJJ, Beijersbergen AGM. The virulence system of Agrobacterium tumefaciens. Annu Rev Phytopathol. 1994;32:157–179. [Google Scholar]
- Huala E, Oeller PW, Liscum E, Han IS, Larsen E, Briggs WR. Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science. 1997;278:2120–2123. doi: 10.1126/science.278.5346.2120. [DOI] [PubMed] [Google Scholar]
- Hungria M, Joseph CM, Phillips DA. Anthocyanins and flavonols, major nod gene inducers from seeds of a black-seeded common bean (Phaseolus vulgaris L.) Plant Physiol. 1991;97:751–758. doi: 10.1104/pp.97.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungria M, Phillips DA. Effects of a seed color mutation on rhizobial nod-gene-inducing flavonoids and nodulation in common bean. Mol Plant Microbe Interact. 1993;6:418–422. [Google Scholar]
- Kim D, Kocz R, Boone L, Keyes WJ, Lynn DG. On becoming a parasite: evaluating the role of wall oxidases in parasitic plant development. Chem Biol. 1998;5:103–117. doi: 10.1016/s1074-5521(98)90144-2. [DOI] [PubMed] [Google Scholar]
- Kuijt J. Parasitic Plants. Berkeley: University of California Press; 1969. [Google Scholar]
- Lynn DG, Chang M. Phenolic signals in cohabitation: implications for plant development. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:497–526. [Google Scholar]
- Lynn DG, Steffens JC, Kamut VK, Graden DW, Shabanowitz J, Riopel JL. Isolation of the first host recognition substance for parasitic angiosperms. J Am Chem Soc. 1981;103:1868–1870. [Google Scholar]
- MacQueen M (1984) Haustorial initiating activity of several simple phenolic compounds. In C Parker, LJ Musselman, RM Polhill, AK Wilson, eds, Proceedings of the Third International Symposium on Parasitic Weeds. International Center for Agricultural Research, Aleppo, Syria, pp 118–122
- Musselman LJ. The biology of Striga, Orobanche, and other root-parasitic weeds. Annu Rev Phytopathol. 1980;18:463–489. [Google Scholar]
- Parker C, Riches CR (1993) Parasitic Weeds of the World: Biology and Control. CAB International, Wallingford, UK
- Press MC, Graves JD. Parasitic Plants. London: Chapman & Hall; 1995. [Google Scholar]
- Pueppke SG. The genetic and biochemical basis for nodulation of legumes by rhizobia. Crit Rev Biotechnol. 1996;16:1–51. doi: 10.3109/07388559609146599. [DOI] [PubMed] [Google Scholar]
- Riopel JL (1979) Experimental studies on induction of haustoria in Agalinis purpurea. In LJ Musselman, AD Worsham, RE Eplee, eds, Second Symposium on Parasitic Weeds. North Carolina State University, Raleigh, pp 165–173
- Riopel JL, Timko MP. Haustorial initiation and differentiation. In: Press MC, Graves JD, editors. Parasitic Plants. London: Chapman & Hall; 1995. pp. 39–79. [Google Scholar]
- Schlaman HRM, Phillips DA, Kondorosi E (1998) Genetic organization and transcriptional regulation of rhizobial nodulation genes. In HP Spaink, A Kondorosi, PJJ Hooykaas, eds, The Rhizobiaceae. Kluwer Academic Press, Dordrecht, The Netherlands, pp 361–386
- Sisqueira JO, Nair MG, Hammerschmidt R, Safir GR. Significance of phenolic compounds in plant-soil-microbial systems. Crit Rev Plant Sci. 1991;10:63–121. [Google Scholar]
- Smith CE, Ruttledge T, Zeng Z, O'Malley RC, Lynn DG. A mechanism for inducing plant development: the genesis of a specific inhibitor. Proc Natl Acad Sci USA. 1996;93:6986–6991. doi: 10.1073/pnas.93.14.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaink HP, Wijffelman CA, Pees E, Okker RJH, Lugtenberg BJJ. Rhizobium nodulation gene nodD as a determinant of host specificity. Nature. 1987;328:337–340. [Google Scholar]
- Yoder JI. Self and cross-compatibility in three species of the hemiparasite Triphysaria (Scrophulariaceae) Environ Exp Bot. 1998;39:77–83. [Google Scholar]