Abstract
A sequential mucosal prime-boost vaccine regimen of oral attenuated (Att) human rotavirus (HRV) priming followed by intranasal (i.n.) boosting with rotavirus protein VP2 and VP6 rotavirus-like particles (2/6-VLPs) has previously been shown to be effective for induction of intestinal antibody-secreting cell (ASC) responses and protection in gnotobiotic pigs. Because serum or fecal antibody titers, but not intestinal ASC responses, can be used as potential markers of protective immunity in clinical vaccine trials, we determined the serum and intestinal antibody responses to this prime-boost rotavirus vaccine regimen and the correlations with protection. Gnotobiotic pigs were vaccinated with one of the two sequential vaccines: AttHRV orally preceding 2/6-VLP (VLP2x) vaccination (AttHRV/VLP2x) or following VLP2x vaccination (VLP2x/AttHRV) given i.n. with a mutant Escherichia coli heat-labile toxin (mLT) as adjuvant. These vaccines were also compared with three i.n. doses of VLP+mLT (VLP3x) and one and three oral doses of AttHRV (AttHRV1x and AttHRV3x, respectively). Before challenge all pigs in the AttHRV/VLP2x group seroconverted to positivity for serum immunoglobulin A (IgA) antibodies. The pigs in this group also had significantly higher (P < 0.05) intestinal IgA antibody titers pre- and postchallenge and IgG antibody titers postchallenge compared to those in the other groups. Statistical analyses of the correlations between serum IgM, IgA, IgG, and virus-neutralizing antibody titers and protection demonstrated that each of these was an indicator of protective immunity induced by the AttHRV3x and the AttHRV/VLP2x regimens. However, only IgA and not IgM or IgG antibody titers in serum were highly correlated (R2 = 0.89; P < 0.001) with the corresponding isotype antibody (IgA) titers in the intestines among all the vaccinated groups, indicating that the IgA antibody titer is probably the most reliable indicator of protection.
Group A rotaviruses (RVs) are the most common dehydrating diarrheal agents of infants and young children worldwide (2, 4). Human RV (HRV) infections range from asymptomatic conditions to severe dehydrating gastroenteritis resulting in hospitalization and death (2, 4). Withdrawal of the live oral rhesus RV tetravalent vaccine (24) because of potential safety concerns (intussusception) has prompted the development and evaluation of recombinant nonreplicating candidate HRV vaccines. A sequential prime-boost vaccine regimen of priming with an oral HRV vaccine followed by intranasal (i.n.) boosting with RV protein VP2 and VP6 RV-like particles (2/6-VLPs) has previously been shown to be effective for induction of intestinal antibody-secreting cell (ASC) responses and protection in gnotobiotic pigs. However, priming and boosting with nonreplicating 2/6-VLPs did not provide protection (39). Data from previous studies with animals and humans have indicated a correlation between the titers of antibodies to RV in serum and the numbers of RV-specific ASCs in the intestinal tissues (5, 33) after RV infection. An earlier study with gnotobiotic pigs orally infected with the virulent Wa strain of HRV also showed that immunoglobulin A (IgA) ASC responses in intestinal tissues were correlated with serum IgA antibody responses (33), presumably reflecting the transit of intestinally derived IgA ASCs in the blood after RV infection. Furthermore, both intestinal IgA ASC numbers (in pigs) and serum IgA antibody titers (in pigs and humans) were correlated with protection against reinfection (33, 35, 43). However, similar correlates between antibody responses and protection have not been evaluated for nonreplicating i.n. RV vaccines or sequential prime-boost vaccine regimens with neonatal pigs or humans. Studies with adult mice showed that the protective immunity against RV infection elicited by 2/6-VLP or chimeric VP6 i.n. vaccines alone is not associated with induction of serum or intestinal RV antibodies (7, 20, 25). The discrepancies in the findings from studies of the protective efficacy of i.n. 2/6-VLP vaccines with naive adult mice (complete protection) compared with those from studies with naive neonatal pigs (no protection) (25, 39) raise an important question for future vaccine trials with humans: are the RV antibodies elicited by nonreplicating vaccines in the intestine and serum an indicator of protective immunity? Previous studies have suggested that antibodies to RV correlate with protection after natural RV infection of humans (35) and after oral infection or vaccination of pigs with HRV (33). We delineated the serum and intestinal antibody responses in neonatal pigs vaccinated with one or three oral doses of attenuated HRV (AttHRV) vaccine alone (AttHRV1x and AttHRV3x, respectively) or i.n. with a nonreplicating RV VLP vaccine alone or after vaccination with a prime-boost sequential vaccine regimen (replicating oral AttHRV and i.n. VLPs) and compared them to the protection rates induced by these same vaccines reported in a recent previous study (42). The similarity between the gastrointestinal physiology of gnotobiotic pigs and humans, the similarity between the development of mucosal immunity in gnotobiotic pigs and humans, the pigs' susceptibilities to infection and disease with several HRV strains to at least 8 weeks of age, and the pigs' development of histopathologic lesions in the small intestine following HRV infection (36) indicate that gnotobiotic pigs are a valuable model for the study of HRV-induced disease and immunity. Gnotobiotic pigs are also devoid of maternal antibodies but are immunocompetent, allowing assessment of true primary immune responses and manipulation of passive maternal antibodies (17, 21, 27). Their gnotobiotic status also ensures that exposure to extraneous RVs or other enteric pathogens is eliminated as a confounding variable.
In this study, we evaluated the magnitudes of the serum and intestinal isotype-specific and virus-neutralizing (VN) antibody responses and analyzed the correlation of these antibody titers with protection.
MATERIALS AND METHODS
RV.
The attenuated cell culture-adapted Wa strain (P1A [8]G1) of HRV was grown in rhesus monkey kidney (MA104) cells (37, 38). Infected cell lysates were harvested as described previously (37) and were used at a dose of 5 × 107 fluorescent focus-forming units for inoculation of gnotobiotic pigs and as the capture antigen for enzyme-linked immunosorbent assay (ELISA) and VN antibody assays (43). Virulent HRV strain Wa, which causes diarrhea in gnotobiotic pigs up to at least 8 weeks of age, was maintained by serial passage of pooled intestinal contents in gnotobiotic pigs (31, 36) and was used for challenge at a dose of 106 median infectious doses (ID50s). The ID50 of the virulent HRV Wa inoculum for gnotobiotic pigs was at least 1 fluorescent focus-forming unit (37). This high infectious dose of heterologous HRV Wa was used to ensure that diarrhea occurred in all age-matched mock-inoculated control pigs after challenge. Virus titers were determined by a cell culture immunofluorescence assay (3).
2/6-VLPs and adjuvant.
The recombinant baculoviruses expressing the individual RV proteins VP2 from the bovine RF strain, provided by Jean Cohen (Virologie Moleculaire et Structurale, Dijon, France), and VP6 from HRV Wa were constructed by using the pBlueBac (version 4.5) system (Invitrogen, Carlsbad, Calif.) and were used to produce 2/6-VLPs by coinfection of Spodoptera frugiperda (Sf9) cells (11, 39). The 2/6-VLPs were purified and characterized as described previously (11, 39) and used at 250 μg per dose. The mutant heat-labile toxin (mLT) adjuvant (mLT-R192G) was provided by John Clements (Tulane University Medical Center, New Orleans, La.) and was administered to the gnotobiotic pigs at 5 μg per dose, as described previously (39). mLT lacks toxicity but retains the adjuvant properties of heat-labile toxin. The mutant has the substitution of an arginine for a glycine at position 192 of the protein. This mutation eliminates the trypsin-sensitive cleavage site in the protein, so the cleavage of the A subunit no longer occurs (12).
Inoculation and challenge of gnotobiotic pigs and sample collection.
Near-term pigs were surgically derived and maintained in gnotobiotic isolator units as described previously (22). The pigs were assigned to one of six treatment groups as follows (Table 1): (i) one oral dose of attenuated HRV Wa followed by two i.n. doses of 2/6-VLP plus mLT (2/6-VLP+mLT) (AttHRV/VLP2x), (ii) two i.n. doses of 2/6-VLP+mLT followed by one oral dose of attenuated HRV Wa (VLP2x/AttHRV), (iii) three oral doses of attenuated HRV Wa (AttHRV3x), (iv) one oral dose of attenuated HRV Wa (AttHRV1x), (v) three i.n. doses of 2/6-VLP+mLT (VLP3x), and (vi) three i.n. doses of mLT alone or oral doses of minimum essential medium (control). Pigs were first inoculated at 3 to 5 days of age and were then reinoculated 10 and 21 days later. At postinoculation day 28 (PID 28) they were challenged with virulent HRV strain Wa and were observed daily for diarrhea. Subsets of pigs from each group were euthanized at PID 28 and postchallenge day 0 (PCD 0) and at PID 35 and PCD 7 (31, 41, 43).
TABLE 1.
Number of gnotobiotic pigs assigned to each vaccine regimena
| Vaccination group | Vaccine(s) | No. of pigs tested:
|
||
|---|---|---|---|---|
| Prechallenge | Postchallenge | Total | ||
| AttHRV/VLP2x | One oral dose of attenuated HRV Wa, two i.n. doses of 2/6-VLP+mLT | 6 | 12 | 18 |
| VLP2x/AttHRV | Two i.n. doses of 2/6-VLP+mLT One oral dose of attenuated HRV Wa | 4 | 6 | 10 |
| AttHRV3x | Three oral doses of attenuated HRV Wa | 9 | 6 | 15 |
| AttHRV1x | One oral dose of attenuated HRV Wa | 4 | 4 | 8 |
| VLP3x | Three i.n. doses of 2/6-VLP+mLT | 4 | 6 | 10 |
| Controls | i.n. doses of mLT alone or oral doses of minimum essential media | 2 | 27 | 29 |
All pigs were bled at each PID until the day of euthanasia.
Blood samples were collected from all groups at PIDs 0, 10, 21, 28, and 35; processed for serum; and kept at −20°C until they were tested. Large and small intestine contents (LICs and SICs, respectively) were collected after euthanasia at PID 28 and at PID 35 and PCD 7. The intestinal contents were diluted 1:2 in medium (minimum essential medium, 1% antibiotic-antimycotic [Gibco], 1% NaHCO2), and 250 μg of trypsin inhibitor (Sigma) per ml and 50 μg of leupeptin (Sigma) per ml were added to inhibit proteolytic enzymes. The suspensions were centrifuged (500 × g for 30 min), and the supernatants were collected and stored at −20°C until they were tested.
Isotype-specific ELISA antibody titers.
Plates were coated with guinea pig hyperimmune serum against IND bovine strain group A RV antigen in 0.1 M carbonate-bicarbonate buffer (pH 9.6) and were incubated at 4°C overnight (27, 33). The plates were blocked with 1% bovine serum albumin or 5% nonfat dry milk, and reagents and samples were added in the following order: (i) semipurified (by sucrose gradient centrifugation) HRV Wa or mock-infected cell culture supernatant; (ii) fourfold serial dilutions of serum or intestinal contents; (iii) biotinylated monoclonal antibodies to porcine IgM, IgA, or IgG (28); and (iv) horseradish peroxidase-conjugated streptavidin (Roche). Antibody titers were calculated as the reciprocal of the highest sample dilution which produced a mean absorbance (A414) greater than the cutoff value (the mean A414 for the negative controls plus 3 standard deviations), after subtraction of the mean A414 for the mock-coated wells from the A414 of the antigen-coated well for each sample.
VN antibody titers.
Serum samples collected from all groups at PIDs 0, 10, 21, 28, and 35 were tested for neutralizing antibodies by a plaque reduction assay, as described previously (3). The VN antibody titers were expressed as the reciprocal of the highest dilution producing a reduction in plaque counts equal to or greater than 80% compared to the counts for the controls.
Assessment of protection.
At PID 28 subsets of pigs from each group were challenged with virulent HRV strain Wa at a dose of 106 ID50s (36). Rectal swab specimens were collected for 6 days after challenge to assess diarrhea and virus shedding. Fecal consistency was scored as follows: 0, normal; 1, pasty; 2, semiliquid; and 3, liquid. Virus shedding was detected by an antigen capture ELISA and a cell culture immunofluorescence assay to detect infectious HRV by using suspensions of the rectal swab fluid processed and tested as described previously (30, 43).
Statistical analysis.
The isotype-specific antibody and VN antibody titers were compared by using the General Linear Model procedure (SAS Institute, Inc., Cary, N.C.) with log10-transformed data. Differences in antibody titers among the vaccination groups were evaluated at PIDs 10, 21, and 28 and at PID 35 and PCD 7 by one-way analysis of variance (ANOVA), followed by Duncan's multiple-range test. Clinical signs postchallenge (the proportion of pigs with diarrhea or shedding virus) were compared by Fisher's exact test. Correlations between groups were analyzed by use of Pearson's and Spearman's correlation coefficients. Statistical analyses were carried out by Statistical Analysis System procedures (SAS Institute, Inc.); significance was assessed at a P value of <0.05 throughout the study.
RESULTS
Seroconversion to positivity for HRV Wa IgA and IgG isotype-specific and VN antibodies was induced earlier in the groups primed with AttHRV.
Pigs were considered to have seroconverted to positivity for antibodies to HRV when antibody titers increased from <4 to ≥16. All pigs were seronegative (titers, <4) for IgM, IgA, and IgG antibodies to HRV at the first inoculation. Seroconversion to positivity for IgM HRV antibodies occurred at PID 10 in 100% of the pigs in all vaccination groups except pigs in the VLP3x group, 90% of which showed seroconversion (Fig. 1). In the control group seroconversion occurred only postchallenge. Seroconversion to positivity for IgA HRV antibodies occurred in 100% of the pigs in the AttHRV/VLP2x group at PID 21 (P < 0.05) and occurred only postchallenge in the other four vaccination groups. Seroconversion to positivity for IgG antibodies to HRV was observed at PID 21 in 100% of the pigs only in the groups primed with AttHRV (AttHRV/VLP2x, AttHRV3x, and AttHRV1x) (P < 0.05) but not until PID 28 in the groups primed with VLPs (VLP3x and VLP2x/AttHRV). The seroconversion to positivity for VN antibody was defined as antibody titer increases from <4 to ≥10. At PIDs 10, 21, and 28, the percentage of pigs that seroconverted to positivity for VN antibodies was significantly greater (P < 0.05) for the groups vaccinated with AttHRV3x, AttHRV/VLP2x, and AttHRV1x than for the groups vaccinated with VLP3x and VLP2x/AttHRV. The VP4 and VP7 neutralizing antigens are present on the intact AttHRV of the first three vaccines, while the VLP3x and VLP2x/AttHRV vaccines lack VP4 and VP7 (2/6-VLP) or the pigs were given AttHRV as the last (third) dose.
FIG. 1.
Seroconversion to positivity for isotype-specific and VN antibodies to HRV Wa in sera of gnotobiotic pigs inoculated with the different vaccine regimens. Arrows indicate the day of challenge. Symbols: ⧫, IgM; □, IgA; ▴, IgG, ×, VN antibody.
The AttHRV/VLP2x and the AttHRV3x vaccine regimens induced the highest isotype-specific antibody titers to HRV in the sera of gnotobiotic pigs.
The geometric mean titers (GMTs) of RV isotype-specific antibody in postvaccination serum samples are summarized in Fig. 2.
FIG. 2.
Titers of isotype-specific antibodies to HRV Wa in sera of gnotobiotic pigs inoculated with the different vaccine regimens. The error bars represent standard errors of the means. Lowercase letters denote significant differences (P < 0.05, by one-way ANOVA and Duncan's multiple-range test) among the vaccine and the control groups.
(i) IgM antibody.
The highest (P < 0.05) serum IgM HRV antibody titers were obtained at PID 10 for the AttHRV-primed groups: for AttHRV3x, the GMT was 34,674; for AttHRV/VLP2x, the GMT was 24,547; and for AttHRV1x, the GMT was 13,804. Thereafter, significantly higher (P < 0.05) IgM titers were obtained at PID 21 in the AttHRV/VLP2x group (GMT = 28,184) and at PID 28 in the VLP2x/AttHRV group (GMT = 9,333) and the AttHRV/VLP2x group (GMT = 6,607). At PID 35 and PCD 7, all vaccination groups except the AttHRV3x group had high IgM antibody titers; the AttHRV3x group had significantly lower titers, reflecting the lower IgM antibody response after repeated oral antigen stimulation and protection against reinfection. The lowest IgM GMT among the vaccination groups was observed in the VLP3x group at all prechallenge PIDs.
(ii) IgA antibody.
The pigs in the AttHRV/VLP2x group had significantly higher (P < 0.05) serum IgA antibody titers at PIDs 21 and 28 (GMTs = 550 and 1,549, respectively). These titers correspond to those that conferred the highest protection rates in these pigs. At PID 35 and PCD 7, significantly higher (P < 0.05) serum IgA antibody titers were observed in the AttHRV/VLP2x group (GMT = 14,454) and the VLP2x/AttHRV group (GMT = 8,128) than in any of the other groups.
(iii) IgG antibody.
Significantly higher (P < 0.05) serum IgG antibody titers were observed in the AttHRV/VLP2x and AttHRV3x groups at PID 21 (GMTs = 11,482 and 9,330, respectively) and PID 28 (GMTs = 19,953 and 17,378, respectively); these were also the groups with the highest protection rates. At PID 35 and PCD 7, all groups had significantly higher (P < 0.05) IgG antibody titers than the controls.
Postchallenge, all vaccination groups except the AttHRV/VLP2x and AttHRV3x groups had significantly increased (P < 0.05) IgM, IgG, and IgA antibody titers compared to those detected prechallenge (PID 28), reflecting the higher degree of protection against reinfection. The AttHRV/VLP2x and AttHRV3x groups did not have significantly increased GMTs of IgM. The control group had only IgM antibodies postchallenge and no detectable IgA and IgG antibodies pre- or postchallenge, consistent with a primary immune response after challenge.
Significantly higher titers of IgA antibodies to HRV in intestinal contents were induced only by the AttHRV/VLP2x vaccine regimen.
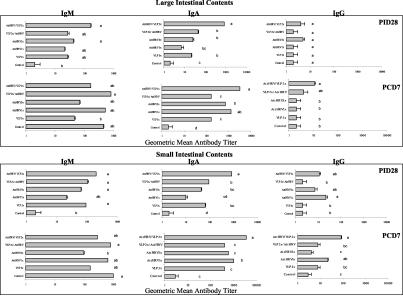
The RV isotype-specific antibody GMTs after vaccination (PID 28) and postchallenge (PID 35 and PCD 7) in SICs and LICs are summarized in Fig. 3.
FIG. 3.
Titers of isotype-specific antibodies to HRV Wa in the intestinal contents of gnotobiotic pigs inoculated with the different vaccine regimens. The error bars represent standard errors of the means. Lowercase letters denote significant differences (P < 0.05 by one-way ANOVA and Duncan's multiple-range test) among the vaccine and the control groups.
(i) IgM antibody.
No statistically significant differences were observed in intestinal IgM antibody titers among the vaccination groups. Prechallenge (PID 28) the highest antibody titers were observed in the SICs and LICs of the AttHRV/VLP2x group (GMTs = 200 and 166, respectively), and postchallenge the highest antibody titers were observed in the LICs of the VLP2x/AttHRV group (GMT = 813) and the SICs of the control group (GMT = 955).
(ii) IgA antibody.
The AttHRV/VLP2x group had significantly higher (P < 0.05) IgA antibody titers in SICs and LICs prechallenge (GMTs = 851 and 851, respectively) and postchallenge (GMTs = 3,631 and 3,236, respectively) compared to those in the SICs and LICs of the other groups and the controls. Again, these titers correspond to those that conferred the highest protection rates in this group. Prechallenge, the AttHRV1x group had the lowest IgA antibody titers in SICs and LICs.
(iii) IgG antibody.
IgG antibody titers in SICs and LICs were low pre- and postchallenge, especially in LICs. Prechallenge, the highest IgG antibody titers were observed in the SICs of the AttHRV1x group (GMT = 22), and postchallenge, the highest IgG antibody titers were observed in the SICs of the AttHRV/VLP2x group (GMT = 85), but they were not significantly higher.
VN antibody titers correlated with RV-specific IgA antibody titers in the sera of the AttHRV/VLP2x and AttHRV3x groups.
Prechallenge, a positive correlation between the VN and serum IgA antibody titers was observed only in the AttHRV/VLP2x group (R2 = 0.69), suggesting that serum IgA antibodies are also neutralizing. Postchallenge, a positive correlation between VN and serum IgM antibody titers was seen in the AttHRV3x group (R2 = 0.97).
The AttHRV/VLP2x and AttHRV3x vaccination groups had significantly reduced levels of virus shedding and diarrhea compared to those for the controls after challenge with virulent HRV strain Wa.
The detailed diarrhea and virus shedding data were previously presented by Yuan et al. (40). The levels of protection against diarrhea and virus shedding after challenge with virulent HRV strain Wa were compared between the vaccinated and the control groups. Control pigs and pigs in the VLP3x group developed virus shedding (100%) and diarrhea (89 to 100%) after challenge. In the AttHRV3x group, 33% virus shedding and diarrhea rates were observed, whereas in the AttHRV1x group, 100% of the pigs shed virus and 60% had diarrhea. In the AttHRV/VLP2x group, the percentages of virus shedding and diarrhea (42 and 50%, respectively) were significantly reduced compared to those for the other groups, but these rates did not differ significantly from those for the AttHRV3x group. The VLP2x/AttHRV group had 83% virus shedding and 67% diarrhea rates.
Higher RV-specific antibody titers in serum were significantly correlated with protection, and higher antibody titers in the SICs and LICs were also associated with higher rates of protection among the vaccination groups.
Because we were able to measure the antibody titers only in the SICs and LICs at the time of challenge (PID 28), when the pigs were killed, the intestinal immune responses to vaccination in individual animals could not be correlated statistically with protection against challenge (16). However, we statistically analyzed the correlation between serum antibody titers at challenge at PID 28 among the challenged pigs with virus shedding and diarrhea after challenge. The IgM, IgA, IgG, and VN antibody titers in serum were significantly inversely correlated with the duration of virus shedding and the duration of diarrhea across all vaccination groups (R2 range, −0.52 to −0.67; P < 0.001).
In addition, postchallenge a positive correlation was observed between higher IgM antibody titers in intestinal contents (an indication of infection in the pigs that lacked protective immunity upon challenge) and a longer duration of shedding and higher mean peak titers of virus shedding among the vaccination groups.
Serum IgA antibody titers, but not IgM or IgG antibody titers, were highly correlated with the corresponding isotype antibody titers in the intestines among the vaccination groups.
Pearson's correlation coefficient analyses were performed to examine the relationship between isotype-specific antibody titers in the serum and SICs or LICs among all samples from all vaccination groups. We found that the serum IgA antibody titers were most closely correlated with the IgA titers in SICs (R2 = 0.88; P < 0.001) or LICs (R2 = 0.89; P < 0.001). The correlation coefficients were much lower for IgM (R2 = 0.43 and P < 0.001 in SICs; R2 = 0.49 and P < 0.001 in LICs) or IgG (R2 = 0.41 and P < 0.001 in SICs; R2 = 0.24 and P < 0.047 in LICs).
DISCUSSION
The sequential administration of a combination of an oral attenuated HRV vaccine and i.n. 2/6-VLPs by using two mucosal inductive sites for immunization (gastrointestinal tract-associated lymphoid tissue and nasal tract-associated lymphoid tissue) has been shown to induce high levels of intestinal ASCs and protective immunity (40). In this study, five groups of pigs were inoculated with 2/6-VLP+mLT, 2/6-VLP+mLT followed by AttHRV, AttHRV followed by 2/6-VLP+mLT, or one or three doses of AttHRV. Our hypothesis was that the immune responses and protection induced by oral priming with a live attenuated vaccine could be enhanced by i.n. booster administration of nonreplicating 2/6-VLPs, which would avoid the need for sequential doses of live vaccine. Alternatively, the initial administration of two doses of 2/6-VLPs might induce intestinal RV antibodies prior to oral boosting with one dose of attenuated RV vaccine and prevent the possible intussusception associated with live virus in infants (24). In this report, serum and intestinal isotype-specific antibodies to HRV and serum neutralizing antibodies were quantitated for the five vaccination groups, and these antibody titers were correlated with the level of protection induced against challenge with virulent HRV strain Wa.
As discussed previously (40), the AttHRV/VLP2x regimen induced moderate rates of protection against virus shedding and diarrhea, and the protection rates were not significantly different from those induced by the AttHRV3x regimen. The VLP2x/AttHRV regimen induced lower protection rates than the AttHRV/VLP2x regimen but higher protection rates than the VLP3x and AttHRV1x regimens. The boosting effect of VLPs on protection in the sequential prime-boost regimen (AttHRV/VLP2x) was observed by comparing the protection rate for this regimen with that for the AttHRV1x regimen. An augmented immunological response to a replicating (AttHRV) vaccine, which effectively increases the virus dose, compared with that of a nonreplicating (VLP) vaccine used to prime the animals and exploitation of a different induction site (the oral site) may be an explanation for the improved immune responses observed in the AttHRV/VLP2x group. The generation of significantly higher neutralizing antibody titers (against VP4 and VP7 on intact virus) upon challenge in the AttHRV/VLP2x group may also have contributed to the higher protection rates. The boosting effects of the 2/6-VLPs on neutralizing antibodies in animals orally primed with live virus may be explained by the development of Th cells against VP6. These VP6-specific Th cells may provide cognate help to VP4- and VP7-specific B cells (13, 23, 32, 40). In this study, one dose of AttHRV was not sufficient to induce a high level of protection against virus shedding and diarrhea. Gorrel and Bishop (15), corroborating the findings from other studies, suggested that for RV vaccines to be effective, they should be multidose to elicit high titers of cross-reactive neutralizing antibodies, an observation in accordance with our data.
Prechallenge, 100% seroconversion to positivity for IgM antibodies to HRV Wa occurred among all the vaccination groups except the VLP3x group. The AttHRV/VLP2x group showed the earliest 100% seroconversion rate to positivity for IgA antibodies at PID 21. This group also had the highest GMT of IgA antibody in sera and the intestines. These data support prior observations for humans (8), in which fecal IgA was shown to be a good marker for protection against RV diarrhea. However, in this group, although serum and intestinal IgA antibody titers prechallenge were significantly higher than those in the AttHRV3x group, the highest protection rate against viral challenge was seen in the latter group (but the difference was not significantly greater). A possible explanation for the higher antibody responses in the AttHRV/VLP2x group than in the AttHRV3x group could be that the antibodies in the intestine induced after oral priming would neutralize the subsequent oral booster dose of AttHRV; the i.n. route of boosting may have prevented or reduced the effects of preexisting antibodies. Yuan et al. (40) also observed higher levels of IgA ASCs in the intestines of the AttHRV/VLP2x group. Although the AttHRV/VLP2x vaccine regimen induced significantly higher serum and intestinal IgA antibody responses, this regimen did not confer better protection than the AttHRV3x regimen.
Johansen et al. (19) established that after primary infection in humans serum IgA antibodies are mainly directed against VP2 and VP6. Similarly, Chang et al. (6) measured isotype-specific ASC responses to individual RV structural and nonstructural proteins and observed that in all RV-inoculated piglets, VP6 induced the highest numbers of ASCs. Therefore, most of the serum and intestinal IgA antibodies observed in the AttHRV/VLP2x group were against VP2 and VP6, as boosted by 2/6-VLPs. The boost against the VP4 and VP7 neutralizing epitopes (which are present on the intact triple-layer AttHRV), such as in the AttHRV3x group, probably occurred to only a limited extent in the AttHRV/VLP2x group after 2/6-VLP boosters. Thus, the higher IgA antibody responses present in the AttHRV/VLP2x group do not necessarily denote higher VN antibody responses. After natural infection of children with HRV or experimental infection of animals with RV, serum and intestinal IgA or IgA ASCs are frequently associated with protection from RV reinfection (8, 14, 39-42); however, in most cases, statistically validated correlations were not established. Jiang et al. (18) reviewed data from a variety of studies with humans and concluded that if antibodies are present in serum at elevated levels, they might be protective or might be an important correlate of protection against disease. In the present study, we observed significant negative correlations between serum IgM, IgA, IgG, and VN antibody titers and the duration of virus shedding and diarrhea. These correlations confirmed the role of serum antibody titers as possible indicators of protective immunity for RV vaccine trials. In addition, the IgA antibody titers in serum were most closely correlated (R2 = 0.89; P < 0.001) with the IgA antibody titers in the intestines among all vaccination groups, indicating that IgA antibody levels rather than IgM or IgG antibody levels are probably the most reliable indicator of protection.
The AttHRV3x group had the highest GMT of VN antibody in serum prechallenge, which corresponded to the titer that conferred the highest protection rate, with a strong negative correlation between VN antibody titers and the duration of virus shedding (R2 = −0.62; P < 0.001). This finding is contrary to that presented in a previous report (33), in which there was no correlation between serum VN antibody titers and the degree of protection in gnotobiotic pigs inoculated with one dose of virulent HRV or two doses of AttHRV and challenged at PID 21. The earlier challenge time in the previous studies may have played a role in this discrepancy, because the VN antibody titers in serum usually do not peak until PID 21 (42).
In conclusion, the AttHRV/VLP2x vaccine conferred moderate but higher rates of protection against RV diarrhea and virus shedding in gnotobiotic pigs compared to those conferred by the AttHRV1x, VLP2x/AttHRV, and vaccine regimens, with the induction of significantly higher serum and intestinal IgA and neutralizing antibody titers. The failure of monovalent live RV vaccines to induce significant protection in children has been attributed to their lack of induction of heterotypic protective antibody responses to multiple RV serotypes. Consequently, a successful RV vaccine may need to induce broadly reactive or neutralizing antibodies to the major serotypes of RV (serotypes G1 to G4) commonly seen in children (29). Natural HRV infections in children induce increased rates of protection from reinfection and diarrhea following each subsequent RV infection, and secondary infections are most often caused by a different G serotype (34). Thus, induction of neutralizing IgA antibodies to homotypic and heterotypic RVs is a goal in the development of effective RV vaccines. HRV strains appear to stimulate broadly reactive serum antibody responses across serotypes only after a second or subsequent infections. Recent results of a phase II trial with RV3 (P2A[6]G3), which possesses antigenic determinants in VP7 cross-reactive with serotype G1 strains (9), showed that it has moderate protective efficacy but induces relatively low antibody responses (1). A 75% level of protection against overall RV infections was noted in preliminary studies of a phase III trial of a reassortant pentavalent vaccine (Rotateq; serotypes G1, G2, G3, G4, and P1A) (26). Use of a combination of vaccines, such as AttHRV (P1A[8]G1) and 2/6-VLP+mLT, might be an alternative approach to decrease the risk of intussusception or other risks associated with the administration of multiple live vaccine doses. The protection rate induced by VLPs combined with AttHRV may be improved by the use of triple-shelled 2/4/6/7-VLPs, alone or in association with AttHRV or 2/6-VLPs. Increased coverage of protection could be achieved by adding additional G serotypes to the 2/4/6/7-VLPs. The use of 2/4/6/7-VLPs to induce neutralizing antibodies against a broad range of heterotypic viruses may provide sufficient priming of the immune system, or the use of 2/6-VLPs for boosting may induce immune responses capable of reducing the severity of disease in the face of subsequent HRV exposure (10).
Acknowledgments
We thank John Clements (Tulane University Medical Center) for providing the mLT-R192G used in this study and Jean Cohen (Virologie Moleculaire et Structurale) for providing the strain RF VP2 baculovirus clone used for the VLPs.
This work was supported by grants from the National Institutes of Health (RO1AI33561 and RO1AI37111). Salaries and research support were provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center, The Ohio State University. Marli S. P. Azevedo is a fellow of Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq), Brasilia, Brazil.
REFERENCES
- 1.Barnes, G. L., J. S. Lund, S. V. Mitchell, L. De Bruyn, L. Piggford, A. L. Smith, J. Furmedge, P. J. Masendycz, H. C. Bugg, N. Bogdanovic-Sakran, J. B. Carlin, and R. F. Bishop. 2002. Early phase II trial of human rotavirus vaccine candidate RV3. Vaccine 20:2950-2956. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett, A. V., III, A. J. Bednarz-Prashad, H. L. DuPont, and L. K. Pickering. 1987. Rotavirus gastroenteritis. Annu. Rev. Med. 38:399-415. [DOI] [PubMed] [Google Scholar]
- 3.Bohl, E. H., K. W. Theil, and L. J. Saif. 1984. Isolation and serotyping of porcine rotaviruses and antigenic comparison with other rotaviruses. J. Clin. Microbiol. 19:105-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandt, C. D., H. W. Kim, W. J. Rodriguez, J. O. Arrobio, B. C. Jeffries, E. P. Stallings, C. Lewis, A. J. Miles, R. M. Chanock, A. Z. Kapikian, and R. H. Parrott. 1983. Pediatric viral gastroenteritis during eight years of study. J. Clin. Microbiol. 18:71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, K. A., J. A. Kriss, C. A. Moser, W. J. Wenner, and P. A. Offit. 2000. Circulating rotavirus-specific antibody-secreting cells (ASCs) predict the presence of rotavirus-specific ASCs in the human small intestinal lamina propria. J. Infect. Dis. 182:1039-1043. [DOI] [PubMed] [Google Scholar]
- 6.Chang, K. O., O. H. Vandal, L. Yuan, D. C. Hodgins, and L. J. Saif. 2001. Antibody-secreting cell responses to rotavirus proteins in gnotobiotic pigs inoculated with attenuated or virulent human rotavirus. J. Clin. Microbiol. 39:2807-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi, A. H., M. Basu, M. M. McNeal, J. D. Clements, and R. L. Ward. 1999. Antibody-independent protection against rotavirus infection of mice stimulated by intranasal immunization with chimeric VP4 or VP6 protein. J. Virol. 73:7574-7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coulson, B. S., K. Grimwood, I. L. Hudson, G. L. Barnes, and R. F. Bishop. 1992. Role of coproantibody in clinical protection of children during reinfection with rotavirus. J. Clin. Microbiol. 30:1678-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coulson, B. S., J. M. Tursi, W. J. McAdam, and R. F. Bishop. 1986. Derivation of neutralizing monoclonal antibodies to human rotaviruses and evidence that an immunodominant neutralization site is shared between serotypes 1 and 3. Virology 154:302-312. [DOI] [PubMed] [Google Scholar]
- 10.Crawford, S. E., M. K. Estes, M. Ciarlet, C. Barone, C. M. O'Neal, J. Cohen, and M. E. Conner. 1999. Heterotypic protection and induction of a broad heterotypic neutralization response by rotavirus-like particles. J. Virol. 73:4813-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crawford, S. E., M. Labbe, J. Cohen, M. H. Burroughs, Y. J. Zhou, and M. K. Estes. 1994. Characterization of virus-like particles produced by the expression of rotavirus capsid proteins in insect cells. J. Virol. 68:5945-5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickinson, B. L., and J. D. Clements. 1995. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect. Immun. 63:1617-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esquivel, F. R., S. Lopez, X. L. Guitierrez, and C. Arias. 2000. The internal rotavirus protein VP6 primes for an enhanced neutralizing antibody response. Arch. Virol. 145:813-825. [DOI] [PubMed] [Google Scholar]
- 14.Feng, N., J. W. Burns, L. Bracy, and H. B. Greenberg. 1994. Comparison of mucosal and systemic humoral immune responses and subsequent protection in mice orally inoculated with a homologous or a heterologous rotavirus. J. Virol. 68:7766-7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorrell, R. J., and R. F. Bishop. 1999. Homotypic and heterotypic serum neutralizing antibody response to rotavirus proteins following natural primary infection and reinfection in children. J. Med. Virol. 57:204-211. [PubMed] [Google Scholar]
- 16.Hein, W. R., and P. J. Griebel. 2003. A road less travelled: large animal models in immunological research. Nat. Rev. Immunol. 3:79-84. [DOI] [PubMed] [Google Scholar]
- 17.Hodgins, D. C., S. Y. Kang, L. deArriba, V. Parreno, L. A. Ward, L. Yuan, T. To, and L. J. Saif. 1999. Effects of maternal antibodies on protection and development of antibody responses to human rotavirus in gnotobiotic pigs. J. Virol. 73:186-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang, B., J. R. Gentsch, and R. I. Glass. 2002. The role of serum antibodies in the protection against rotavirus disease: an overview. Clin. Infect. Dis. 34:1351-1361. [DOI] [PubMed] [Google Scholar]
- 19.Johansen, K., L. Granqvist, K. Karlen, G. Stintzing, I. Uhnoo, and L. Svensson. 1994. Serum IgA immune response to individual rotavirus polypeptides in young children with rotavirus infection. Arch. Virol. 138:247-259. [DOI] [PubMed] [Google Scholar]
- 20.McNeal, M. M., J. L. VanCott, A. H. Choi, M. Basu, J. A. Flint, S. C. Stone, J. D. Clements, and R. L. Ward. 2002. CD4 T cells are the only lymphocytes needed to protect mice against rotavirus shedding after intranasal immunization with a chimeric VP6 protein and the adjuvant LT(R192G). J. Virol. 76:560-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehrazar, K., and Y. B. Kim. 1988. Total parenteral nutrition in germfree colostrum-deprived neonatal miniature piglets: a unique model to study the ontogeny of the immune system. J. Parenter. Enteral Nutr. 12:563-568. [DOI] [PubMed] [Google Scholar]
- 22.Meyer, R. C., E. H. Bohl, and E. M. Kohler. 1964. Procurement and maintenance of germ-free swine for microbiological investigations. Appl. Microbiol. 12:295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milich, D. R., A. McLachlan, G. B. Thornton, and J. L. Hughes. 1987. Antibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T cell site. Nature 329:547-549. [DOI] [PubMed] [Google Scholar]
- 24.Murphy, T. V., P. M. Gargiullo, M. S. Massoudi, D. B. Nelson, A. O. Jumaan, C. A. Okoro, L. R. Zanardi, S. Setia, E. Fair, C. W. LeBaron, M. Wharton, J. R. Livengood, and J. R. Livingood. 2001. Intussusception among infants given an oral rotavirus vaccine. N. Engl. J. Med. 344:564-572. [DOI] [PubMed] [Google Scholar]
- 25.O'Neal, C. M., J. D. Clements, M. K. Estes, and M. E. Conner. 1998. Rotavirus 2/6 viruslike particles administered intranasally with cholera toxin, Escherichia coli heat-labile toxin (LT), and LT-R192G induce protection from rotavirus challenge. J. Virol. 72:3390-3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orellana, C. 2003. Rotavirus vaccine shows promise. Lancet Infect. Dis. 3:396. [DOI] [PubMed] [Google Scholar]
- 27.Parreno, V., D. C. Hodgins, L. de Arriba, S. Y. Kang, L. Yuan, L. A. Ward, T. L. To, and L. J. Saif. 1999. Serum and intestinal isotype antibody responses to Wa human rotavirus in gnotobiotic pigs are modulated by maternal antibodies. J. Gen. Virol. 80(Pt 6):1417-1428. [DOI] [PubMed] [Google Scholar]
- 28.Paul, P. S., W. L. Mengeling, C. E. Malstrom, and R. A. Van Deusen. 1989. Production and characterization of monoclonal antibodies to porcine immunoglobulin gamma, alpha, and light chains. Am. J. Vet. Res. 50:471-479. [PubMed] [Google Scholar]
- 29.Perez-Schael, I., D. Garcia, M. Gonzalez, R. Gonzalez, N. Daoud, M. Perez, W. Cunto, A. Z. Kapikian, and J. Flores. 1990. Prospective study of diarrheal diseases in Venezuelan children to evaluate the efficacy of rhesus rotavirus vaccine. J. Med. Virol. 30:219-229. [DOI] [PubMed] [Google Scholar]
- 30.Saif, L., L. Yuan, L. Ward, and T. To. 1997. Comparative studies of the pathogenesis, antibody immune responses, and homologous protection to porcine and human rotaviruses in gnotobiotic piglets. Adv. Exp. Med. Biol. 412:397-403. [DOI] [PubMed] [Google Scholar]
- 31.Saif, L. J., L. A. Ward, L. Yuan, B. I. Rosen, and T. L. To. 1996. The gnotobiotic piglet as a model for studies of disease pathogenesis and immunity to human rotaviruses. Arch. Virol. Suppl. 12:153-161. [DOI] [PubMed] [Google Scholar]
- 32.Scherle, P. A., and W. Gerhard. 1988. Differential ability of B cells specific for external vs. internal influenza virus proteins to respond to help from influenza virus-specific T-cell clones in vivo. Proc. Natl. Acad. Sci. USA 85:4446-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.To, T. L., L. A. Ward, L. Yuan, and L. J. Saif. 1998. Serum and intestinal isotype antibody responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J. Gen. Virol. 79(Pt 11):2661-2672. [DOI] [PubMed] [Google Scholar]
- 34.Velazquez, F. R., D. O. Matson, J. J. Calva, L. Guerrero, A. L. Morrow, S. Carter-Campbell, R. I. Glass, M. K. Estes, L. K. Pickering, and G. M. Ruiz-Palacios. 1996. Rotavirus infections in infants as protection against subsequent infections. N. Engl. J. Med. 335:1022-1028. [DOI] [PubMed] [Google Scholar]
- 35.Velazquez, F. R., D. O. Matson, M. L. Guerrero, J. Shults, J. J. Calva, A. L. Morrow, R. I. Glass, L. K. Pickering, and G. M. Ruiz-Palacios. 2000. Serum antibody as a marker of protection against natural rotavirus infection and disease. J. Infect. Dis. 182:1602-1609. [DOI] [PubMed] [Google Scholar]
- 36.Ward, L. A., B. I. Rosen, L. Yuan, and L. J. Saif. 1996. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J. Gen. Virol. 77(Pt 7):1431-1441. [DOI] [PubMed] [Google Scholar]
- 37.Ward, L. A., L. Yuan, B. I. Rosen, T. L. To, and L. J. Saif. 1996. Development of mucosal and systemic lymphoproliferative responses and protective immunity to human group A rotaviruses in a gnotobiotic pig model. Clin. Diagn. Lab. Immunol. 3:342-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wyatt, R. G., W. D. James, E. H. Bohl, K. W. Theil, L. J. Saif, A. R. Kalica, H. B. Greenberg, A. Z. Kapikian, and R. M. Chanock. 1980. Human rotavirus type 2: cultivation in vitro. Science 207:189-191. [DOI] [PubMed] [Google Scholar]
- 39.Yuan, L., A. Geyer, D. C. Hodgins, Z. Fan, Y. Qian, K. O. Chang, S. E. Crawford, V. Parreno, L. A. Ward, M. K. Estes, M. E. Conner, and L. J. Saif. 2000. Intranasal administration of 2/6-rotavirus-like particles with mutant Escherichia coli heat-labile toxin (LT-R192G) induces antibody-secreting cell responses but not protective immunity in gnotobiotic pigs. J. Virol. 74:8843-8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan, L., C. Iosef, M. S. Azevedo, Y. Kim, Y. Qian, A. Geyer, T. V. Nguyen, K. O. Chang, and L. J. Saif. 2001. Protective immunity and antibody-secreting cell responses elicited by combined oral attenuated Wa human rotavirus and intranasal Wa 2/6-VLPs with mutant Escherichia coli heat-labile toxin in gnotobiotic pigs. J. Virol. 75:9229-9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan, L., S. Y. Kang, L. A. Ward, T. L. To, and L. J. Saif. 1998. Antibody-secreting cell responses and protective immunity assessed in gnotobiotic pigs inoculated orally or intramuscularly with inactivated human rotavirus. J. Virol. 72:330-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan, L., and L. J. Saif. 2002. Induction of mucosal immune responses and protection against enteric viruses: rotavirus infection of gnotobiotic pigs as a model. Vet. Immunol. Immunopathol. 87:147-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan, L., L. A. Ward, B. I. Rosen, T. L. To, and L. J. Saif. 1996. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J. Virol. 70:3075-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]