Abstract
Calcium is an important second messenger in the phospholipase C (PLC) signal transduction pathway. Calcium signaling is involved in many biological processes, including muscle contraction, cellular activation, and cellular proliferation. Dendritic cell (DC) maturation is induced by many different stimuli, including bacterial lipopolysaccharide (LPS), bacterial toxins, inflammatory cytokines, prostaglandins, as well as calcium mobilization. In the present study, we determined the role of the PLC signal transduction pathway in the activation and maturation of human monocyte-derived DCs (MDDCs) induced by diverse agonists. We found that signaling through PLC activates MDDCs to mature and is necessary for LPS, cholera toxin, dibutyryl-cyclic AMP, prostaglandin E2, and the calcium ionophore A23187 to induce MDDC maturation. The results of the present study along with the results of other studies indicate that multiple signaling pathways are involved in the activation of DCs and that inhibition of any of these pathways inhibits the maturation of DCs.
Dendritic cells (DCs) are the most potent antigen-presenting cells (APCs) known and are probably the only APCs capable of initiating functional primary immune responses in vivo. Immature DCs residing in peripheral tissues are highly efficient at capturing antigen (for a review, see reference 3). After receiving an activation signal, DCs migrate to organized lymphoid tissues, lose the ability to take up new antigens, and mature into potent APCs (3). The potency of mature DCs for the presentation of antigens stems from their increased expression of the major histocompatibility complex and costimulatory and adhesion molecules, along with their ability to secrete cytokines and chemokines (3). Monocyte-derived DCs (MDDCs), produced by culturing monocytes with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4), are phenotypically equivalent to immature DCs residing in peripheral tissues (16). When MDDCs are incubated with activating agonists such as lipopolysaccharide (LPS) or cholera toxin (CT), they undergo maturation and take on the phenotypic characteristics of mature DCs found in secondary lymphoid tissues (16). DC biology is a rapidly expanding field; however, little is known about the signaling processes that control maturation.
Understanding of the signaling process that leads to DC maturation is important for determining how these cells initiate immune responses to foreign antigens. The signaling pathways induced by LPS in MDDCs have recently been studied (1). Several signaling pathways in MDDCs were found to be activated by LPS, including the p38 stress-activated kinase (p38 SAPK) pathway, the extracellular signal-regulated kinase (ERK) pathway, the PI3 kinase pathway, and the NF-κB pathway (1). However, only the p38 SAPK, PI3 kinase, and NF-κB pathways were found to be involved in maturation (1). Although many of the signaling pathways activated by LPS in MDDCs and other cells have been identified, little is known about the signaling pathways in MDDCs activated by other agonists such as CT and prostaglandin E2 (PGE2).
CT is an AB5 enterotoxin produced by pathogenic strains of Vibrio cholerae. CT consists of a 27-kDa catalytic A domain anchored in a ring of five identical 11.7-kDa B subunits (14). The B pentamer targets CT to cell membranes by binding to GM1 gangliosides (11). The A domain of CT exploits the host protein retention and degradation pathways to gain access to the cytoplasm (for a review, see reference 10). In the cytosol, the A1 subunit catalyzes the transfer of an ADP-ribose from NAD to stimulatory α subunits of G proteins (Gsα). After ADP-ribosylation, Gsα binds to adenylate cyclase and constitutively activates it, leading to a sustained increase in the intracellular cyclic AMP (cAMP) concentration (4). Previously, we showed that the ability of CT to elevate intracellular cAMP levels is responsible for its activation of MDDCs, as the activation was mimicked by dibutyryl-cAMP (d-cAMP) and forskolin and was blocked by an inhibitor of cAMP-dependent protein kinase A (PKA) (2). In addition, PGE2, which also activates adenylate cyclase, induces MDDC maturation (15).
Although our previous study showed that elevated intracellular cAMP levels are necessary for CT to activate MDDCs, it did not determine if the PKA pathway is the only pathway activated by CT. In this regard, d-cAMP mimics the effects of CT on PKA activation; however, the PKA pathway is not the only signaling pathway activated by CT. For instance, CT has been shown to increase arachidonic acid metabolism and to interact with ADP-ribosylation factors. In addition, the B pentamer of CT activates signaling pathways by cross-linking GM1 gangliosides on the cell surface.
Prostaglandins also signal through PKA by activating adenylate cyclase; however, these arachidonic acid derivatives also activate other signaling pathways. As calcium signaling is important in many immune system functions and has been shown to activate MDDCs to mature (5, 8, 12), we determined if the phospholipase C (PLC)-calcium signaling pathway is involved in the activation of MDDCs induced by various agonists. We found that the PLC-calcium signaling pathway is activated in MDDCs by the activating agonists LPS, CT, d-cAMP, PGE2, and A23187 (a calcium ionophore), as these agonists increase the levels of production of inositol 1,4,5-triphosphate (IP3). In addition, we determined that the PLC-calcium signaling pathway contributes to the activation of MDDCs induced by these agonists, as sequestration of calcium or inhibition of PLC or protein kinase C inhibits the maturation of MDDCs induced by these agonists. Our data, along with the results of other studies, indicate that multiple signaling pathways are involved in the activation of DCs and that inhibition of any of these pathways inhibits the maturation of DCs.
MATERIALS AND METHODS
Reagents.
BAPT-AM (1,2-bis(2-aminophenoxy)-ethane-N,N,N′,N′, tetraacetic acid-tetrakis(acetoxymethyl ester) D609, cyclosporine, d-cAMP, A23187, thapsigargin, and PGE2 were purchased from Sigma (St. Louis, Mo.). Xestospongin was purchased from Calbiochem (San Diego, Calif.). CT and Pasteurella multocida toxin (PMT) were purchased from List Biological Laboratories (Campbell, Calif.).
DC culture medium.
DC culture medium consisted of RPMI 1640 medium (Life Technologies, Carlsbad, Calif.) supplemented with 2 mM l-glutamine (Sigma), 1% nonessential amino acids (Life Technologies), 1% sodium pyruvate (Life Technologies), 50 μM 2-mercaptoethanol (Sigma), 50 μg of gentamicin (Life Technologies) per ml, and 10% fetal calf serum (Life Technologies).
DC preparations.
All human specimens were obtained under informed consent, as approved by the University of Maryland Baltimore Institutional Review Board. Monocyte-derived DCs were generated as described previously (16), with minor modifications. Briefly, human peripheral blood mononuclear cells were isolated by Ficoll-Hypaque density gradient centrifugation and were enriched for CD14+ monocytes by negative selection with a cocktail of monoclonal antibodies from StemCell Technologies (Vancouver, British Columbia, Canada) according to the instructions of the manufacturer. Typically, greater than 80% of the cells were CD14+ after enrichment (data not shown). The isolated monocytes were allowed to adhere to plastic by plating 106 cells per/ml in RPMI 1640 medium for 2 h. Adherent monocytes were washed with RPMI 1640 medium and were then cultured at 106 cells per/ml in DC culture medium supplemented with 50 ng of recombinant GM-CSF (rGM-CSF) per ml and 1,000 U of recombinant IL-4 (rIL-4; R&D Systems, Minneapolis, Minn.) per ml. After adherence to the plastic, typically greater than 90% of the adherent cells were CD14+ (data not shown). Within 24 h after the addition of rGM-CSF and rIL-4, CD14 was undetectable on the cells (data not shown).
Cell treatments.
Agonists and inhibitors were added directly to MDDC cultures in individual wells. The cells were harvested, washed, and stained for phenotypic analysis by flow cytometry. For reagents suspended in dimethyl sulfoxide (DMSO), an equal volume of DMSO was added to control cultures. At the volumes tested, DMSO had no stimulatory or inhibitory effects on the MDDCs (data not shown).
Flow cytometry.
Cells were incubated for 30 min at 4°C with murine monoclonal antibodies specific for CD80, CD83, CD86, and HLA-DR (BD Pharmingen, San Diego, Calif.); washed; and then fixed with 2% paraformaldehyde for analysis with a FACScalibur flow cytometer (BD Pharmingen). Data analysis was carried out with FlowJo software (Tree Star Inc., San Carlos, Calif.). Incubation with equal concentrations of isotype-specific control antibodies did not raise the fluorescence intensity above the autofluorescent background levels (data not shown).
Calculation of percent activation.
The fraction of MDDCs that responded by upregulation of activation markers on the cell surface was calculated with Flowjo software by overlaying the histograms of treated and untreated MDDCs and Overton subtraction of the curves.
Calculation of percent inhibition.
Percent inhibition was calculated with the following formula: [(X − Y)/X] × 100, where X equals the fraction of cells that upregulated a marker in the absence of the inhibitor and Y equals the fraction of cells that upregulated a marker in the presence of the inhibitor.
Endotoxin quantitation.
Endotoxin levels in the cell medium and the dilutions of the agonists applied were quantified by the Limulus assay (Bio-Whittaker, Walkersville, Md.). There was no more than 40 pg of endotoxin per ml in the medium or the final dilutions of the agonists used in the studies (excluding LPS). This concentration of endotoxin is approximately 2- to 10-fold lower than the minimal concentration required to induce detectable activation of MDDCs in vitro (data not shown).
IP3 quantitation.
On day 4 of culture, individual wells of MDDCs were treated with 0.5 μg of LPS or CT per ml, 0.5 mM d-cAMP, 10 μM PGE2, 150 ng of A23187 per ml, or 1 μg of P. multocida toxin (PMT) per ml. At 1, 3, 6, and 12 h after the addition of the agonists the cells were harvested, washed, and resuspended in 1 ml of phosphate-buffered saline. The cells were then incubated for 20 min with 0.2 ml of ice-cold 20% perchloric acid. The cell lysates were then pelleted by centrifugation at 2,000 × g for 15 min at 4°C. The supernatants were removed, placed in glass tubes, and neutralized with ice-cold 10 M KOH containing 60 mM HEPES buffer and phenol red. The supernatants were centrifuged at 200 × g for 15 min at 4°C to sediment KClO4. The supernatants were then removed and assayed for the IP3 concentration by a radiometric assay according to the instructions of the manufacturer (Amersham, Little Chalfont, England).
RESULTS
Elevated calcium levels contribute to the maturation of MDDCs.
Calcium is an important second messenger in many signaling pathways, and calcium ionophores have been shown to induce MDDC maturation (8). In this regard, the calcium ionophore A23187 (150 ng/ml) activates MDDCs to mature, as measured by the increased levels of expression of the activation markers CD80, CD83, CD86, and HLA-DR (8). In addition, activation induced by A23187 is blocked by the calcineurin inhibitor cyclosporine (8). In initial experiments, we determined the MDDC activation and maturation profiles induced by LPS, CT, cAMP, PGE2, and A23187 and determined if the IP3-independent calcium releaser thapsigargin (18) also activates MDDCs. For these experiments, we used day 4 MDDCs. In preliminary studies, we found that at day 4, MDDCs display all of the characteristics of the more traditional day 7 MDDCs but are more responsive to the agonists tested (data not shown). These results are in agreement with those of a recent study showing that monocytes become immature DCs within 24 h of incubation with GM-CSF and IL-4 and readily mature into potent APCs when stimulated with activating agonists (6). Importantly, these “fast DCs” are just as potent APCs as the more traditional day 7 MDDCs (6).
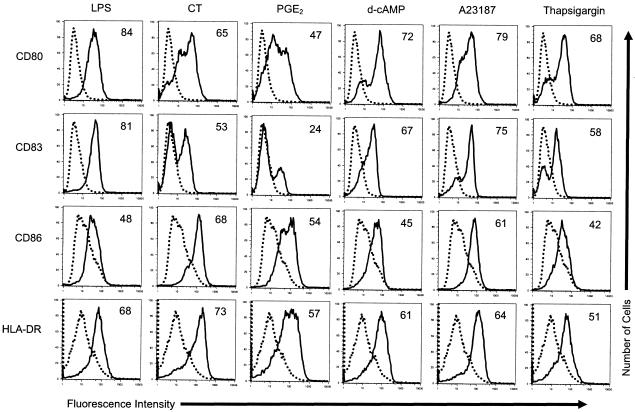
Day 4 MDDCs were incubated with 0.5 μg of LPS per ml, 0.5 μg of CT per ml, 10 μM PGE2, 500 μM d-cAMP, 150 ng of A23187 per ml, or 10 μM thapsigargin. Twenty hours later, the cells were harvested and activation was quantified by measurement of surface marker upregulation by flow cytometry. Figure 1 shows the typical expression profiles of CD80, CD83, CD86, and HLA-DR on MDDCs induced by these concentrations of LPS, CT, PGE2, d-cAMP, and A23187. Figure 1 also shows that thapsigargin activates MDDCs, as judged by increased levels of expression of the indicated activation markers. The fraction of MDDCs that increased the levels of expression of these activation markers was concentration dependent for all of the agonists tested, as this fraction increased with increasing concentration (data not shown). In other experiments, we found that the MDDCs activated by each of these agonists were more potent APCs than untreated MDDCs in mixed lymphocyte reactions (data not shown). It should also be noted that at time points after 24 h, an increased number of dead cells was detected in cultures of MDDCs stimulated with A23187 (determined by trypan blue exclusion and light scatter profiles). This phenomenon was not detected in cultures of MDDCs stimulated by the other agonists (data not shown).
FIG. 1.
Thapsigargin induces MDDCs to maturation. The cell surface expression of the indicated markers on untreated MDDCs (dotted histograms) or MDDCs treated with the indicated agonists (solid histograms) is shown. Day 4 MDDCs were incubated with 0.5 μg of LPS per ml, 0.5 μg of CT per ml, 10 μM PGE2, 500 μM d-cAMP, 150 ng of A23187 per ml, or 10 μM thapsigargin for 20 h. The cells were then harvested and stained for four-color flow cytometry with phycoerythrin-labeled anti-CD80, fluorescein isothiocynate-labeled anti-CD83, cytochrome-labeled anti-CD86, and anti-HLA-DR APCs. The percentage of cells that increased the levels of expression of the indicated markers (percent activation [the numbers in the histograms]) was calculated as described in Materials and Methods. Incubation with equal concentrations of isotype-specific control antibodies did not raise the fluorescence intensity above autofluorescent background levels (data not shown). The data shown are from one of three experiments performed, with similar results obtained in each experiment.
Next, we determined if calcium signaling is important for the activation of MDDCs induced by LPS, CT, d-cAMP, or PGE2. For these experiments, day 4 MDDCs were incubated with 0.5 μg of LPS per ml, 0.5 μg of CT per ml, 10 μM PGE2, 500 μM d-cAMP, or 150 ng of A23187 per ml with or without a prior 1-h incubation with the membrane-permeating calcium chelator BAPT-AM at 10 μM (17), the IP3-mediated calcium release inhibitor xestospongin at 10 μM (9), or the calcineurin inhibitor cyclosporine at 5 μM. Twenty hours later, the cells were harvested for flow cytometry. Inhibition was calculated as described in Materials and Methods. As shown in Table 1, BAPT-AM, xestospongin, and cyclosporine each inhibited the activation of MDDCs induced by LPS, CT, PGE2, d-cAMP, and A23187. In this regard, the fraction of cells that increased the levels of expression of CD80, CD83, CD86, and HLA-DR in response to these agonists was lower in the presence of these inhibitors.
TABLE 1.
Inhibition of MDDC maturationlegend
| Inhibitor (concn. [μM]) | Marker | % Inhibition
|
||||
|---|---|---|---|---|---|---|
| LPS | CT | d-cAMP | PGE2 | A23187 | ||
| BAPT-AM (10) | CD80 | 91.0 (0.5) | 76.5 (2.5) | 91.1 (3.0) | 74.0 (5.3) | 100 (0.0) |
| CD83 | 84.4 (0.7) | 76.9 (9.4) | 96.6 (1.9) | 83.4 (7.2) | 96.8 (2.5) | |
| CD86 | 87.3 (3.5) | 47.4 (20.2) | 81.7 (4.3) | 88.6 (6.5) | 100 (0.0) | |
| HLA-DR | 95.8 (1.8) | 78.6 (7.8) | 80.3 (1.7) | 84.1 (8.9) | 98.6 (1.2) | |
| Xestospongin (10) | CD80 | 71.9 (6.5) | 73.2 (3.5) | 77.3 (5.2) | 92.6 (1.2) | 74.2 (2.4) |
| CD83 | 44.2 (7.2) | 72.0 (6.4) | 74.4 (7.9) | 45.6 (7.6) | 55.4 (2.1) | |
| CD86 | 58.5 (14.0) | 56.3 (17.7) | 55.2 (18.8) | 46.8 (3.3) | 70.8 (11.2) | |
| HLA-DR | 67.4 (12.5) | 78.3 (8.2) | 82.2 (6.8) | 76.2 (11.1) | 86.1 (9.4) | |
| Cyclosporine (5) | CD80 | 55.6 (1.3) | 99.6 (0.1) | 99.3 (0.5) | 86.7 (5.7) | 100 (0.0) |
| CD83 | 30.4 (2.1) | 77.8 (8.9) | 77.2 (10.7) | 63.6 (10.6) | 94.2 (4.5) | |
| CD86 | 50.2 (12.5) | 60.0 (13.8) | 64.0 (14.6) | 82.4 (5.3) | 99.1 (0.6) | |
| HLA-DR | 41.6 (10.5) | 42.9 (5.1) | 45.2 (6.7) | 43.6 (16.4) | 82.6 (2.6) | |
| D609 (100) | CD80 | 87.1 (5.8) | 83.5 (5.4) | 91.8 (1.4) | 82.0 (7.9) | 95.7 (3.2) |
| CD83 | 79.6 (2.8) | 83.9 (2.0) | 89.0 (3.1) | 70.8 (9.6) | 76.7 (2.0) | |
| CD86 | 92.4 (6.2) | 67.4 (14.8) | 76.6 (9.5) | 60.5 (17.8) | 94.8 (4.2) | |
| HLA-DR | 65.2 (19.1) | 65.4 (14.4) | 45.6 (3.9) | 78.9 (10.8) | 72.3 (3.7) | |
aDay 4 MDDCs were incubated with 0.5 μg of LPS per ml, 0.5 μg of CT per ml, 10 μM PGE2, 500 μM d-cAMP, or 150 ng of A23187 per ml with or without a prior 1-h incubation with the indicated concentrations of the indicated inhibitors for 20 h. The cells were then harvested and stained for four-color flow cytometry with phycoerythrin-labeled anti-CD80, fluorescein isothiocyanate labeled anti-CD83, cytochrome-labeled anti-CD86, and anti-HLA-DR APCs. The percent inhibition was calculated as described in Materials and Methods. For reagents suspended in DMSO, control cultures were incubated with an equal volume of DMSO. The DMSO concentrations used did not activate or inhibit the activation of these cells (data not shown). The results are the means (standard errors of the means) of at least three experiments performed with cells from different donors.
It is surprising that xestospongin inhibits the activation of MDDCs induced by A23187, as calcium ionophores elevate intracellular calcium levels by importing calcium from the extracellular space, while xestospongin inhibits calcium release from the endoplasmic reticulum (ER). It is possible that the increase in intracellular calcium levels obtained with A23187 triggers calcium-induced calcium release from the ER and that xestospongin blocks this release and therefore partially inhibits the effects of A23187. Alternatively, elevated cytoplasmic calcium levels may provide a feedback mechanism that activates PLC to produce IP3 and diacylglycerol (DAG). Together, the results of these experiments indicate that calcium signaling contributes to the activation of MDDCs induced by all of the agonists tested.
PLC contributes to the maturation of MDDCs.
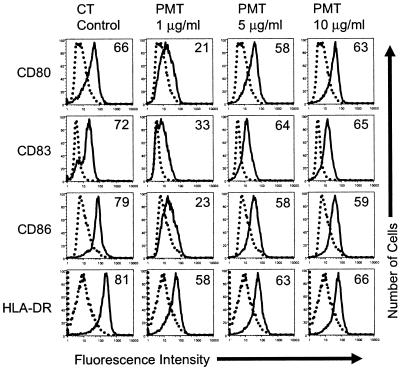
PLC cleaves phosphatidylinositol 4,5-bisphosphate into IP3 and DAG, and therefore, the cleavage by PLC is upstream of calcium release in signal transduction pathways. For this reason, we determined if PLC signaling activates MDDCs to mature and if an inhibitor of PLC inhibits the maturation of MDDCs induced by LPS, CT, PGE2, d-cAMP, or A23187. To determine if the activation of PLC activates MDDCs, we treated MDDCs with PMT. This toxin ADP-ribosylates the α subunit of Gq proteins and subsequently activates PLC-b1 (19). For these experiments, day 4 MDDCs were incubated with increasing concentrations of PMT. Twenty hours later, the cells were harvested and activation was quantified by surface marker upregulation, as described in Materials and Methods. As shown in Fig. 2, PMT activates MDDCs in a concentration-dependent manner. This result indicates that signaling through PLC activates MDDCs.
FIG. 2.
PMT activates MDDCs to mature. Cell surface expression of the indicated markers on untreated MDDCs (dotted histograms) or MDDCs treated with the indicated agonists (solid histograms) is shown. Day 4 MDDCs were incubated with the indicated concentrations of PMT for 20 h. The cells were then harvested and stained for four-color flow cytometry with phycoerythrin-labeled anti-CD80, fluorescein isothiocyanate-labeled anti-CD83, cytochrome-labeled anti-CD86, and anti-HLA-DR APCs. The percentage of cells that increased the expression of the indicated markers (percent activation [the numbers in the histograms]) was calculated as described in Materials and Methods. The data shown are from one of six experiments performed, with similar results obtained in each experiment.
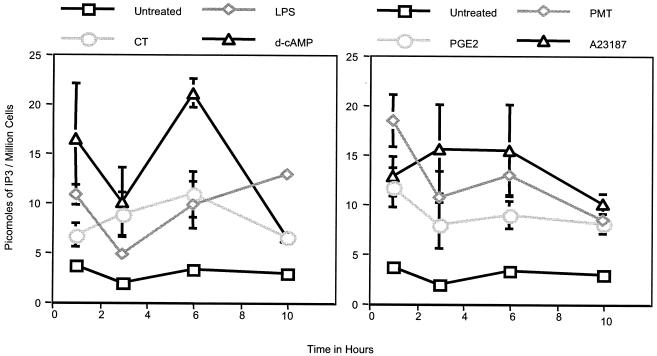
To determine if the activation of MDDCs with LPS, CT, d-cAMP, PGE2, A23187, or PMT induces PLC signaling, day 4 MDDCs were incubated with 0.5 μg of LPS per ml, 0.5 μg of CT per ml, 10 μM PGE2, 500 μM d-cAMP, 150 ng of A23187 per ml, or 1 μg of PMT (as a positive control) per ml for various times. The cells were then harvested and analyzed for their intracellular IP3 contents, as described in Materials and Methods. As shown in Fig. 3, the level of IP3 in MDDCs incubated with these agonists was elevated as early as 1 h after treatment and remained elevated for at least 10 h. It is interesting that A23187 initiates IP3 production, as calcium release from the ER is IP3 dependent and therefore downstream of PLC activation in signaling pathways. As A23187 imports calcium from outside the cell, it would be expected to bypass IP3-mediated calcium release from the ER. As was mentioned above, it is possible that the increased intracellular calcium levels induced by A23187 provide positive feedback for PLC activation. In any case, these results indicate that all of the agonists tested activate PLC in MDDCs; however, they do not show that the activation of PLC contributes to the maturation of MDDCs.
FIG. 3.
Activated MDDCs have elevated intracellular IP3 levels. Day 4 MDDCs were stimulated with 0.5 μg of LPS per ml, 0.5 μg of CT per ml, 10 μM PGE2, 500 μM d-cAMP, 150 ng of A23187 per ml, or 1 μg of PMT per ml. At the indicated times, the cells were harvested and intracellular IP3 levels were quantified by a radiometric assay, as described in Materials and Methods. The results shown are the means and standard errors of the means for MDDCs from three different donors.
To determine if signaling through PLC plays a role in the activation of MDDCs induced by these agonists, we incubated day 4 MDDCs with 0.5 μg LPS per ml, 0.5 μg of CT per ml, 10 μM PGE2, 500 μM d-cAMP, or 150 ng of A23187 per ml with or without a prior 1-h incubation with the PLC inhibitor D609 at 100 μM (13). Twenty hours later, the cells were harvested for flow cytometry. Inhibition was calculated as described in Materials and Methods. Table 1 shows that D609 inhibits the maturation of MDDCs induced by LPS, CT, PGE2, d-cAMP, and A23187. Again, it is surprising that D609 inhibited the maturation of MDDCs induced by the calcium ionophore A23187. However, these results are in agreement with both the results of the xestospongin inhibition experiments (Table 1) and the intracellular IP3 measurements presented above (Fig. 2). It should also be noted that inhibition of PLC blocks the production of DAG, which cooperates with calcium for the activation of protein kinase C, an important mediator of calcium-induced signaling. All told, these results indicate that calcium release from IP3-gated stores in the ER initiated through PLC signaling is a mechanism common to all of the MDDC-activating agonists tested.
DISCUSSION
Until recently, little was known about the intracellular and intercellular signal transduction pathways that lead to DC activation and maturation. In eukaryotic cells, numerous signal transduction mechanisms control cell functions. These systems are often mechanistically complex and involve a large degree of overlap and cross talk between pathways. The p38 SAPK, PI3K, ERK, and NF-κB pathways have been shown to be activated in MDDCs by LPS (1), whereas the adenylate cyclase-PKA pathway was shown to be activated by CT (2). As calcium signaling is important in many immune system functions, we determined if the PLC-calcium signaling pathway is involved in the maturation of MDDCs induced by various agonists, including LPS, CT, d-cAMP, PGE2, and A23187. The results of the present study demonstrate that the PLC-calcium signaling pathway is involved in the maturation of MDDCs induced by the agonists listed above.
The abilities of both the calcium ionophore A23187 and the IP3-independent calcium releaser thapsigargin to activate MDDCs to mature clearly demonstrate that sustained increases in cytoplasmic calcium levels induce the maturation of these cells. The importance of calcium release from IP3-gated stores in the activation and maturation of MDDCs induced by the activating agonists LPS, CT, d-cAMP, PGE2, and A23187 is supported by four separate observations. First, all of these agonists increased the levels of IP3 production by the stimulated MDDCs. Second, the cell-permeating calcium chelator BAPT-AM inhibits the maturation of MDDCs induced by these agonists. Third, the IP3-dependent calcium release inhibitor xestospongin inhibits the maturation of MDDCs induced by these agonists, and fourth, the calcineurin inhibitor cyclosporine inhibits the maturation of MDDCs induced by these agonists.
It is surprising that xestospongin inhibits the maturation of MDDCs induced by A23187. In this regard, calcium ionophores such as A23187 pump extracellular calcium into the cell. For this reason, an inhibitor that blocks calcium release from IP3-gated intracellular stores, such as xestospongin, would not be expected to inhibit the actions of A23187. At least two possible explanations could account for this result. First, the increased cytoplasmic calcium concentration induced by A23187 could cause calcium-induced calcium release from intracellular stores. This phenomenon is well documented in muscle cells and neurons (7). Second, increased cytoplasmic calcium levels may induce positive feedback that activates PLC to cleave phosphatidylinositol 4,5-bisphosphate into IP3 and DAG.
PLC is the enzyme responsible for liberating the second messengers IP3 and DAG from the plasma membrane. For this reason, PLC activation is upstream of calcium release in many signal transduction pathways. The ability of PMT to induce MDDC maturation demonstrates that sustained PLC signaling induces the maturation of MDDCs. The ability of the PLC inhibitor D609 to inhibit the maturation of MDDCs induced by all of the agonists tested indicates the involvement of PLC in the activation and maturation induced by these agonists. Again, it is surprising that D609 inhibits the maturation of MDDCs induced by A23187, as PLC activation is involved upstream of calcium release in signaling pathways. However, as mentioned above, the increased cytoplasmic calcium levels induced by A23187 may induce a positive feedback that activates PLC. This possibility is supported by the increase in the levels of IP3 production induced by A23187.
There is a third possibility that may explain how A23187 and thapsigargin induce PLC signaling. In other studies, we found that the MDDCs activated by the agonists tested in this study release soluble factors that act in an autocrine and/or a paracrine fashion on the MDDCs (unpublished data). These factors include but are not limited to prostaglandins and nitric oxide. Interestingly, the actions of these soluble factors are required for the maturation of the MDDCs induced by the agonists. Therefore, the activation of the PLC pathway induced by A23187 or the other agonists might be due to the effects of these soluble factors.
In summary, the results of this study strongly suggest that signaling through the PLC-calcium signaling pathway is necessary for the activation and maturation of MDDCs induced by the agonists LPS, CT, d-cAMP, PGE2, and A23187. However, the results of other studies show that this is not the only pathway activated by these agonists and is not the only pathway required for the activation of the MDDCs that they induce (1, 2). For instance, LPS activates the p38 SAPK, PI3K, and NF-κB pathways; and inhibition of any of these pathways blocks the activation of MDDCs induced by LPS (1). Likewise, CT, d-cAMP, and PGE2 activate the cAMP-dependent PKA pathway; and inhibition of this pathway blocks their ability to activate of MDDC (2; submitted for publication). All told, it appears that contributions from multiple signaling pathways are necessary for DC maturation. The dissection of these pathways and identification of the transcription factors that they induce will substantially increase the understanding of DC maturation and how these cells initiate immune responses.
Acknowledgments
This work was supported by NIH grants AI38192 and AI43046 to George K. Lewis.
REFERENCES
- 1.Ardeshna, K. M., A. R. Pizzey, S. Devereux, and A. Khwaja. 2000. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood 96:1039-1046. [PubMed] [Google Scholar]
- 2.Bagley, K. C., S. F. Abdelwahab, R. G. Tuskan, T. R. Fouts, and G. K. Lewis. 2002. Cholera toxin and heat-labile enterotoxin activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cyclic AMP-dependent pathway. Infect. Immun. 70:5533-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 4.Cassel, D., and Z. Selinger. 1977. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc. Natl. Acad. Sci. USA 74:3307-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czerniecki, B. J., C. Carter, L. Rivoltini, G. K. Koski, H. I. Kim, D. E. Weng, J. G. Roros, Y. M. Hijazi, S. Xu, S. A. Rosenberg, and P. A. Cohen. 1997. Calcium ionophore-treated peripheral blood monocytes and dendritic cells rapidly display characteristics of activated dendritic cells. J. Immunol. 159:3823-3837. [PubMed] [Google Scholar]
- 6.Dauer, M., B. Obermaier, J. Herten, C. Haerle, K. Pohl, S. Rothenfusser, M. Schnurr, S. Endres, and A. Eigler. 2003. Mature dendritic cells derived from human monocytes within 48 hours: a novel strategy for dendritic cell differentiation from blood precursors. J. Immunol. 170:4069-4076. [DOI] [PubMed] [Google Scholar]
- 7.Endo, M., M. Tanaka, and Y. Ogawa. 1970. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature 228:34-36. [DOI] [PubMed] [Google Scholar]
- 8.Faries, M. B., I. Bedrosian, S. Xu, G. Koski, J. G. Roros, M. A. Moise, H. Q. Nguyen, F. H. Engels, P. A. Cohen, and B. J. Czerniecki. 2001. Calcium signaling inhibits interleukin-12 production and activates CD83(+) dendritic cells that induce Th2 cell development. Blood 98:2489-2497. [DOI] [PubMed] [Google Scholar]
- 9.Gafni, J., J. A. Munsch, T. H. Lam, M. C. Catlin, L. G. Costa, T. F. Molinski, and I. N. Pessah. 1997. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5- trisphosphate receptor. Neuron 19:723-733. [DOI] [PubMed] [Google Scholar]
- 10.Hazes, B., and R. J. Read. 1997. Accumulating evidence suggests that several AB-toxins subvert the endoplasmic reticulum-associated protein degradation pathway to enter target cells. Biochemistry 36:11051-11054. [DOI] [PubMed] [Google Scholar]
- 11.Heyningen, S. V. 1974. Cholera toxin: interaction of subunits with ganglioside GM1. Science 183:656-657. [DOI] [PubMed] [Google Scholar]
- 12.Koski, G. K., G. N. Schwartz, D. E. Weng, B. J. Czerniecki, C. Carter, R. E. Gress, and P. A. Cohen. 1999. Calcium mobilization in human myeloid cells results in acquisition of individual dendritic cell-like characteristics through discrete signaling pathways. J. Immunol. 163:82-92. [PubMed] [Google Scholar]
- 13.Muller-Decker, K. 1989. Interruption of TPA-induced signals by an antiviral and antitumoral xanthate compound: inhibition of a phospholipase C-type reaction. Biochem. Biophys. Res. Commun. 162:198-205. [DOI] [PubMed] [Google Scholar]
- 14.Ohtomo, N., T. Muraoka, A. Tashiro, Y. Zinnaka, and K. Amako. 1976. Size and structure of the cholera toxin molecule and its subunits. J. Infect. Dis. 133(Suppl.):31-40. [DOI] [PubMed] [Google Scholar]
- 15.Rieser, C., G. Bock, H. Klocker, G. Bartsch, and M. Thurnher. 1997. Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J. Exp. Med. 186:1603-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi, T., K. Fukuda, J. Pan, H. Kodama, M. Sano, S. Makino, T. Kato, T. Manabe, and S. Ogawa. 1999. Characterization of insulin-like growth factor-1-induced activation of the JAK/STAT pathway in rat cardiomyocytes. Circ. Res. 85:884-891. [DOI] [PubMed] [Google Scholar]
- 18.Thastrup, O., H. Linnebjerg, P. J. Bjerrum, J. B. Knudsen, and S. B. Christensen. 1987. The inflammatory and tumor-promoting sesquiterpene lactone, thapsigargin, activates platelets by selective mobilization of calcium as shown by protein phosphorylations. Biochim. Biophys. Acta 927:65-73. [DOI] [PubMed] [Google Scholar]
- 19.Wilson, B. A., X. Zhu, M. Ho, and L. Lu. 1997. Pasteurella multocida toxin activates the inositol triphosphate signaling pathway in Xenopus oocytes via G(q)alpha-coupled phospholipase C-beta1. J. Biol. Chem. 272:1268-1275. [DOI] [PubMed] [Google Scholar]