Abstract
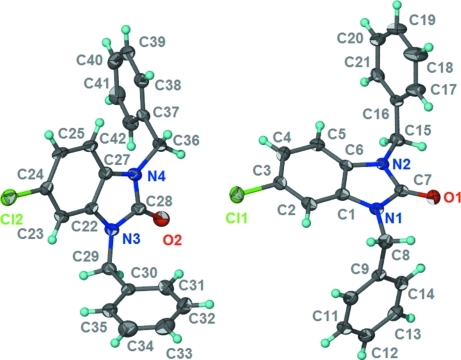
In both independent molecules of the title compound, C21H17ClN2O, the aromatic rings of the benzyl substituents are located on opposite sides of the benzimidazole ring systems. In one molecule, the rings are aligned at 77.0 (1) and 78.1 (1)° with respect to the fused-ring system, whereas in the other molecule the rings are aligned at 76.0 (1) and 76.9 (1)°. There is an intermolecular Cl⋯O contact of 3.086 (1) Å.
Related literature
For the structure of monobenzyl-benzimidazol-3-one, see: Ouzidan et al. (2011 ▶).
Experimental
Crystal data
C21H17ClN2O
M r = 348.82
Monoclinic,
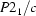
a = 11.0380 (4) Å
b = 9.2863 (3) Å
c = 33.2679 (13) Å
β = 92.297 (2)°
V = 3407.3 (2) Å3
Z = 8
Mo Kα radiation
μ = 0.24 mm−1
T = 293 K
0.08 × 0.04 × 0.03 mm
Data collection
Bruker X8 APEXII diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.981, T max = 0.993
22403 measured reflections
5950 independent reflections
3942 reflections with I > 2σ(I)
R int = 0.073
Refinement
R[F 2 > 2σ(F 2)] = 0.058
wR(F 2) = 0.159
S = 1.03
5950 reflections
451 parameters
H-atom parameters constrained
Δρmax = 0.77 e Å−3
Δρmin = −0.36 e Å−3
Data collection: APEX2 (Bruker, 2008 ▶); cell refinement: SAINT (Bruker, 2008 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811029084/bt6817sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811029084/bt6817Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811029084/bt6817Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
We thank Université Sidi Mohamed Ben Abdallah, Université Mohammed V-Agdal and the University of Malaya for supporting this study.
supplementary crystallographic information
Comment
A recent study reported the synthesis of monobenzyl-benzimidazol-3-one by the reaction of benzyl chloride on benzimidazol-3-one (Ouzidan et al., 2011). The use of double the molar quantity of benzyl choride on 5-chlorobenzimidazol-3-one yielded the expected title dibenzyl analog (Scheme I, Fig. 1). In both independent molecules, the aromatic rings of the benzyl substituent lie of opposite sides of the planar benzimidazole fused-ring. In one molecule, the rings are aligned at 77.0 (1)° and 78.1 (1)° with respect to the fused-ring whereas in the other, the rings are aligned at 76.0 (1)° and 76.9 (1)°.
Experimental
To 5-chloro-1H-benzimidazol-2(3H)-one (0.2 g, 1.18 mmol), potassium carbonate (0.40 g, 2.80 mmol), and tetra-n-butylammonium bromide (0.08 g, 0.23 mmol) in DMF (15 ml) was added benzyl chloride (0.33 g, 2.6 mmol). Stirring was continued at room temperature for 6 h. The salts were removed by filtration and the filtrate concentrated under reduced pressure. The residue was separated by chromatography on a column of silica gel with ethyl acetate/hexane (1/2) as eluent. The compound was recrystallized from hexane.
Refinement
Carbon-bound H-atoms were placed in calculated positions (C—H 0.93–0.97 Å) and were included in the refinement in the riding model approximation, with U(H) set to 1.2Ueq(C).
Omitted from the refinement were (-1 0 2), (1 0 2), (0 1 2), (0 0 2), (0 1 1) and (-1 1 2) owing to bad agreement between observed and calculated structure factors.
Figures
Fig. 1.
Anisotropic displacement ellipsoid plot (Barbour, 2001) of the two molecules of C21H17ClN2O at the 70% probability level; hydrogen atoms are drawn as spheres of an arbitrary radius.
Crystal data
| C21H17ClN2O | F(000) = 1456 |
| Mr = 348.82 | Dx = 1.360 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 2455 reflections |
| a = 11.0380 (4) Å | θ = 2.5–24.4° |
| b = 9.2863 (3) Å | µ = 0.24 mm−1 |
| c = 33.2679 (13) Å | T = 293 K |
| β = 92.297 (2)° | Prism, colorless |
| V = 3407.3 (2) Å3 | 0.08 × 0.04 × 0.03 mm |
| Z = 8 |
Data collection
| Bruker X8 APEXII diffractometer | 5950 independent reflections |
| Radiation source: fine-focus sealed tube | 3942 reflections with I > 2σ(I) |
| graphite | Rint = 0.073 |
| φ and ω scans | θmax = 25.0°, θmin = 1.9° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −13→13 |
| Tmin = 0.981, Tmax = 0.993 | k = −11→10 |
| 22403 measured reflections | l = −39→39 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.058 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.159 | H-atom parameters constrained |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.071P)2 + 2.3735P] where P = (Fo2 + 2Fc2)/3 |
| 5950 reflections | (Δ/σ)max = 0.001 |
| 451 parameters | Δρmax = 0.77 e Å−3 |
| 0 restraints | Δρmin = −0.36 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 0.34242 (8) | 0.76198 (10) | 0.10621 (3) | 0.0370 (3) | |
| Cl2 | −0.16981 (8) | 1.64706 (10) | 0.03835 (3) | 0.0392 (3) | |
| O1 | 0.6486 (2) | 0.2235 (3) | 0.23982 (7) | 0.0360 (6) | |
| O2 | 0.1395 (2) | 0.9431 (3) | 0.06445 (8) | 0.0391 (7) | |
| N1 | 0.5083 (2) | 0.3850 (3) | 0.21135 (8) | 0.0290 (7) | |
| N2 | 0.6912 (2) | 0.3771 (3) | 0.18714 (9) | 0.0286 (7) | |
| N3 | −0.0049 (2) | 1.1228 (3) | 0.05254 (9) | 0.0287 (7) | |
| N4 | 0.1826 (2) | 1.1884 (3) | 0.06987 (9) | 0.0299 (7) | |
| C1 | 0.5083 (3) | 0.4824 (4) | 0.17969 (10) | 0.0277 (8) | |
| C2 | 0.4193 (3) | 0.5703 (4) | 0.16282 (10) | 0.0297 (8) | |
| H2 | 0.3415 | 0.5730 | 0.1725 | 0.036* | |
| C3 | 0.4529 (3) | 0.6548 (4) | 0.13040 (10) | 0.0282 (8) | |
| C4 | 0.5684 (3) | 0.6555 (4) | 0.11589 (11) | 0.0332 (9) | |
| H4 | 0.5868 | 0.7160 | 0.0947 | 0.040* | |
| C5 | 0.6573 (3) | 0.5652 (4) | 0.13307 (11) | 0.0324 (9) | |
| H5 | 0.7353 | 0.5636 | 0.1235 | 0.039* | |
| C6 | 0.6258 (3) | 0.4786 (4) | 0.16458 (10) | 0.0256 (8) | |
| C7 | 0.6200 (3) | 0.3167 (4) | 0.21566 (10) | 0.0288 (8) | |
| C8 | 0.4026 (3) | 0.3441 (4) | 0.23430 (10) | 0.0325 (9) | |
| H8A | 0.3534 | 0.4287 | 0.2387 | 0.039* | |
| H8B | 0.4300 | 0.3070 | 0.2604 | 0.039* | |
| C9 | 0.3263 (3) | 0.2313 (4) | 0.21252 (10) | 0.0264 (8) | |
| C10 | 0.2142 (3) | 0.2636 (4) | 0.19518 (11) | 0.0320 (9) | |
| H10 | 0.1824 | 0.3557 | 0.1977 | 0.038* | |
| C11 | 0.1489 (3) | 0.1595 (4) | 0.17398 (11) | 0.0350 (9) | |
| H11 | 0.0736 | 0.1825 | 0.1622 | 0.042* | |
| C12 | 0.1940 (3) | 0.0227 (4) | 0.17016 (11) | 0.0364 (9) | |
| H12 | 0.1501 | −0.0465 | 0.1556 | 0.044* | |
| C13 | 0.3058 (3) | −0.0117 (4) | 0.18826 (11) | 0.0355 (9) | |
| H13 | 0.3364 | −0.1047 | 0.1863 | 0.043* | |
| C14 | 0.3710 (3) | 0.0919 (4) | 0.20903 (10) | 0.0326 (9) | |
| H14 | 0.4461 | 0.0687 | 0.2210 | 0.039* | |
| C15 | 0.8138 (3) | 0.3320 (4) | 0.17933 (11) | 0.0316 (9) | |
| H15A | 0.8174 | 0.3031 | 0.1514 | 0.038* | |
| H15B | 0.8342 | 0.2487 | 0.1959 | 0.038* | |
| C16 | 0.9066 (3) | 0.4487 (4) | 0.18787 (10) | 0.0254 (8) | |
| C17 | 0.9235 (3) | 0.5040 (5) | 0.22652 (11) | 0.0429 (10) | |
| H17 | 0.8761 | 0.4707 | 0.2471 | 0.051* | |
| C18 | 1.0100 (4) | 0.6077 (5) | 0.23439 (13) | 0.0555 (13) | |
| H18 | 1.0211 | 0.6436 | 0.2604 | 0.067* | |
| C19 | 1.0807 (3) | 0.6594 (4) | 0.20421 (12) | 0.0419 (10) | |
| H19 | 1.1393 | 0.7292 | 0.2098 | 0.050* | |
| C20 | 1.0637 (3) | 0.6071 (4) | 0.16622 (11) | 0.0359 (9) | |
| H20 | 1.1104 | 0.6420 | 0.1457 | 0.043* | |
| C21 | 0.9772 (3) | 0.5022 (4) | 0.15803 (11) | 0.0320 (9) | |
| H21 | 0.9664 | 0.4671 | 0.1319 | 0.038* | |
| C22 | −0.0032 (3) | 1.2731 (4) | 0.05389 (10) | 0.0252 (8) | |
| C23 | −0.0928 (3) | 1.3738 (4) | 0.04580 (10) | 0.0285 (8) | |
| H23 | −0.1721 | 1.3468 | 0.0390 | 0.034* | |
| C24 | −0.0587 (3) | 1.5167 (4) | 0.04828 (10) | 0.0269 (8) | |
| C25 | 0.0588 (3) | 1.5596 (4) | 0.05836 (10) | 0.0307 (8) | |
| H25 | 0.0784 | 1.6570 | 0.0595 | 0.037* | |
| C26 | 0.1466 (3) | 1.4569 (4) | 0.06670 (10) | 0.0301 (8) | |
| H26 | 0.2257 | 1.4841 | 0.0738 | 0.036* | |
| C27 | 0.1156 (3) | 1.3146 (4) | 0.06442 (10) | 0.0268 (8) | |
| C28 | 0.1094 (3) | 1.0696 (4) | 0.06260 (10) | 0.0299 (8) | |
| C29 | −0.1116 (3) | 1.0311 (4) | 0.04639 (10) | 0.0301 (8) | |
| H29A | −0.0863 | 0.9373 | 0.0370 | 0.036* | |
| H29B | −0.1649 | 1.0730 | 0.0257 | 0.036* | |
| C30 | −0.1805 (3) | 1.0127 (4) | 0.08438 (10) | 0.0280 (8) | |
| C31 | −0.1280 (3) | 0.9383 (4) | 0.11718 (12) | 0.0390 (10) | |
| H31 | −0.0511 | 0.8989 | 0.1152 | 0.047* | |
| C32 | −0.1877 (4) | 0.9221 (4) | 0.15238 (12) | 0.0450 (10) | |
| H32 | −0.1513 | 0.8723 | 0.1739 | 0.054* | |
| C33 | −0.3026 (4) | 0.9806 (5) | 0.15555 (13) | 0.0489 (11) | |
| H33 | −0.3434 | 0.9706 | 0.1793 | 0.059* | |
| C34 | −0.3563 (4) | 1.0533 (5) | 0.12353 (13) | 0.0475 (11) | |
| H34 | −0.4336 | 1.0918 | 0.1256 | 0.057* | |
| C35 | −0.2953 (3) | 1.0695 (4) | 0.08817 (12) | 0.0371 (9) | |
| H35 | −0.3321 | 1.1192 | 0.0667 | 0.045* | |
| C36 | 0.3085 (3) | 1.1791 (4) | 0.08465 (11) | 0.0316 (9) | |
| H36A | 0.3262 | 1.0804 | 0.0924 | 0.038* | |
| H36B | 0.3192 | 1.2386 | 0.1085 | 0.038* | |
| C37 | 0.3976 (3) | 1.2263 (4) | 0.05408 (10) | 0.0275 (8) | |
| C38 | 0.4760 (3) | 1.3383 (4) | 0.06241 (11) | 0.0314 (9) | |
| H38 | 0.4722 | 1.3870 | 0.0868 | 0.038* | |
| C39 | 0.5609 (3) | 1.3799 (4) | 0.03490 (12) | 0.0387 (10) | |
| H39 | 0.6130 | 1.4566 | 0.0407 | 0.046* | |
| C40 | 0.5674 (4) | 1.3065 (4) | −0.00092 (12) | 0.0411 (10) | |
| H40 | 0.6247 | 1.3327 | −0.0193 | 0.049* | |
| C41 | 0.4894 (4) | 1.1950 (5) | −0.00948 (12) | 0.0416 (10) | |
| H41 | 0.4943 | 1.1458 | −0.0337 | 0.050* | |
| C42 | 0.4036 (3) | 1.1548 (4) | 0.01745 (11) | 0.0352 (9) | |
| H42 | 0.3500 | 1.0801 | 0.0111 | 0.042* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0347 (5) | 0.0362 (6) | 0.0402 (5) | 0.0082 (4) | 0.0016 (4) | 0.0039 (4) |
| Cl2 | 0.0399 (5) | 0.0284 (6) | 0.0497 (6) | 0.0047 (4) | 0.0066 (4) | 0.0039 (4) |
| O1 | 0.0320 (14) | 0.0387 (16) | 0.0369 (14) | −0.0017 (12) | −0.0031 (11) | 0.0085 (12) |
| O2 | 0.0315 (14) | 0.0280 (16) | 0.0577 (18) | 0.0055 (12) | 0.0008 (12) | 0.0016 (13) |
| N1 | 0.0250 (15) | 0.0309 (18) | 0.0312 (16) | −0.0041 (13) | 0.0011 (12) | 0.0009 (13) |
| N2 | 0.0198 (14) | 0.0301 (18) | 0.0356 (17) | −0.0008 (13) | −0.0011 (12) | 0.0049 (13) |
| N3 | 0.0226 (15) | 0.0261 (18) | 0.0374 (17) | −0.0011 (13) | 0.0002 (12) | −0.0005 (13) |
| N4 | 0.0205 (15) | 0.0319 (19) | 0.0373 (17) | 0.0009 (13) | 0.0014 (12) | 0.0021 (13) |
| C1 | 0.0258 (18) | 0.025 (2) | 0.0323 (19) | −0.0032 (16) | 0.0009 (15) | −0.0028 (16) |
| C2 | 0.0228 (18) | 0.030 (2) | 0.036 (2) | 0.0030 (16) | 0.0009 (15) | −0.0078 (17) |
| C3 | 0.0293 (19) | 0.019 (2) | 0.036 (2) | 0.0039 (15) | −0.0029 (15) | −0.0025 (16) |
| C4 | 0.034 (2) | 0.032 (2) | 0.034 (2) | −0.0004 (17) | 0.0013 (16) | 0.0058 (17) |
| C5 | 0.0253 (19) | 0.035 (2) | 0.037 (2) | −0.0041 (17) | 0.0012 (15) | 0.0005 (17) |
| C6 | 0.0199 (17) | 0.026 (2) | 0.0308 (19) | −0.0032 (15) | −0.0039 (14) | −0.0029 (15) |
| C7 | 0.028 (2) | 0.029 (2) | 0.0294 (19) | −0.0061 (16) | −0.0041 (15) | −0.0024 (17) |
| C8 | 0.032 (2) | 0.038 (2) | 0.0282 (19) | −0.0009 (17) | 0.0045 (15) | −0.0019 (17) |
| C9 | 0.0237 (18) | 0.028 (2) | 0.0284 (19) | −0.0016 (15) | 0.0091 (14) | 0.0002 (15) |
| C10 | 0.0276 (19) | 0.031 (2) | 0.038 (2) | 0.0022 (17) | 0.0055 (16) | 0.0024 (17) |
| C11 | 0.0220 (18) | 0.044 (3) | 0.039 (2) | −0.0030 (17) | 0.0007 (16) | 0.0032 (18) |
| C12 | 0.039 (2) | 0.035 (2) | 0.035 (2) | −0.0101 (18) | 0.0020 (17) | −0.0018 (17) |
| C13 | 0.045 (2) | 0.025 (2) | 0.037 (2) | −0.0007 (18) | 0.0072 (18) | 0.0018 (17) |
| C14 | 0.0296 (19) | 0.037 (2) | 0.031 (2) | 0.0005 (17) | 0.0014 (15) | 0.0065 (17) |
| C15 | 0.0218 (18) | 0.033 (2) | 0.039 (2) | 0.0016 (16) | −0.0010 (15) | 0.0000 (17) |
| C16 | 0.0170 (16) | 0.029 (2) | 0.0297 (18) | −0.0004 (15) | 0.0006 (14) | −0.0001 (15) |
| C17 | 0.038 (2) | 0.060 (3) | 0.031 (2) | −0.021 (2) | 0.0109 (17) | −0.0048 (19) |
| C18 | 0.050 (3) | 0.077 (3) | 0.040 (2) | −0.029 (2) | 0.008 (2) | −0.024 (2) |
| C19 | 0.032 (2) | 0.042 (3) | 0.052 (3) | −0.0153 (19) | 0.0048 (18) | −0.008 (2) |
| C20 | 0.0266 (19) | 0.040 (2) | 0.042 (2) | −0.0051 (18) | 0.0062 (16) | 0.0070 (18) |
| C21 | 0.0224 (18) | 0.044 (2) | 0.0298 (19) | 0.0029 (17) | −0.0001 (15) | 0.0023 (17) |
| C22 | 0.0250 (18) | 0.025 (2) | 0.0261 (18) | −0.0022 (16) | 0.0072 (14) | 0.0016 (15) |
| C23 | 0.0221 (18) | 0.034 (2) | 0.0293 (19) | −0.0020 (16) | 0.0018 (14) | −0.0025 (16) |
| C24 | 0.0302 (19) | 0.028 (2) | 0.0230 (17) | 0.0006 (16) | 0.0089 (14) | 0.0019 (15) |
| C25 | 0.035 (2) | 0.027 (2) | 0.031 (2) | −0.0110 (17) | 0.0089 (16) | 0.0000 (16) |
| C26 | 0.0259 (18) | 0.033 (2) | 0.032 (2) | −0.0083 (17) | 0.0056 (15) | 0.0019 (16) |
| C27 | 0.0250 (18) | 0.028 (2) | 0.0274 (18) | 0.0008 (16) | 0.0063 (14) | 0.0004 (15) |
| C28 | 0.0233 (18) | 0.034 (2) | 0.033 (2) | −0.0017 (17) | 0.0062 (15) | 0.0008 (17) |
| C29 | 0.0276 (19) | 0.025 (2) | 0.037 (2) | −0.0024 (16) | −0.0019 (15) | −0.0032 (16) |
| C30 | 0.0288 (19) | 0.020 (2) | 0.035 (2) | −0.0052 (15) | −0.0018 (15) | −0.0013 (15) |
| C31 | 0.030 (2) | 0.041 (3) | 0.046 (2) | 0.0028 (18) | −0.0019 (17) | 0.0043 (19) |
| C32 | 0.051 (3) | 0.043 (3) | 0.040 (2) | −0.006 (2) | −0.0014 (19) | 0.0093 (19) |
| C33 | 0.055 (3) | 0.049 (3) | 0.044 (2) | −0.010 (2) | 0.014 (2) | −0.001 (2) |
| C34 | 0.034 (2) | 0.048 (3) | 0.061 (3) | 0.003 (2) | 0.014 (2) | 0.001 (2) |
| C35 | 0.030 (2) | 0.035 (2) | 0.047 (2) | −0.0013 (17) | 0.0017 (17) | 0.0068 (18) |
| C36 | 0.0208 (18) | 0.040 (2) | 0.034 (2) | 0.0010 (16) | 0.0002 (15) | 0.0036 (17) |
| C37 | 0.0177 (17) | 0.029 (2) | 0.036 (2) | 0.0032 (15) | 0.0010 (14) | 0.0019 (16) |
| C38 | 0.0260 (19) | 0.032 (2) | 0.037 (2) | 0.0043 (17) | 0.0013 (15) | −0.0012 (17) |
| C39 | 0.027 (2) | 0.028 (2) | 0.060 (3) | 0.0050 (17) | 0.0038 (18) | 0.0095 (19) |
| C40 | 0.038 (2) | 0.043 (3) | 0.044 (2) | 0.008 (2) | 0.0166 (18) | 0.013 (2) |
| C41 | 0.045 (2) | 0.046 (3) | 0.034 (2) | 0.010 (2) | 0.0107 (18) | −0.0003 (18) |
| C42 | 0.031 (2) | 0.031 (2) | 0.043 (2) | 0.0032 (17) | −0.0011 (17) | −0.0048 (18) |
Geometric parameters (Å, °)
| Cl1—C3 | 1.745 (3) | C17—H17 | 0.9300 |
| Cl2—C24 | 1.745 (3) | C18—C19 | 1.382 (6) |
| O1—C7 | 1.214 (4) | C18—H18 | 0.9300 |
| O2—C28 | 1.222 (4) | C19—C20 | 1.360 (5) |
| N1—C7 | 1.389 (4) | C19—H19 | 0.9300 |
| N1—C1 | 1.389 (4) | C20—C21 | 1.384 (5) |
| N1—C8 | 1.470 (4) | C20—H20 | 0.9300 |
| N2—C7 | 1.376 (4) | C21—H21 | 0.9300 |
| N2—C6 | 1.389 (4) | C22—C23 | 1.380 (5) |
| N2—C15 | 1.450 (4) | C22—C27 | 1.397 (5) |
| N3—C28 | 1.384 (4) | C23—C24 | 1.380 (5) |
| N3—C22 | 1.396 (4) | C23—H23 | 0.9300 |
| N3—C29 | 1.461 (4) | C24—C25 | 1.386 (5) |
| N4—C28 | 1.383 (5) | C25—C26 | 1.380 (5) |
| N4—C27 | 1.393 (4) | C25—H25 | 0.9300 |
| N4—C36 | 1.459 (4) | C26—C27 | 1.367 (5) |
| C1—C2 | 1.379 (5) | C26—H26 | 0.9300 |
| C1—C6 | 1.410 (5) | C29—C30 | 1.511 (5) |
| C2—C3 | 1.396 (5) | C29—H29A | 0.9700 |
| C2—H2 | 0.9300 | C29—H29B | 0.9700 |
| C3—C4 | 1.381 (5) | C30—C35 | 1.383 (5) |
| C4—C5 | 1.396 (5) | C30—C31 | 1.398 (5) |
| C4—H4 | 0.9300 | C31—C32 | 1.375 (5) |
| C5—C6 | 1.377 (5) | C31—H31 | 0.9300 |
| C5—H5 | 0.9300 | C32—C33 | 1.388 (6) |
| C8—C9 | 1.511 (5) | C32—H32 | 0.9300 |
| C8—H8A | 0.9700 | C33—C34 | 1.375 (6) |
| C8—H8B | 0.9700 | C33—H33 | 0.9300 |
| C9—C10 | 1.377 (5) | C34—C35 | 1.387 (5) |
| C9—C14 | 1.392 (5) | C34—H34 | 0.9300 |
| C10—C11 | 1.382 (5) | C35—H35 | 0.9300 |
| C10—H10 | 0.9300 | C36—C37 | 1.508 (5) |
| C11—C12 | 1.373 (5) | C36—H36A | 0.9700 |
| C11—H11 | 0.9300 | C36—H36B | 0.9700 |
| C12—C13 | 1.388 (5) | C37—C38 | 1.375 (5) |
| C12—H12 | 0.9300 | C37—C42 | 1.392 (5) |
| C13—C14 | 1.372 (5) | C38—C39 | 1.391 (5) |
| C13—H13 | 0.9300 | C38—H38 | 0.9300 |
| C14—H14 | 0.9300 | C39—C40 | 1.377 (6) |
| C15—C16 | 1.510 (5) | C39—H39 | 0.9300 |
| C15—H15A | 0.9700 | C40—C41 | 1.369 (6) |
| C15—H15B | 0.9700 | C40—H40 | 0.9300 |
| C16—C21 | 1.379 (5) | C41—C42 | 1.381 (5) |
| C16—C17 | 1.390 (5) | C41—H41 | 0.9300 |
| C17—C18 | 1.374 (5) | C42—H42 | 0.9300 |
| C7—N1—C1 | 110.4 (3) | C19—C20—C21 | 120.2 (3) |
| C7—N1—C8 | 123.3 (3) | C19—C20—H20 | 119.9 |
| C1—N1—C8 | 125.9 (3) | C21—C20—H20 | 119.9 |
| C7—N2—C6 | 110.5 (3) | C16—C21—C20 | 121.1 (3) |
| C7—N2—C15 | 124.7 (3) | C16—C21—H21 | 119.4 |
| C6—N2—C15 | 124.6 (3) | C20—C21—H21 | 119.4 |
| C28—N3—C22 | 109.7 (3) | C23—C22—N3 | 131.5 (3) |
| C28—N3—C29 | 123.2 (3) | C23—C22—C27 | 121.3 (3) |
| C22—N3—C29 | 126.7 (3) | N3—C22—C27 | 107.2 (3) |
| C28—N4—C27 | 110.2 (3) | C22—C23—C24 | 116.6 (3) |
| C28—N4—C36 | 123.6 (3) | C22—C23—H23 | 121.7 |
| C27—N4—C36 | 126.0 (3) | C24—C23—H23 | 121.7 |
| C2—C1—N1 | 132.2 (3) | C23—C24—C25 | 122.8 (3) |
| C2—C1—C6 | 121.3 (3) | C23—C24—Cl2 | 117.9 (3) |
| N1—C1—C6 | 106.4 (3) | C25—C24—Cl2 | 119.3 (3) |
| C1—C2—C3 | 116.1 (3) | C26—C25—C24 | 119.5 (3) |
| C1—C2—H2 | 121.9 | C26—C25—H25 | 120.2 |
| C3—C2—H2 | 121.9 | C24—C25—H25 | 120.2 |
| C4—C3—C2 | 123.4 (3) | C27—C26—C25 | 119.0 (3) |
| C4—C3—Cl1 | 118.3 (3) | C27—C26—H26 | 120.5 |
| C2—C3—Cl1 | 118.4 (3) | C25—C26—H26 | 120.5 |
| C3—C4—C5 | 119.9 (3) | C26—C27—N4 | 132.5 (3) |
| C3—C4—H4 | 120.1 | C26—C27—C22 | 120.7 (3) |
| C5—C4—H4 | 120.1 | N4—C27—C22 | 106.8 (3) |
| C6—C5—C4 | 117.9 (3) | O2—C28—N4 | 127.1 (3) |
| C6—C5—H5 | 121.1 | O2—C28—N3 | 126.8 (3) |
| C4—C5—H5 | 121.1 | N4—C28—N3 | 106.1 (3) |
| C5—C6—N2 | 131.8 (3) | N3—C29—C30 | 112.3 (3) |
| C5—C6—C1 | 121.4 (3) | N3—C29—H29A | 109.2 |
| N2—C6—C1 | 106.9 (3) | C30—C29—H29A | 109.2 |
| O1—C7—N2 | 127.4 (3) | N3—C29—H29B | 109.2 |
| O1—C7—N1 | 126.8 (3) | C30—C29—H29B | 109.2 |
| N2—C7—N1 | 105.8 (3) | H29A—C29—H29B | 107.9 |
| N1—C8—C9 | 111.6 (3) | C35—C30—C31 | 118.0 (3) |
| N1—C8—H8A | 109.3 | C35—C30—C29 | 121.8 (3) |
| C9—C8—H8A | 109.3 | C31—C30—C29 | 120.2 (3) |
| N1—C8—H8B | 109.3 | C32—C31—C30 | 121.4 (4) |
| C9—C8—H8B | 109.3 | C32—C31—H31 | 119.3 |
| H8A—C8—H8B | 108.0 | C30—C31—H31 | 119.3 |
| C10—C9—C14 | 118.9 (3) | C31—C32—C33 | 119.5 (4) |
| C10—C9—C8 | 121.6 (3) | C31—C32—H32 | 120.2 |
| C14—C9—C8 | 119.5 (3) | C33—C32—H32 | 120.2 |
| C9—C10—C11 | 120.2 (3) | C34—C33—C32 | 120.0 (4) |
| C9—C10—H10 | 119.9 | C34—C33—H33 | 120.0 |
| C11—C10—H10 | 119.9 | C32—C33—H33 | 120.0 |
| C12—C11—C10 | 120.7 (3) | C33—C34—C35 | 120.1 (4) |
| C12—C11—H11 | 119.6 | C33—C34—H34 | 119.9 |
| C10—C11—H11 | 119.6 | C35—C34—H34 | 119.9 |
| C11—C12—C13 | 119.5 (4) | C30—C35—C34 | 120.9 (4) |
| C11—C12—H12 | 120.3 | C30—C35—H35 | 119.5 |
| C13—C12—H12 | 120.3 | C34—C35—H35 | 119.5 |
| C14—C13—C12 | 119.7 (4) | N4—C36—C37 | 113.2 (3) |
| C14—C13—H13 | 120.1 | N4—C36—H36A | 108.9 |
| C12—C13—H13 | 120.1 | C37—C36—H36A | 108.9 |
| C13—C14—C9 | 120.9 (3) | N4—C36—H36B | 108.9 |
| C13—C14—H14 | 119.5 | C37—C36—H36B | 108.9 |
| C9—C14—H14 | 119.5 | H36A—C36—H36B | 107.8 |
| N2—C15—C16 | 112.9 (3) | C38—C37—C42 | 119.1 (3) |
| N2—C15—H15A | 109.0 | C38—C37—C36 | 120.4 (3) |
| C16—C15—H15A | 109.0 | C42—C37—C36 | 120.5 (3) |
| N2—C15—H15B | 109.0 | C37—C38—C39 | 120.8 (3) |
| C16—C15—H15B | 109.0 | C37—C38—H38 | 119.6 |
| H15A—C15—H15B | 107.8 | C39—C38—H38 | 119.6 |
| C21—C16—C17 | 118.3 (3) | C40—C39—C38 | 119.5 (4) |
| C21—C16—C15 | 121.4 (3) | C40—C39—H39 | 120.3 |
| C17—C16—C15 | 120.2 (3) | C38—C39—H39 | 120.3 |
| C18—C17—C16 | 120.1 (3) | C41—C40—C39 | 120.0 (4) |
| C18—C17—H17 | 120.0 | C41—C40—H40 | 120.0 |
| C16—C17—H17 | 120.0 | C39—C40—H40 | 120.0 |
| C17—C18—C19 | 120.9 (4) | C40—C41—C42 | 120.8 (4) |
| C17—C18—H18 | 119.6 | C40—C41—H41 | 119.6 |
| C19—C18—H18 | 119.6 | C42—C41—H41 | 119.6 |
| C20—C19—C18 | 119.4 (4) | C41—C42—C37 | 119.8 (4) |
| C20—C19—H19 | 120.3 | C41—C42—H42 | 120.1 |
| C18—C19—H19 | 120.3 | C37—C42—H42 | 120.1 |
| C7—N1—C1—C2 | 177.0 (4) | C28—N3—C22—C23 | 178.8 (3) |
| C8—N1—C1—C2 | 4.2 (6) | C29—N3—C22—C23 | −8.1 (6) |
| C7—N1—C1—C6 | −2.1 (4) | C28—N3—C22—C27 | 1.0 (4) |
| C8—N1—C1—C6 | −174.9 (3) | C29—N3—C22—C27 | 174.0 (3) |
| N1—C1—C2—C3 | −179.5 (3) | N3—C22—C23—C24 | −177.0 (3) |
| C6—C1—C2—C3 | −0.4 (5) | C27—C22—C23—C24 | 0.6 (5) |
| C1—C2—C3—C4 | −1.4 (5) | C22—C23—C24—C25 | −0.1 (5) |
| C1—C2—C3—Cl1 | 177.0 (3) | C22—C23—C24—Cl2 | −179.8 (2) |
| C2—C3—C4—C5 | 2.0 (6) | C23—C24—C25—C26 | −0.6 (5) |
| Cl1—C3—C4—C5 | −176.5 (3) | Cl2—C24—C25—C26 | 179.2 (3) |
| C3—C4—C5—C6 | −0.6 (5) | C24—C25—C26—C27 | 0.7 (5) |
| C4—C5—C6—N2 | 178.6 (3) | C25—C26—C27—N4 | 178.0 (3) |
| C4—C5—C6—C1 | −1.2 (5) | C25—C26—C27—C22 | −0.1 (5) |
| C7—N2—C6—C5 | −179.6 (4) | C28—N4—C27—C26 | −177.6 (4) |
| C15—N2—C6—C5 | −4.8 (6) | C36—N4—C27—C26 | 7.7 (6) |
| C7—N2—C6—C1 | 0.2 (4) | C28—N4—C27—C22 | 0.7 (4) |
| C15—N2—C6—C1 | 175.0 (3) | C36—N4—C27—C22 | −174.0 (3) |
| C2—C1—C6—C5 | 1.7 (5) | C23—C22—C27—C26 | −0.5 (5) |
| N1—C1—C6—C5 | −179.0 (3) | N3—C22—C27—C26 | 177.6 (3) |
| C2—C1—C6—N2 | −178.1 (3) | C23—C22—C27—N4 | −179.1 (3) |
| N1—C1—C6—N2 | 1.2 (4) | N3—C22—C27—N4 | −1.0 (4) |
| C6—N2—C7—O1 | 178.9 (3) | C27—N4—C28—O2 | 179.6 (3) |
| C15—N2—C7—O1 | 4.0 (6) | C36—N4—C28—O2 | −5.6 (6) |
| C6—N2—C7—N1 | −1.5 (4) | C27—N4—C28—N3 | −0.1 (4) |
| C15—N2—C7—N1 | −176.3 (3) | C36—N4—C28—N3 | 174.7 (3) |
| C1—N1—C7—O1 | −178.1 (3) | C22—N3—C28—O2 | 179.8 (3) |
| C8—N1—C7—O1 | −5.1 (5) | C29—N3—C28—O2 | 6.4 (5) |
| C1—N1—C7—N2 | 2.2 (4) | C22—N3—C28—N4 | −0.5 (4) |
| C8—N1—C7—N2 | 175.3 (3) | C29—N3—C28—N4 | −173.9 (3) |
| C7—N1—C8—C9 | −90.5 (4) | C28—N3—C29—C30 | 92.6 (4) |
| C1—N1—C8—C9 | 81.5 (4) | C22—N3—C29—C30 | −79.6 (4) |
| N1—C8—C9—C10 | −108.5 (4) | N3—C29—C30—C35 | 112.5 (4) |
| N1—C8—C9—C14 | 70.1 (4) | N3—C29—C30—C31 | −66.6 (4) |
| C14—C9—C10—C11 | −1.4 (5) | C35—C30—C31—C32 | −0.3 (6) |
| C8—C9—C10—C11 | 177.2 (3) | C29—C30—C31—C32 | 178.8 (3) |
| C9—C10—C11—C12 | 0.5 (5) | C30—C31—C32—C33 | 0.1 (6) |
| C10—C11—C12—C13 | 0.8 (5) | C31—C32—C33—C34 | 0.4 (6) |
| C11—C12—C13—C14 | −1.3 (5) | C32—C33—C34—C35 | −0.6 (6) |
| C12—C13—C14—C9 | 0.5 (5) | C31—C30—C35—C34 | 0.2 (5) |
| C10—C9—C14—C13 | 0.9 (5) | C29—C30—C35—C34 | −179.0 (3) |
| C8—C9—C14—C13 | −177.8 (3) | C33—C34—C35—C30 | 0.3 (6) |
| C7—N2—C15—C16 | −116.9 (4) | C28—N4—C36—C37 | 112.1 (4) |
| C6—N2—C15—C16 | 68.9 (4) | C27—N4—C36—C37 | −73.9 (4) |
| N2—C15—C16—C21 | −118.9 (4) | N4—C36—C37—C38 | 120.9 (4) |
| N2—C15—C16—C17 | 61.7 (4) | N4—C36—C37—C42 | −60.6 (4) |
| C21—C16—C17—C18 | −1.0 (6) | C42—C37—C38—C39 | −0.4 (5) |
| C15—C16—C17—C18 | 178.4 (4) | C36—C37—C38—C39 | 178.1 (3) |
| C16—C17—C18—C19 | 0.5 (7) | C37—C38—C39—C40 | −0.8 (5) |
| C17—C18—C19—C20 | 0.3 (7) | C38—C39—C40—C41 | 0.9 (6) |
| C18—C19—C20—C21 | −0.6 (6) | C39—C40—C41—C42 | 0.1 (6) |
| C17—C16—C21—C20 | 0.7 (5) | C40—C41—C42—C37 | −1.3 (6) |
| C15—C16—C21—C20 | −178.7 (3) | C38—C37—C42—C41 | 1.4 (5) |
| C19—C20—C21—C16 | 0.1 (6) | C36—C37—C42—C41 | −177.0 (3) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BT6817).
References
- Barbour, L. J. (2001). J. Supramol. Chem. 1, 189–191.
- Bruker (2008). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Ouzidan, Y., Essassi, E. M., Luis, S. V., Bolte, M. & El Ammari, L. (2011). Acta Cryst. E67, o1822. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811029084/bt6817sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811029084/bt6817Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811029084/bt6817Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report