Abstract
All the non-H atoms of the title compound, C10H10N2O2, are nearly in the same plane with a maximum deviation of 0.093 (1) Å. In the crystal, adjacent molecules are linked by pairs of intermolecular N—H⋯O hydrogen bonds, generating inversion dimers with R 2 2(14) ring motifs.
Related literature
For background to and applications of pyrrole derivatives, see: Fischer & Orth (1934 ▶). For the Knoevenagel condensation reaction and its applications, see: Knoevenagel (1898 ▶); Bigi et al. (1999 ▶). For the synthesis of related compounds, see: Knizhnikov et al. (2007 ▶); Sarda et al. (2009 ▶). For related structures, see: Ye et al. (2009 ▶); Wang & Jian (2008 ▶); Zhang et al. (2009 ▶).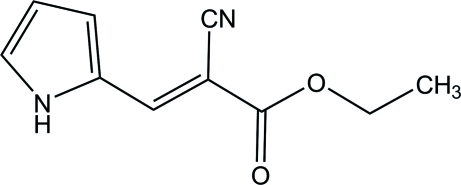
Experimental
Crystal data
C10H10N2O2
M r = 190.20
Monoclinic,
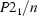
a = 6.2811 (2) Å
b = 9.4698 (3) Å
c = 16.3936 (5) Å
β = 92.645 (3)°
V = 974.06 (5) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 293 K
0.30 × 0.20 × 0.15 mm
Data collection
Oxford Diffraction Xcalibur Sapphire3 diffractometer
Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2010 ▶) T min = 0.971, T max = 0.986
18157 measured reflections
1908 independent reflections
1574 reflections with I > 2σ(I)
R int = 0.032
Refinement
R[F 2 > 2σ(F 2)] = 0.039
wR(F 2) = 0.113
S = 1.06
1908 reflections
128 parameters
H-atom parameters constrained
Δρmax = 0.12 e Å−3
Δρmin = −0.19 e Å−3
Data collection: CrysAlis PRO (Oxford Diffraction, 2010 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2009 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811028790/is2752sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811028790/is2752Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811028790/is2752Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1A⋯O1i | 0.86 | 2.09 | 2.874 (2) | 151 |
Symmetry code: (i)  .
.
Acknowledgments
The authors thank the Director of the University Sophisticated Instrumentation Facility, University of Jammu, Jammu Tawi, India, for the X-ray data collection. HY gratefully acknowledges Yeungnam University for providing the opportunity to work as an International Research Professor.
supplementary crystallographic information
Comment
The chemistry of pyrrole compounds and biological activities of the related compounds has been extensively studied (Fischer & Orth, 1934). The Knoevenagel condensation is an important carbon–carbon bond forming reaction in organic synthesis (Knoevenagel, 1898). Ever since its discovery, the Knoevenagel reaction has been widely used in organic synthesis to prepare coumarins and their derivatives, which are important intermediates in the synthesis of cosmetics, perfumes and pharmaceuticals (Bigi et al., 1999). With the view of biological importance the title compound was synthesized and reported here its crystal structure.
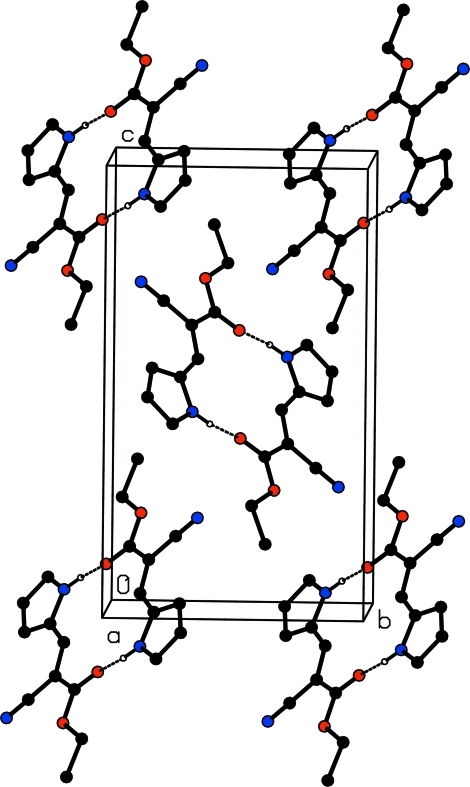
Bond lengths and bond angles are comparable with the similar crystal structures solved earlier (Ye et al., 2009; Wang & Jian, 2008; Zhang et al., 2009). All the non-hydrogen atoms in the molecule are nearly in the same plane with the maximum out-of-plane deviation of 0.093 (1) Å (r.m.s. deviation = 0.04 Å). The crystal packing is stabilized by N—H···O intermolecular interactions, generating a centrosymmetric dimer of R22(14) ring.
Experimental
A solution of pyrrole-2-aldehyde (1 mol), ethyl cyanoacetate (1.2 mol) and piperidine (0.1 ml) in ethanol (20 ml) was stirred at room temperature for 8 h. After removal of the volatiles in vacuo, orange solid was obtained in quantitative yield. A sample for analysis was obtained by recrystallization from EtOAc as pale yellow needles: 1H NMR (300 MHz, CDCl3) δ p.p.m.: 1.38 t (3H, CH3), 4.35 q (2H, CH2), 6.41 m (1H, CH), 6.92 m (1H, CH), 7.22 m (1H, CH), 7.98 s (1H, HC=C), 9.92 s (1H, NH).
Refinement
All H atoms were refined using a riding model, with d(C—H) = 0.93 Å for aromatic, 0.97 Å for CH2 and 0.96 Å for CH3, and d(N—H) = 0.86 Å, and with Uiso(H) = 1.2Ueq(C, N) or 1.5Ueq(methylC)
Figures
Fig. 1.
The molecular structure of the title compound, showing 30% probability displacement ellipsoids.
Fig. 2.
A molecular packing view of the title compound, showing intermolecular interactions. For clarity, hydrogen atoms which are not involved in hydrogen bonding have been omitted.
Crystal data
| C10H10N2O2 | F(000) = 400 |
| Mr = 190.20 | Dx = 1.297 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2yn | Cell parameters from 7544 reflections |
| a = 6.2811 (2) Å | θ = 3.5–29.0° |
| b = 9.4698 (3) Å | µ = 0.09 mm−1 |
| c = 16.3936 (5) Å | T = 293 K |
| β = 92.645 (3)° | Rectangular, light yellow |
| V = 974.06 (5) Å3 | 0.30 × 0.20 × 0.15 mm |
| Z = 4 |
Data collection
| Oxford Diffraction Xcalibur Sapphire3 diffractometer | 1908 independent reflections |
| Radiation source: fine-focus sealed tube | 1574 reflections with I > 2σ(I) |
| graphite | Rint = 0.032 |
| Detector resolution: 16.1049 pixels mm-1 | θmax = 26.0°, θmin = 3.5° |
| ω scans | h = −7→7 |
| Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2010) | k = −11→11 |
| Tmin = 0.971, Tmax = 0.986 | l = −20→20 |
| 18157 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.039 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.113 | H-atom parameters constrained |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0623P)2 + 0.1138P] where P = (Fo2 + 2Fc2)/3 |
| 1908 reflections | (Δ/σ)max < 0.001 |
| 128 parameters | Δρmax = 0.12 e Å−3 |
| 0 restraints | Δρmin = −0.19 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | −0.1517 (2) | 0.73171 (17) | 0.56284 (9) | 0.0557 (4) | |
| H1 | −0.2260 | 0.7170 | 0.6099 | 0.067* | |
| C2 | −0.2045 (3) | 0.82705 (18) | 0.50230 (9) | 0.0596 (4) | |
| H2 | −0.3208 | 0.8879 | 0.5007 | 0.072* | |
| C3 | −0.0526 (2) | 0.81600 (16) | 0.44393 (9) | 0.0522 (4) | |
| H3 | −0.0488 | 0.8687 | 0.3962 | 0.063* | |
| C4 | 0.0926 (2) | 0.71274 (13) | 0.46903 (7) | 0.0403 (3) | |
| C5 | 0.2764 (2) | 0.65298 (13) | 0.43588 (8) | 0.0403 (3) | |
| H5 | 0.3441 | 0.5838 | 0.4679 | 0.048* | |
| C6 | 0.36856 (19) | 0.68137 (13) | 0.36460 (7) | 0.0390 (3) | |
| C7 | 0.2877 (2) | 0.78645 (15) | 0.30869 (8) | 0.0433 (3) | |
| C8 | 0.5592 (2) | 0.60070 (14) | 0.34340 (7) | 0.0400 (3) | |
| C9 | 0.8147 (2) | 0.56123 (16) | 0.24473 (8) | 0.0502 (4) | |
| H9A | 0.7809 | 0.4614 | 0.2415 | 0.060* | |
| H9B | 0.9363 | 0.5737 | 0.2827 | 0.060* | |
| C10 | 0.8645 (3) | 0.61655 (18) | 0.16209 (9) | 0.0593 (4) | |
| H10A | 0.7448 | 0.6008 | 0.1247 | 0.089* | |
| H10B | 0.9870 | 0.5683 | 0.1429 | 0.089* | |
| H10C | 0.8937 | 0.7159 | 0.1657 | 0.089* | |
| N1 | 0.02503 (19) | 0.66332 (12) | 0.54287 (7) | 0.0472 (3) | |
| H1A | 0.0873 | 0.5982 | 0.5717 | 0.057* | |
| N2 | 0.2208 (2) | 0.87126 (15) | 0.26485 (8) | 0.0631 (4) | |
| O1 | 0.63859 (15) | 0.50906 (11) | 0.38609 (6) | 0.0539 (3) | |
| O2 | 0.63298 (14) | 0.64028 (10) | 0.27206 (5) | 0.0454 (3) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0510 (8) | 0.0684 (10) | 0.0490 (8) | 0.0055 (7) | 0.0159 (6) | −0.0045 (7) |
| C2 | 0.0537 (9) | 0.0672 (10) | 0.0587 (9) | 0.0183 (7) | 0.0105 (7) | 0.0000 (8) |
| C3 | 0.0544 (8) | 0.0562 (9) | 0.0464 (8) | 0.0119 (7) | 0.0072 (6) | 0.0048 (6) |
| C4 | 0.0420 (7) | 0.0431 (7) | 0.0359 (6) | −0.0008 (6) | 0.0034 (5) | −0.0025 (5) |
| C5 | 0.0402 (7) | 0.0420 (7) | 0.0386 (7) | 0.0013 (5) | 0.0018 (5) | −0.0001 (5) |
| C6 | 0.0375 (7) | 0.0422 (7) | 0.0375 (6) | −0.0014 (5) | 0.0024 (5) | 0.0005 (5) |
| C7 | 0.0414 (7) | 0.0477 (8) | 0.0412 (7) | 0.0014 (6) | 0.0068 (5) | 0.0012 (6) |
| C8 | 0.0388 (7) | 0.0434 (7) | 0.0380 (7) | −0.0012 (5) | 0.0032 (5) | 0.0002 (5) |
| C9 | 0.0450 (7) | 0.0541 (8) | 0.0526 (8) | 0.0072 (6) | 0.0133 (6) | 0.0026 (7) |
| C10 | 0.0608 (9) | 0.0625 (10) | 0.0565 (9) | 0.0038 (7) | 0.0227 (7) | 0.0043 (7) |
| N1 | 0.0487 (7) | 0.0524 (7) | 0.0412 (6) | 0.0062 (5) | 0.0091 (5) | 0.0043 (5) |
| N2 | 0.0660 (9) | 0.0669 (8) | 0.0570 (8) | 0.0129 (7) | 0.0092 (6) | 0.0184 (7) |
| O1 | 0.0540 (6) | 0.0611 (6) | 0.0474 (6) | 0.0163 (5) | 0.0092 (4) | 0.0123 (5) |
| O2 | 0.0425 (5) | 0.0507 (6) | 0.0437 (5) | 0.0047 (4) | 0.0120 (4) | 0.0066 (4) |
Geometric parameters (Å, °)
| C1—N1 | 1.3386 (18) | C6—C8 | 1.4753 (18) |
| C1—C2 | 1.371 (2) | C7—N2 | 1.1447 (17) |
| C1—H1 | 0.9300 | C8—O1 | 1.2080 (15) |
| C2—C3 | 1.386 (2) | C8—O2 | 1.3316 (15) |
| C2—H2 | 0.9300 | C9—O2 | 1.4528 (16) |
| C3—C4 | 1.3869 (19) | C9—C10 | 1.4991 (19) |
| C3—H3 | 0.9300 | C9—H9A | 0.9700 |
| C4—N1 | 1.3827 (16) | C9—H9B | 0.9700 |
| C4—C5 | 1.4165 (18) | C10—H10A | 0.9600 |
| C5—C6 | 1.3546 (18) | C10—H10B | 0.9600 |
| C5—H5 | 0.9300 | C10—H10C | 0.9600 |
| C6—C7 | 1.4301 (18) | N1—H1A | 0.8600 |
| N1—C1—C2 | 108.49 (12) | O1—C8—O2 | 124.05 (12) |
| N1—C1—H1 | 125.8 | O1—C8—C6 | 123.60 (11) |
| C2—C1—H1 | 125.8 | O2—C8—C6 | 112.35 (11) |
| C1—C2—C3 | 107.40 (13) | O2—C9—C10 | 107.37 (12) |
| C1—C2—H2 | 126.3 | O2—C9—H9A | 110.2 |
| C3—C2—H2 | 126.3 | C10—C9—H9A | 110.2 |
| C2—C3—C4 | 108.22 (13) | O2—C9—H9B | 110.2 |
| C2—C3—H3 | 125.9 | C10—C9—H9B | 110.2 |
| C4—C3—H3 | 125.9 | H9A—C9—H9B | 108.5 |
| N1—C4—C3 | 105.90 (12) | C9—C10—H10A | 109.5 |
| N1—C4—C5 | 119.30 (12) | C9—C10—H10B | 109.5 |
| C3—C4—C5 | 134.80 (12) | H10A—C10—H10B | 109.5 |
| C6—C5—C4 | 129.78 (12) | C9—C10—H10C | 109.5 |
| C6—C5—H5 | 115.1 | H10A—C10—H10C | 109.5 |
| C4—C5—H5 | 115.1 | H10B—C10—H10C | 109.5 |
| C5—C6—C7 | 122.53 (12) | C1—N1—C4 | 110.00 (12) |
| C5—C6—C8 | 118.95 (11) | C1—N1—H1A | 125.0 |
| C7—C6—C8 | 118.53 (11) | C4—N1—H1A | 125.0 |
| N2—C7—C6 | 178.93 (14) | C8—O2—C9 | 115.85 (10) |
| N1—C1—C2—C3 | −0.38 (18) | C7—C6—C8—O1 | −179.34 (12) |
| C1—C2—C3—C4 | 0.31 (18) | C5—C6—C8—O2 | −179.66 (11) |
| C2—C3—C4—N1 | −0.12 (16) | C7—C6—C8—O2 | 0.57 (17) |
| C2—C3—C4—C5 | 178.72 (15) | C2—C1—N1—C4 | 0.31 (18) |
| N1—C4—C5—C6 | 178.23 (12) | C3—C4—N1—C1 | −0.11 (16) |
| C3—C4—C5—C6 | −0.5 (3) | C5—C4—N1—C1 | −179.17 (12) |
| C4—C5—C6—C7 | 1.1 (2) | O1—C8—O2—C9 | 2.78 (19) |
| C4—C5—C6—C8 | −178.64 (12) | C6—C8—O2—C9 | −177.13 (10) |
| C5—C6—C8—O1 | 0.4 (2) | C10—C9—O2—C8 | 176.96 (11) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1A···O1i | 0.86 | 2.09 | 2.874 (2) | 151 |
Symmetry codes: (i) −x+1, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: IS2752).
References
- Bigi, F., Chesini, L., Maggi, R. & Sartori, G. (1999). J. Org. Chem. 64, 1033–1035. [DOI] [PubMed]
- Fischer, H. & Orth, H. (1934). Die Chemie des Pyrrols, Vol. 1, pp. 333. Leipzig: Akademische Verlagsgesellschaft.
- Knizhnikov, V. A., Borisova, N. E., Yurashevich, N. Y., Popova, L. A., Cherny, A. Yu., Zubreichuk, Z. P. & Reshetova, M. D. (2007). Russ. J. Org. Chem. 43, 855–860.
- Knoevenagel, E. (1898). Berichte, 31, 2585–2595.
- Oxford Diffraction (2010). CrysAlis PRO Oxford Diffraction Ltd, Yarnton, England.
- Sarda, S. R., Jadhav, W. N., Tekale, S. U., Jadhav, G. V., Patil, B. R., Suryawanshi, G. S. & Pawar, R. P. (2009). Lett. Org. Chem. 6, 481–484.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Wang, J.-G. & Jian, F.-F. (2008). Acta Cryst. E64, o2145. [DOI] [PMC free article] [PubMed]
- Ye, Y., Shen, W.-L. & Wei, X.-W. (2009). Acta Cryst. E65, o2636. [DOI] [PMC free article] [PubMed]
- Zhang, S.-J., Zheng, X.-M. & Hu, W.-X. (2009). Acta Cryst. E65, o2351. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811028790/is2752sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811028790/is2752Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811028790/is2752Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report