Abstract
We evaluated alamarBlue as a metabolic indicator in a standardized assay for the measurement of serum bactericidal activity (SBA) to Haemophilus influenzae type b (Hib) using sera containing natural and vaccine-induced anticapsular (polyribosylribitol phosphate) antibodies. SBA assays with a colorimetric and a fluorometric end point in the presence of alamarBlue were developed and compared to a standard SBA assay, where colony counts are performed to determine the titer (12). A colorimetric end point required a spectrophotometer, whereas a fluorometric end point required a fluorometer. Prevaccination sera (n = 27) and postvaccination sera (n = 13) were tested by all three methodologies, and the SBA titers obtained in the presence of alamarBlue were compared to those from the standard method. Both the colorimetric and the fluorometric SBA titers were significantly correlated (r = 0.87 and r = 0.95, respectively) with those of the standard assay (≥50% killing as the SBA titer end point), and titers were not significantly different when compared to those of the standard assay (P > 0.68). However, the fluorometric end point had superior performance and ease of titer determination compared to the colorimetric end point (95 versus 87% of SBA titers were within 2 dilutions of the standard titer). Hib SBA assays with alamarBlue are reproducible, faster (same-day assay), and easier to perform than the standardized assay, which requires manual or automated colony counts. These semiautomated methodologies result in increased sample throughput and collection of data in digital formats that can be exported to data analysis programs for determination of SBA titers.
Haemophilus influenzae type b (Hib) is a respiratory pathogen which causes a variety of clinical manifestations, including meningitis, bacteremic pneumonia, epiglottitis, septicemia, cellulitis, and osteoarticular infections (2). The disease primarily affects young children of <5 years of age, causing up to 500,000 deaths each year worldwide (10). However, with the use of polyribosylribitol phosphate-protein conjugate vaccines, the incidence of invasive Hib in the United States has declined dramatically from 20,000 cases in 1987 to 227 cases in 2000 (4). These successful formulations have also been introduced as part of the routine vaccination regimens of other countries as part of the Expanded Program for Immunizations. Currently, there is a concerted effort towards further reduction of Hib disease in the United States and in the Americas. In the United States there are a few populations that have reported a higher incidence of invasive Hib disease, despite the introduction of successful Hib conjugate vaccines (3, 4, 6). Unlike the United States, introduction of this type of vaccine has been delayed in many countries due to difficulties in recognition of disease burden and concerns about vaccine cost. In addition, active surveillance through both epidemiologic and immunogenicity studies is helpful in monitoring progress in Hib disease reduction after introduction of the vaccine.
Immunological evaluation of vaccine-induced protection includes the measurement of vaccine-induced antibodies by enzyme-linked immunosorbent assay (ELISA). Minimal levels for short-term protection (as determined for unconjugated polysaccharide vaccines) are ≥0.15 μg/ml, and for long-term protection they are ≥1.0 μg/ml (5, 8). Antibody function is measured by serum bactericidal activity (SBA) rather than by ELISA. The current SBA assay relies on the measurement of Hib viability after addition of active complement and serum antibodies (12). This method is highly reproducible, but it requires overnight incubation to allow for the formation of CFU, it has a lower sample throughput, and it requires either manual or automated counting of CFU for the determination of SBA titers. In addition, data analysis is time-consuming, as the interface with data analysis programs is not automated. In this study, we evaluated a metabolic indicator (alamarBlue) to develop both a colorimetric and a fluorometric SBA assay for Hib, which is a highly fastidious organism. Mountzouros and Howell used this type of indicator for SBA assays specific for Neisseria meningitidis serogroup B (11). In this study, we determined that alamarBlue is suitable for same-day SBA titer determinations (fluorometric end point), it is highly reproducible, and it allows for automated data collection.
MATERIALS AND METHODS
Serum samples.
A total of 40 serum samples were tested by the standardized SBA assay and the alamarBlue SBA assays (colorimetric and fluorometric end points). Twelve normal healthy donor sera (age range = 24 to 62 years) were obtained through Emory Donor Services, Atlanta, Ga. We also tested 15 prevaccination sera and 13 postvaccination sera from study participants (age range = 6 to 73 years) who received a single dose of HibTITER vaccine (HbOC; Wyeth-Lederle Vaccines and Pediatrics, West Henrietta, N.Y.). These sera were collected before vaccination and 8 weeks postvaccination as part of a colonization intervention study in Alaska. All participants or their legal guardians provided informed consent. Participants had no history of Hib disease, although they had history of Hib oropharyngeal colonization. Participants of age 22 to 73 (n = 9) had no previous history of Hib vaccination. Sera were stored frozen at −70°C in 500-μl aliquots until use. The quality control serum used was PSAB-90, kindly donated by Claudette Thompson, Dana Farber Cancer Institute, Boston, Mass. The SBA titer of either 2,048 or 4,096 for PSAB-90 was determined by testing (n = 7) in parallel with the control preparation, bacterial polysaccharide immune globulin (SBA titer, 16,384) previously used as the quality control preparation for the standard SBA assay (12). The immunoglobulin G ELISA concentration of PSAB-90 (mean ± standard deviation [SD]) was determined to be 60.3 ± 9.45 μg/ml after 35 independent measurements against the standard reference serum, lot 1983 (provided by Carl Frasch, Center for Biological Evaluation and Review, Food and Drug Administration, Bethesda, Md.).
Standard SBA assay.
We performed the standardized SBA assay, which measures the direct bactericidal activity of serum antibodies as previously described by Romero-Steiner et al. (12), which is a modification of the method described by Schlesinger et al. (13). This assay was used as the standard method for comparison of SBA titers generated by the assays containing alamarBlue. Specifically, serum samples were tested in duplicate at a starting dilution of 1:8. Twofold serum serial dilutions were made in 10 μl of Hanks buffer with Ca2+ and Mg2+ (Life Technologies, Grand Island, N.Y.) supplemented with 2% Fildes enrichment (BBL, Becton Dickinson and Co., Sparks, Md.). A frozen aliquot of Hib strain GB 3292 (12) was diluted to yield 1,000 bacteria in a 20-μl volume (amount added to each well). An active source of complement was added after a preincubation period of 15 min at 37°C and 5% CO2. The complement used (25 μl per well) was sterile serum from 3- to 4-week-old baby rabbits (Pel-Freez, Brown Deer, Wis.), previously used as an efficient source of complement in Hib SBA assays (12, 14). An additional 25 μl of the dilution buffer as described above was added to each well. The final volume per well was 80 μl. After an incubation of 1 h at 37°C in 5% CO2, an aliquot of 5 μl from each well was plated onto chocolate agar plates (ChocII; BBL) and allowed to incubate at 37°C in 5% CO2 for 16 h. Viability counts of CFU were performed on each individual serum dilution, and SBA titers were determined. SBA titers were defined as the reciprocal of the serum dilution that resulted in ≥50% killing compared to the growth in complement controls. The predetermined SBA titer of 4,096 ± 1 dilution was consistently obtained with the PSAB-90 serum control.
alamarBlue SBA.
alamarBlue (Trek Diagnostics, Westlake, Ohio) is a patented (9) metabolic indicator that is commercially available. This metabolic indicator is not toxic or inhibitory to Hib. In its oxidized form alamarBlue is blue, and when it is reduced by the bacterium it changes to a pink compound. The amount of reduced alamarBlue can be measured in a spectrophotometer at 490 nm, under the assay conditions described for a colorimetric SBA assay. The reduced compound is also fluorescent (emission wavelength = 590 nm) if excited by a UV light source at a wavelength of 530 nm for a fluorometric SBA assay. The amount of reduced alamarBlue is proportional to cell growth, which in an SBA assay is directly proportional to the number of Hib bacteria surviving serum bactericidal killing.
For the alamarBlue SBA assays, the same protocol described for the standard SBA assay was followed, except that 25 μl of an alamarBlue buffer was added to each well after the addition of complement. The alamarBlue buffer included 16% alamarBlue, 64% Hanks buffer (containing Ca2+ and Mg2+ and 2% Fildes enrichment) and 20% brain heart infusion (BHI) broth (BBL). Typically, alamarBlue was diluted daily, as described, in a 10-ml volume and kept in the dark until used. BHI broth was needed to increase the growth of the surviving bacteria. This allowed for a fluorometric endpoint determination the same day as the assay performance. Incubation time for the fluorometric assay was 6 h at 37°C and 5% CO2. After the 6-h incubation period, assay plates were read in a fluorometer (model FL 600; BIO-TEK Instruments Inc., Winooski, Vt.). However, plates were allowed to incubate for a total of 19 to 22 h and read in a spectrophotometer (model ELX808; BIO-TEK) in order to read the colorimetric end point. Since the alamarBlue SBA assay required absorbance and fluorescence readings, reagent blanks were included in all plates. These blanks contained all assay reagents except bacteria. Similar to the standardized SBA assay, serum dilution titers were compared to complement control wells. These wells contained all assay reagents including bacteria and complement source but did not contain immune sera.
In order to obtain SBA titers similar to those of the standard SBA assay, the SBA titers for the colorimetric assay were defined as the reciprocal of the serum dilution at the inflection point of the bactericidal curve. This inflection point corresponded to the serum dilution prior to the plateau of each serum's absorbance curve. This plateau was defined as the serum dilution where the bacterial growth was in the same range as the absorbance observed in the complement controls (mean optical density at 490 nm [OD490] ± SD = 0.37 ± 0.08). SBA titers for the fluorometric assay were defined, following the same format as the standard SBA assay, as the reciprocal of the serum dilution with ≤50% of the fluorescent units (FU) detected in the complement controls. Regardless of the end point, the SBA assay using alamarBlue is an indirect method for the measurement of metabolism of the surviving bacteria after SBA.
Statistical analysis.
Correlations between SBA assays were determined by Pearson's product moment correlation coefficient by use of Sigma Plot software, version 8.0 (SPSS, Inc., Chicago, Ill.). Significant differences among assays were determined by Student's t test or the Mann-Whitney rank sum test for data not normally distributed. Comparisons between paired data were done by chi-square or Fisher's two-tailed exact test, using EpiInfo software version 6 (Centers for Disease Control and Prevention [CDC], Atlanta, Ga.). The significance level was set at a P value of <0.05. All SBA titers were determined as discontinuous titers. Percent agreements between standard and test (alamarBlue) SBA titers were calculated for SBA titers at >8 (a/a+c) or ≤8 (d/b+d) using two-by-two tables, where a is the number of sera reported as >8 by both assays, b is the number of sera reported as ≤8 by the standard assay but >8 by the test assay, c is the number of sera reported as >8 by the standard assay but ≤8 by the test assay, and d is the number of sera reported as ≤8 by both assays.
RESULTS
alamarBlue SBA optimization.
Multiple assay conditions were tested with the control serum, PSAB-90, to optimize the assay conditions that would allow for both colorimetric and fluorometric end points from the same assay plate. The metabolic activity (reduction of alamarBlue) of Hib was dependent on the type of buffer used at the time of addition of alamarBlue to the assay plate. We evaluated four different buffers containing the same amount of alamarBlue but various amounts of BHI broth (84, 20, 10, and 0%). The optimal buffer was found to contain 20% BHI broth, 64% Hanks buffer (containing Ca2+ and Mg2+ and 2% Fildes enrichment), and 16% alamarBlue. Incubation times tested were 0, 2, 4, 6, 7, 8, 19, 22, and 24 h of incubation at 37°C in 5% CO2. The optimal time for the fluorometric assay was 6 h, and for the colorimetric assay it was 19 to 22 h. Reduction of alamarBlue by Hib was directly proportional to the number of bacteria present in the assay wells. Bacterial inocula of 1,000, 5,000, and 10,000 bacteria per well were tested in parallel. Although 10,000 bacteria per well allowed for a more rapid reduction of alamarBlue under most media conditions, the use of 1,000 bacteria per well allowed for both a fluorometric (6 h) and a colorimetric (19 to 22 h) readout of the same assay plate when the alamarBlue buffer was supplemented with 20% BHI broth. The higher bacterial inoculum resulted in a 4- to 5-h fluorometric readout and 10- to 12-h colorimetric readouts. The optimal assay conditions for both colorimetric and fluorometric end points are described in Materials and Methods. Once the optimal assay conditions were defined, a panel of 40 human sera and a quality control serum (PSAB-90) were tested for evaluation of the assay performance in comparison to the standard SBA assay.
Reproducibility of Hib SBA assays containing alamarBlue.
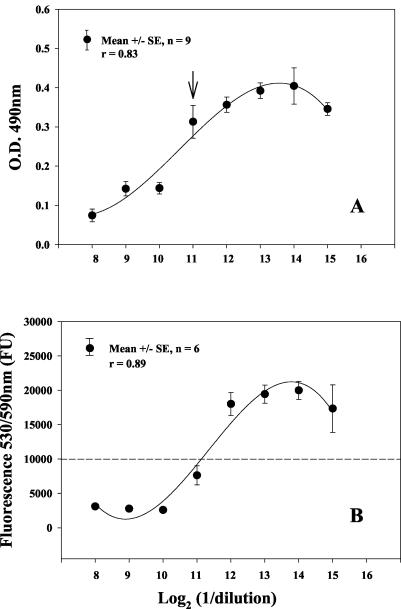
The reproducibility of both the colorimetric and the fluorometric assays was evaluated using the quality control serum PSAB-90, as shown in Fig. 1. For this control serum, the range (mean ± SD) of absorbance measured at 490 nm was 0.4 ± 0.14 to 0.07 ± 0.05 (Fig. 1A). The overall mean OD ± SD values for reagent blanks, complement control, and blanked complement control wells were 0.20 ± 0.07, 0.58 ± 0.08, and 0.37 ± 0.08, respectively. The coefficients of variance for reagent blanks, complement control, and blanked complement control wells were 35, 13.8, and 22%, respectively. The colorimetric SBA assay had a very restricted curve range. This narrow range made the interpretation of the SBA titers more difficult than in the fluorometric SBA assay. The range (mean ± SD) of the fluorometric SBA assay measured at 6 h under the conditions described was from 19,985 ± 3,221 FU for complement controls to 3,106 ± 525 FU for reagent blanks. The coefficients of variance for complement controls and reagent blanks were 16.1 and 16.9, respectively, with the fluorometric end point. Figure 1B gives the fluorescence signals observed for PSAB-90. In the fluorometric SBA assay, data were not blanked, since there was a high signal-to-noise ratio (6.4). In both alamarBlue SBA assays, the calculated median titer for PSAB-90 was 2,048 ± 1 dilution. This median titer was 1 dilution lower than the median titer observed with the standard SBA assay. The minimal level of quantification was a titer of 8 for the standard SBA assay as well as for the alamarBlue SBA assays. A titer lower than 8 was assigned a titer of 4 to allow for statistical analysis. Table 1 gives the standard SBA colony counts (CFU) that corresponded to the colorimetric and fluorometric SBA end points after a 6-h and a 19-h incubation period for the control serum, PSAB-90.
FIG. 1.
Serum bactericidal dilution curve for the serum control, PSAB-90, read under colorimetric optimal conditions (A) and under fluorometric optimal conditions (B). The arrow in panel A shows the inflection point in the SBA assay absorbance curve used to define the SBA titer compared to that in corresponding complement control wells. The dashed line in panel B indicates the 50% threshold used to define the SBA titer compared to that in complement control wells.
TABLE 1.
Comparison of a Hib standard SBA assay with fluorometric and colorimetric end points of an SBA assay using alamarBlue
| Parameter | Standard SBA (CFU/5-μl aliquot) | Fluorometric SBA (FU/well)a | Colorimetric SBA OD490 |
|---|---|---|---|
| Colony count or end point for: | |||
| PSAB-90 dilution | |||
| 1:512 | 24 | 3,468 | 0.162 |
| 1:1,024 | 27 | 3,100 | 0.208 |
| 1:2,048b | 43 | 4,590 | 0.327 |
| 1:4,096 | 65 | 14,244 | 0.362 |
| 1:8,192 | 90 | 21,267 | 0.410 |
| Complement wells | 116 | 20,750 | 0.367 |
| Blank wells | NAc | 2,590 | 0.156 |
| Range | 0 to 150 | 19,985 ± 3,221 to 3,106 ± 525 | 0.20 ± 0.07 to 0.37 ± 0.08 |
| MLQc | 8 | 8 | 8 |
| Total assay time (h)d | 24 | 8 | 21 |
| Instrumentation | Dissecting scope or automated reader | Fluorometer | Spectrophotometer (ELISA reader) |
| Correlation (r)e | NA | 0.95 | 0.87 |
For the standard SBA assay, values are numbers of CFU per 5-μl aliquot; for the fluorometric SBA, values are numbers of nonbanked fluorescence units measured at 530 and 5990 nm wavelength (excitation and emission) per well; and for the colorimetric SBA, values are ODs. The PSAB-90 titer is in bold.
NA, nonapplicable.
MLQ, minimum level of quantification (SBA titer).
Values are numbers of hours. Total assay time includes estimates for assay setup, SBA incubation, and titer determination and quantification.
r values, Pearson’s product moment correlation coefficient with standard assay, are listed. Absorbance values were blanked.
Correlation with standard SBA assay.
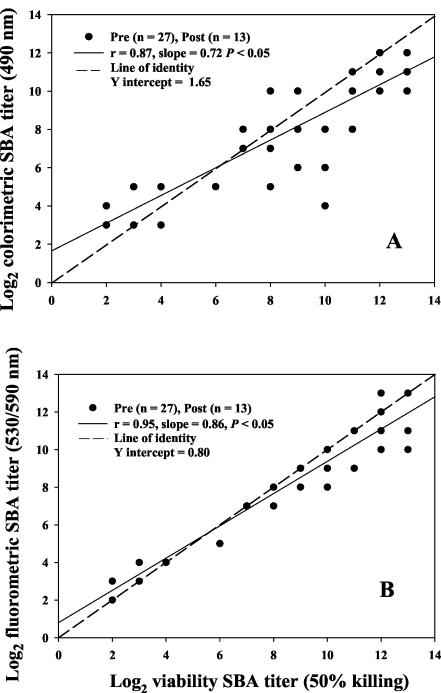
We evaluated the performance of the alamarBlue SBA assays by direct comparison to the standard assay. Figure 2 gives the correlations between the colorimetric and standard assays (r = 0.87; Fig. 2A) and between the fluorometric and standard assays (r = 0.95; Fig. 2B). Both assays significantly (P < 0.05) correlated with the standard assay. However, the fluorometric SBA assay had a significantly higher (chi-square P = 0.01) number of SBA titers (17 of 40) on the line of identity than did the colorimetric assay (7 of 40). Table 2 gives the overall cumulative percentages of SBA titers in agreement at ±1, ±2, or ≥3 dilutions from the standard SBA assay.
FIG. 2.
Comparison of SBA titers (n = 40 human serum samples) obtained by either the colorimetric SBA (A) or the fluorometric SBA (B) with the standard SBA. The dotted line represents the identity line, and the solid line represents the regression line. Correlation coefficients were determined by Pearson's product moment correlation coefficient. The percent agreement between the standard SBA and the colorimetric end point for titers of >8 was 97%, and for titers of ≤8 it was 50%. Similarly, the percent agreement for fluorometric titers of >8 was 100%, and for titers of ≤8 it was 83%.
TABLE 2.
Cumulative percentage of serum samples within a given dilution difference compared to that with the standard SBA assay
| Dilution difference | Fluorometric SBA (n = 40)
|
Colorimetric SBA (n = 40)
|
||
|---|---|---|---|---|
| No. in group | Cumulative % | No. in group | Cumulative % | |
| 0 | 17 | 42.5 | 7a | 17.5 |
| ±1 | 15 | 80.0 | 17 | 60.0 |
| ±2 | 7 | 97.5 | 10 | 67.5 |
| ≥3 | 1 | 100 | 6 | 100 |
Number of sera in agreement with the standard assay was significantly lower in the colorimetric SBA assay (P < 0.05) when compared to the fluorometric SBA. Significance between pairs was determined by chi-square. However, when all of the colorimetric or fluorometric SBA titers (log2 transformed) were compared (Student's t test) to the standard SBA titers, there were no significant differences (P range = 0.67 to 0.86) observed.
DISCUSSION
Serum bactericidal activity can be measured using a standard assay that requires bacterial colony counts for the determination of the serum SBA titer. This process is time-consuming, it requires special growth medium, and it is labor-intensive for use in large immunogenicity studies. Alternatively, a chromogenic oxidation-reduction (redox) compound such as alamarBlue can be used to monitor bacterial survival. AlamarBlue is a metabolic indicator formulated to quantitatively measure the proliferation of a variety of human or animal cells, bacteria, mycobacteria, and fungi (1, 7, 15, 16). It consists of an indicator that yields a colorimetric redox change and a fluorescent signal in response to metabolic activity.
AlamarBlue is blue in its oxidized form. When reduced by bacteria or tissue culture cells, it changes to a bright pink color that can be measured at 570 nm in the visible range or in the fluorometric UV range at 590 nm. The oxidized blue state can only be read at 600 to 630 nm in the visible range, and it is not fluorometric. Under the Hib SBA assay conditions (buffers are dark amber in color), the oxidized state was green instead of blue and the optimal wavelength for measurement of the reduced pink state was 490 nm. Other wavelengths tested were 405, 450, 562, and 620 nm (data not shown).
Use of alamarBlue in Hib SBA assays was found to be simple and practical for the measurement of SBA specific to Hib. However, improved assay performance was found (Fig. 2B) with the fluorometric readout (530/590 nm [excitation/emission UV wavelength]). The use of alamarBlue in Hib SBA assays offers a number of advantages over the standard SBA assay, such as (i) rapid instrument collection of results (40 to 60 s per 96-well plate), (ii) interface to computer software, (iii) 1-day assay for the fluorometric end point, (iv) potential for higher throughput of serum samples, (v) use of instruments available in most laboratory settings, and (vi) elimination of manual or automated viability counts and consequently the use of agar plates (ChocII). The main disadvantages of using alamarBlue in Hib SBA assays were observed for the colorimetric end point rather than the fluorometric end point. These disadvantages were (i) high sensitivity to buffer conditions, especially for the colorimetric end point; (ii) incubation times require close monitoring for the fluorometric assay (6 h ± 30 min); (iii) SBA titer determination in the colorimetric assay is difficult and may require plotting the SBA curve or a data analysis program to avoid subjectivity; and (iv) a narrow absorbance range for the colorimetric endpoint (blanked OD490 range, 0.07 to 0.4).
In the evaluation of alamarBlue as a metabolic indicator of SBA activity for Hib, we found this compound to be highly stable under the SBA assay conditions and under the recommended storage conditions (4°C in the dark until expiration date). This compound was not toxic to Hib and did not result in bacterial cell death in the absence of anti-polyribosylribitol phosphate antibodies. Bacterial viability counts were identical in the presence or absence of alamarBlue after a 1-h incubation period (data not shown).
Due to its superior performance, we recommend the fluorometric over the colorimetric SBA assay for the measurement of Hib SBA titers. SBA titers can be determined the same day as assay performance. Although the fluorometric end point requires a fluorometer, the SBA titers were easily defined and highly comparable to the standard SBA titers. We are currently evaluating the use of this metabolic indicator for meningococcal SBA (serogroups A, C, Y, and W135). The use of alamarBlue in measuring Hib SBA should facilitate broader application of this immunological assay for the evaluation of Hib vaccines.
Acknowledgments
Patricia Gomez de Leon was sponsored by a sabbatical grant from the Universidad Nacional Autónoma de México for a 6-month internship at the CDC. Willie Spear was sponsored by a training fellowship from the Oak Ridge Institute of Technology. Nekeidra Brown was sponsored by a Ferguson fellowship from the Minority Health Foundation, CDC. HibTITER vaccine was provided by Wyeth-Lederle Vaccines and Pediatrics as part of a cooperative research agreement with the CDC.
The findings of this research do not represent any endorsement of any commercial product by the CDC. The investigators do not have any conflict of interest with the manufacturers of the reagent evaluated in this study.
REFERENCES
- 1.Ahmed, S. A., R. M. Gogal, Jr., and J. E. Walsh. 1994. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 170:211-224. [DOI] [PubMed] [Google Scholar]
- 2.Carlone, G. M., B. A. Perkins, T. Popovic, N. Rosenstein, and S. Romero-Steiner. 2002. Haemophilus influenzae type b, Neisseria meningitidis, Streptococcus pneumoniae, and Corynebacterium diphtheriae vaccines, p. 418-431. In N. R. Rose, R. G. Hamilton, and B. Detrick (ed.), Manual of clinical laboratory immunology, 6th ed. American Society for Microbiology, Washington, D.C.
- 3.Centers for Disease Control and Prevention. 1996. Progress toward elimination of Haemophilus influenzae type B disease among infants and children—United States, 1987-1995. Morb. Mortal. Wkly. Rep. 45:901-906. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2002. Summary of notifiable diseases—United States, 2000. Morb. Mortal. Wkly. Rep. 49:1-102. [PubMed] [Google Scholar]
- 5.Fothergill, L., and J. Wright. 1933. Influenzal meningitis: relation of age incidence to the bactericidal power of blood against the causal organism. J. Immunol. 24:273-284. [Google Scholar]
- 6.Galil, K., R. Singleton, O. S. Levine, M. A. Fitzgerald, L. Bulkow, M. Getty, B. A. Perkins, and A. Parkinson. 1999. Reemergence of invasive Haemophilus influenzae type b disease in remote Alaska. J. Infect. Dis. 179:101-106. [DOI] [PubMed] [Google Scholar]
- 7.Jorgensen, J. H., J. A. Skweres, S. K. Mishra, M. L. McElmeel, L. A. Maher, R. Mulder, M. V. Lancaster, and D. L. Pierson. 1997. Development of an antimicrobial susceptibility testing method suitable for performance during space flight. J. Clin. Microbiol. 35:2093-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Käythy, H., H. Peltola, V. Kankako, and P. H. Mäkelä. 1983. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J. Infect. Dis. 147:1100. [DOI] [PubMed] [Google Scholar]
- 9.Lancaster, M. V., and R. D. Fields. March1996. Antibiotic and cytotoxic drug susceptibility testing using resazurin and poising agents. U.S. patent 5,501,959.
- 10.Levine, O., J. Wenger, Y. B. Perkins, N. Rosenstein, and A. Schuchat. 1998. Haemophilus influenzae type B infection. Bull. W. H. O. 76(Suppl. 2):131-132. [PMC free article] [PubMed] [Google Scholar]
- 11.Mountzouros, K. T., and A. P. Howell. 2000. Detection of complement-mediated antibody-dependent bactericidal activity in a fluorescence-based serum bactericidal assay for group B Neisseria meningitidis. J. Clin. Microbiol. 38:2878-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero-Steiner, S., J. Fernandez, C. Biltoft, M. E. Wohl, J. Feris, S. Balter, O. S. Levine, and G. M. Carlone. 2001. Functional antibody activity elicited by fractional doses of Haemophilus influenzae type b conjugate vaccine (polyribosylribitol phosphate-tetanus conjugate). Clin. Diagn. Lab. Immunol. 8:1115-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlesinger, Y., D. M. Granoff, et al. 1992. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. JAMA 267:1489-1494. [PubMed] [Google Scholar]
- 14.Schneerson, R., and J. B. Robbins. 1975. Induction of serum Haemophilus influenzae type b capsular antibodies in adult volunteers fed cross-reacting Escherichia coli O75:K100:H5. N. Engl. J. Med. 292:1093-1096. [DOI] [PubMed] [Google Scholar]
- 15.Tiballi, R. N., X. He, L. T. Zarins, S. G. Revankar, and C. A. Kaufman. 1995. Use of a colorimetric system for yeast susceptibility testing. J. Clin. Microbiol. 33:915-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yajko, D. M., J. J. Madej, M. V. Lancaster, C. A. Sanders, V. L. Cawthon, B. Gee, A. Babst, and W. K. Hadley. 1995. Colorimetric method for determining MICs of antimicrobial agents for Mycobacterium tuberculosis. J. Clin. Microbiol. 33:2324-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]