Abstract
Previous work in our laboratory showed that the ESAT-6 protein of Mycobacterium tuberculosis and Mycobacterium bovis induces strong antibody responses in a large proportion (∼90%) of experimentally or naturally infected nonhuman primates. Here, the antibody response to ESAT-6 in tuberculous monkeys was characterized at the epitope level by measuring antibodies to overlapping, synthetic peptides spanning the ESAT-6 sequence. The antibody response against the COOH-terminal portion of the protein was the strongest in both experimentally and naturally infected animals. Moreover, these antibodies became detectable the earliest during experimental infection, suggesting an ordered expansion of ESAT-6-specific B-cell clones in the course of infection. The data support use of synthetic peptides in lieu of the full-length ESAT-6 protein in diagnostic antibody detection assays.
Tuberculosis (TB) not only is one of the human plagues but also affects a variety of animals, with great consequences to wildlife, agriculture, and zoo keeping (4, 9, 10). Moreover, TB in captive nonhuman primates greatly affects research endeavors. Outbreaks of TB in research monkey colonies, even when only suspected, are economically costly. The losses are caused not only by the elevated costs of animal losses but also by the expenses associated with disrupted research, lost time, and sometimes delayed release of new products into the market. As a result, strict control guidelines have been implemented (2). However, effective TB control requires accurate diagnostic methods, and the current method for diagnosing TB in living nonhuman primates, i.e., the tuberculin skin test, has limitations (8). A new method, which is based on detection of gamma interferon in whole blood (5), is still being evaluated. Thus, we have begun to characterize the antibody response to Mycobacterium tuberculosis antigens to evaluate the prospect of serologic methods for the diagnosis of TB in monkeys.
In previous work members of our group showed that 6-kDa early secretory antigenic target (ESAT-6), a low-molecular-weight protein secreted by virulent M. tuberculosis and Mycobacterium bovis (6, 12), induced strong antibody responses in >90% of experimentally infected monkeys (1). An outbreak of M. bovis infection in a nonhuman primate research facility provided the opportunity to characterize the antibody response to ESAT-6 in naturally infected macaques. We found that almost 90% of the animals exhibiting TB lesions at necropsy had anti-ESAT-6 immunoglobulin G (IgG) antibody (7). These studies strongly imply that an antibody detection assay utilizing ESAT-6 has a place in the diagnosis of TB in nonhuman primates. The present study was conducted to further characterize the IgG antibody response to ESAT-6 at the epitope level in experimentally infected and naturally infected nonhuman primates.
Three groups of nonhuman primates were included in the study. The first comprised 11 animals (4 cynomolgus macaques, 5 rhesus macaques, and 2 African Green monkeys) infected experimentally with 100 CFU of M. tuberculosis Erdman by the intratracheal route (1). Rhesus and African Green monkeys were obtained from closed breeding colonies; cynomolgus monkeys were feral, of Mauritian origin. Experimental infection was conducted using biosafety level 3 operating procedures and policies in a biosafety level 3 facility with approval of and oversight by the Institutional Environmental Health and Safety Office. TB was confirmed at necropsy by histopathology of major organs, acid-fast or fluorescent staining of infected tissue, culture methods, and bacterial nucleic acid amplification, as previously described (1). A second group of animals included 15 naturally infected monkeys (12 cynomolgus macaques and 3 rhesus macaques). Adult male, feral cynomolgus macaques of Mauritian origin and adult male and female rhesus macaques were housed in animal holding facilities at Stanford University, which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. These animals, which became infected with M. bovis during an outbreak of TB in the animal facility, were confirmed to have TB at necropsy by histopathology of major organs and acid-fast staining of infected tissue (7). The infectious agent, which was isolated from characteristic lesions, was identified as M. bovis on the basis of standard methods (genotyping with the insertion sequence IS6110 [13] and resistance to pyrazinamide). A third group comprised five baboons imported from Tanzania by Worldwide Primates, Inc. (Miami, Fla.), an importer of nonhuman primates registered with the Centers for Disease Control and Prevention. These animals were confirmed to have TB based on M. tuberculosis-specific nucleic acid amplification from infected organs. Animal use protocols were reviewed and approved by Institutional Animal Care and Use Committees in accordance with the Guide for the Care and Use of Laboratory Animals.
Sera were collected serially from experimentally infected animals over the course of infection. The number of serial sera ranged from 5 to 17 per animal. Only one serum sample was available from naturally infected animals. The ESAT-6 protein of M. tuberculosis was expressed as a polyhistidine-tagged fusion product in Escherichia coli and purified to near-homogeneity as previously described (3). Eight overlapping peptides (P1 to P8) spanning the full-length protein were also used. Peptides were synthesized as 24-mers (except P1, which was a 20-mer) with a sequence overlap of 14 amino acid residues (Fig. 1).
FIG. 1.
Amino acid sequence of synthetic, overlapping peptides spanning the sequence of ESAT-6 protein. Each line represents a peptide sequence. Peptide names are listed at left.
We first evaluated by enzyme-linked immunosorbent assay (ELISA) reactivities to ESAT-6-derived peptides by sera serially collected from 11 experimentally infected animals. Representative antibody profiles obtained with two animals are shown in Fig. 2. Sera from different animals reacted with different numbers of peptides (three to eight peptides) (data not shown). Despite this heterogeneous recognition, it was apparent that the COOH-terminal half of the ESAT-6 protein reacted with sera from most animals (Fig. 3, solid bars). The P8 peptide, which contains the most COOH-terminal sequences of ESAT-6, was recognized by sera from 10 of 11 animals that reacted with the full-length protein. The second-most-reactive peptide was P5, which is also located in the COOH-terminal half of the protein. The least-reactive peptide was P1 (only 3 reactors out of 11), which included the most NH2-terminal ESAT-6 sequences. Similar results were obtained from tests of serial sera from 13 cynomolgus macaques infected with a lower dose of M. tuberculosis (∼25 CFU): all animals tested reacted with the P8 peptide, while only three reacted with the P1 peptide (data not shown).
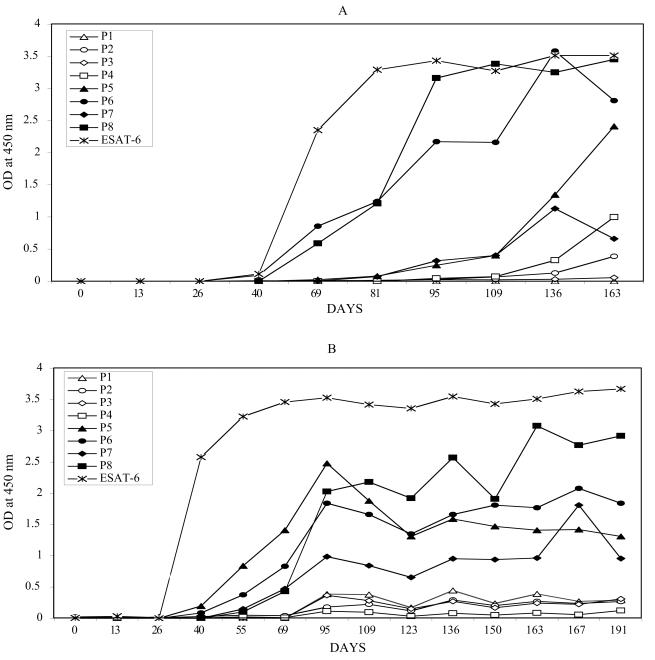
FIG. 2.
Profiles of serum IgG antibody to ESAT-6 protein and ESAT-6-derived peptides in one rhesus macaque (A) and one cynomolgus macaque (B). IgG antibody against ESAT-6 protein and eight ESAT-6-derived, overlapping peptides (P1 to P8; see Fig. 1) was measured by ELISA in serum samples collected serially at times postinfection, as indicated. For coating of microtiter plates, recombinant protein or synthetic peptides were used at a concentration of 1.0 μg/ml (200 μl/well). ELISA was performed as previously described (1, 11). Data from two representative animals are shown, one animal per panel. Open symbols are used for antibody reactivity to peptides spanning the NH2-terminal half of ESAT-6; solid symbols correspond to peptides spanning the COOH-terminal half of the protein.
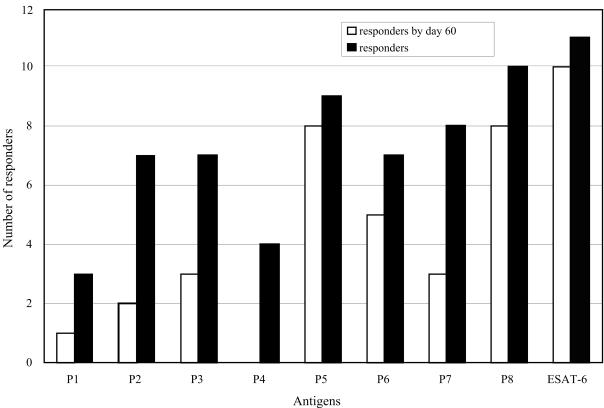
FIG. 3.
The antibody response to ESAT-6 peptides in experimentally infected animals. Antibody levels against ESAT-6 and ESAT-6-derived peptides were measured by ELISA in serum samples from 11 monkeys experimentally infected with M. tuberculosis. Cumulative numbers of animals whose sera gave OD450 readings above cutoff (responders) for each antigen are shown. For ESAT-6, the cutoff value was the mean plus 3 standard deviations of OD450 values obtained with sera from 51 negative-control, uninfected animals. For synthetic peptides, a cutoff of an OD450 of 0.3 was arbitrarily chosen, because the negative control sera gave extremely low readings when tested with synthetic peptides. Open bars represent numbers of responders for the indicated antigen by day 60 of infection; solid bars indicate the total number of responders for the indicated antigen.
The COOH-terminal sequences of ESAT-6 were recognized earliest in infection (Fig. 3, open bars). The earliest antibody response was to the P8 and P5 peptides (60 [± 10] and 60 [± 15] days postinfection), while antibody recognition of the NH2-terminal half of ESAT-6 occurred 2 weeks to 3 months later. The P1 peptide was typically recognized last.
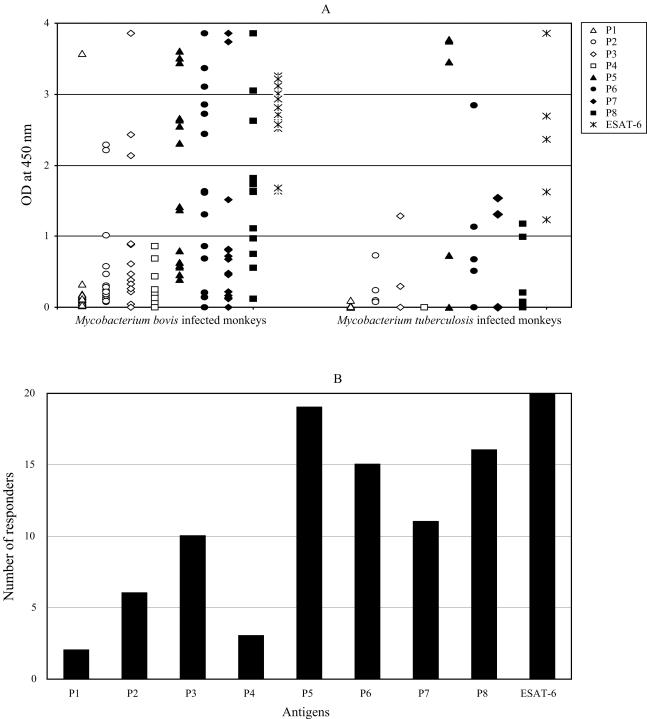
We next tested ESAT-6-reactive sera from 20 nonhuman primates naturally infected with tuberculous mycobacteria. The levels of antibodies to the COOH-terminal half of ESAT-6 (P5 through P8 peptides) were higher than those of the antibodies directed against the P1-P4 region of ESAT-6 (Fig. 4A). Differences in mean optical densities at 450 nm (OD450s) were statistically significant between groups (mean OD450 = 0.4 [95% confidence interval: 0.175 to 0.625] for peptides P1 through P4; mean OD450 = 1.67 [95% confidence interval: 1.414 to 1.933] for P5, P6, and P8 peptides; P < 0.0001). Moreover, a greater number of sera reacted with the COOH-terminal half (peptides P5 to P8) than with the NH2-terminal half (peptides P1 to P4) of the protein (Fig. 4B). All sera reacted with peptides P5 and P8 together (data not shown).
FIG. 4.
The antibody response to ESAT-6 peptides in naturally infected animals. (A) Antibody levels against ESAT-6 and ESAT-6-derived peptides were measured by ELISA in serum samples from 20 monkeys naturally infected with M. tuberculosis or with M. bovis. Because of potential differences in the response to M. bovis versus M. tuberculosis infection, raw data are shown for the two groups of infected animals separately. Each data point represents one serum sample tested against the antigen indicated. Open symbols are used for data obtained with peptides spanning to the NH2-terminal half of ESAT-6; solid symbols correspond to peptides spanning the COOH-terminal half of the protein. (B) Specific antibody levels were not statistically different between the two groups of animals (with the exception of the intergroup analysis of the antibody levels to P8; P = 0.02). Thus, this panel shows the cumulative number of responders to ESAT-6 and ESAT-6-derived peptides in the two groups of naturally infected animals, calculated as described in the legend to Fig. 3.
The data reported here clearly indicate that the ESAT-6 antigen of M. tuberculosis and M. bovis contains multiple B-cell epitopes and that antibodies produced by nonhuman primates predominantly react with sequences in the COOH-terminal half of the protein. Moreover, antibodies against the COOH-terminal peptides become detectable earlier in infection than those generated against the NH2-terminal half of the protein. These data strongly suggest an ordered expansion of epitope-specific B-cell clones during the course of tuberculous infection of nonhuman primates. Since the serodominance of COOH-terminal peptides was observed also with sera from naturally infected monkeys, it appears likely that recent infection should be associated with failure to seroreact with the NH2-terminal epitopes of ESAT-6.
We have previously shown that almost 90% of animals diagnosed at necropsy as having TB had serum IgG antibody against ESAT-6 (7). In relation to developing an ESAT-6-based serodiagnostic test for TB in monkeys, the observation that all ESAT-6-positive sera reacted to P5 plus P8 peptides makes it possible to consider use of synthetic peptides in lieu of full-length protein in order to reduce the costs of test manufacturing.
Acknowledgments
We thank Robert Peters for promoting our collaborative work on TB in monkeys. We also thank Karl Drlica for critical reading of the manuscript.
This work was supported in part by NIH grant AI36989 (to M.L.G.).
REFERENCES
- 1.Brusasca, P. N., R. L. Peters, S. Motzel, H. Klein, and M. L. Gennaro. 2003. Antigen recognition by serum antibodies in non-human primates experimentally infected with Mycobacterium tuberculosis. Comp. Med. 53:165-172. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1993. Tuberculosis in imported nonhuman primates—United States, June 1990-May 1993. Morb. Mortal. Wkly. Rep. 42:572-576. [PubMed] [Google Scholar]
- 3.Colangeli, R., A. Heijbel, A. Williams, C. Manca, J. Chan, K. Lyashchenko, and M. L. Gennaro. 1998. Three-step purification of lipopolysaccharide-free, polyhistidine-tagged recombinant antigens of Mycobacterium tuberculosis. J. Chromatogr. B. 714:223-235. [DOI] [PubMed] [Google Scholar]
- 4.de Lisle, G. W., R. G. Bengis, S. M. Schmitt, and D. J. O'Brien. 2002. Tuberculosis in free-ranging wildlife: detection, diagnosis and management. Rev. Sci. Tech. 21:317-334. [DOI] [PubMed] [Google Scholar]
- 5.Desem, N., and S. L. Jones. 1998. Development of a human gamma interferon enzyme immunoassay and comparison with tuberculin skin testing for detection of Mycobacterium tuberculosis infection. Clin. Diagn. Lab. Immunol. 5:531-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harboe, M., T. Oettinger, H. G. Wiker, I. Rosenkrands, and P. Andersen. 1996. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect. Immun. 64:16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanaujia, G. V., M. A. Garcia, D. M. Bouley, R. Peters, and M. L. Gennaro. 2003. Detection of anti-ESAT-6 antibody for diagnosis of tuberculosis in non-human primates. Comp. Med. 53:472-476. [PubMed] [Google Scholar]
- 8.McLaughlin, R. M., and G. E. Marrs. 1978. Tuberculin testing in nonhuman primates: OT vs. PPD, p. 123-128. In R. J. Montali (ed.), Mycobacterial infections of zoo animals. Smithsonian Institution Press, Washington, D.C.
- 9.Montali, R. J., S. K. Mikota, and L. I. Cheng. 2001. Mycobacterium tuberculosis in zoo and wildlife species. Rev. Sci. Tech. 20:291-303. [DOI] [PubMed] [Google Scholar]
- 10.O'Reilly, L. M., and C. J. Darborn. 1995. The epidemiology of Mycobacterium bovis infection in animals and man: a review. Tuber. Lung Dis. 76(Suppl. 1):1-46. [DOI] [PubMed] [Google Scholar]
- 11.Silva, V. M. C., G. Kanaujia, M. L. Gennaro, and D. Menzies. 2003. Factors associated with humoral response to ESAT-6, 38kDa and 14kDa antigens in patients with a spectrum of tuberculosis. Int. J. Tuberc. Lung Dis. 7:478-484. [PubMed] [Google Scholar]
- 12.Sørensen, A. L., S. Nagai, G. Houen, P. Andersen, and Å. B. Andersen. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.vanEmbden, J. D., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, and P. M. Small. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]