Abstract
The molecule of the title compound [systematic name: N-(carbamoylmethyl)prop-2-enamide], C5H8N2O2, which can be radically polymerized to polymers with thermoresponsive behavior in aqueous solution, consists of linked essentially planar acrylamide and amide segments [maximum deviations = 0.054 (1) and 0.009 (1) Å] with an angle of 81.36 (7)° between their mean planes. In the crystal, N—H⋯O hydrogen bonding leads to an infinite two-dimensional network along (100).
Related literature
For the first preparation of the title compound, see: Haas & Schuler (1964 ▶). For the properties of polymers of the title compound in aquous solution, see: Haas et al. (1967 ▶, 1970a ▶,b ▶,c ▶,d
▶); Marstokk et al. (1998 ▶); Nagaoka et al. (2007 ▶); Ohnishi et al. (2007 ▶); Seuring & Agarwal (2010 ▶); Glatzel et al. (2010 ▶). For the structure of the related compound, 2-(2-acrylamidoacetamido)acetic acid monohydrate, see Gao et al. (2007 ▶).
Experimental
Crystal data
C5H8N2O2
M r = 128.13
Monoclinic,

a = 15.938 (2) Å
b = 4.8055 (4) Å
c = 8.4920 (12) Å
β = 98.109 (11)°
V = 643.91 (14) Å3
Z = 4
Mo Kα radiation
μ = 0.10 mm−1
T = 100 K
0.23 × 0.19 × 0.09 mm
Data collection
Stoe IPDS 2T diffractometer
Absorption correction: integration (X-RED; Stoe & Cie, 2006 ▶) T min = 0.991, T max = 0.997
6001 measured reflections
1362 independent reflections
1065 reflections with I > 2σ(I)
R int = 0.049
Refinement
R[F 2 > 2σ(F 2)] = 0.032
wR(F 2) = 0.085
S = 0.97
1362 reflections
115 parameters
All H-atom parameters refined
Δρmax = 0.18 e Å−3
Δρmin = −0.15 e Å−3
Data collection: X-AREA (Stoe & Cie, 2006 ▶); cell refinement: X-AREA; data reduction: X-AREA; program(s) used to solve structure: SIR92 (Altomare et al., 1994 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: DIAMOND (Brandenburg, 2007 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶), PLATON (Spek, 2009 ▶) and WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811029758/sj5179sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811029758/sj5179Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811029758/sj5179Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N5—H5⋯O4i | 0.850 (19) | 2.062 (19) | 2.8946 (14) | 166.3 (15) |
| N8—H8B⋯O9ii | 0.881 (17) | 2.081 (17) | 2.9494 (14) | 168.2 (15) |
| N8—H8A⋯O9iii | 0.927 (18) | 1.971 (18) | 2.8855 (14) | 168.6 (16) |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
Acknowledgments
We acknowledge financial support from Philipps-Universität Marburg, Germany.
supplementary crystallographic information
Comment
N-acryloyl glycinamide was first synthesized by Haas and Schuler (1964). It can be polymerized radically to obtain polymers that exhibit thermoresponsive behavior in aqueous solution. The polymers show gelatin-like thermoreversible gelation (Haas & Schuler, 1964; Haas et al., 1967, 1970a, 1970b, 1970c, 1970d; Marstokk et al., 1998; Seuring & Agarwal, 2010; Glatzel et al., 2010) and an upper critical solution temperature (Seuring & Agarwal, 2010; Ohnishi et al., 2007; Nagaoka et al., 2007) in water. These phenomena rely on intermolecular hydrogen bonding. Therefore, investigating the intermolecular hydrogen bonding between monomer units in the crystal may contribute to the understanding of interpolymer interactions.
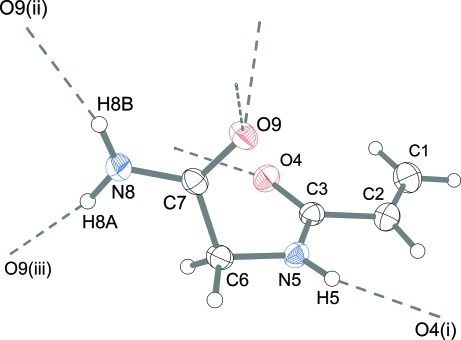
The molecular structure of the title compound shows two planar parts with C1, C2, C3, O4, N5, C6 and C6, C7, N8, O9 in plane. The angle between these mean planes is 81.36 (7)°. In the packing the molecule forms three hydrogen bonds to three different neighbouring molecules. For details see Table 1. The intermolecular N5—H5···O4i contacts form an infinite chain in the (0 1 0) direction. Two of these chains are linked via N8—H8A···O9iii and N8—H8B···O9ii interactions, respectively (symmetry codes: (i) x, y + 1, z; (ii) -x + 1, y - 1/2, -z + 3/2; (iii) x, -y + 1/2, z + 1/2). Herein the N8—H8A···O9iii hydrogen bonds form a second chain with direction (0 0 1), and hydrogen bonded rings are generated. In conclusion, a two dimensional hydrogen bond network has been formed with the hydrophobic tails of the molecules as border planes.
For the crystal structure of a related compound, 2-(2-acrylamidoacetamido)acetic acid monohydrate, see Gao et al. (2007).
Experimental
N-acryloyl glycinamide has been prepared according to the route of Haas and Schuler (1964). However, reagent ratios, workup and purification have been modified as follows.
In a 1 l three-necked round-bottom flask equipped with a mechanical stirrer glycinamide hydrochloride (23.11 g, 209 mmol) and potassium carbonate (56.7 g, 410 mmol) were dissolved in 125 ml of water. The solution was cooled in an ice bath and acryloyl chloride (16.65 ml, 205 mmol) dissolved in 250 ml of diethylether was added dropwise over 30 min with fast stirring (300 rpm). The suspension was further stirred at RT for 2 h. The diethylether was removed by rotary evaporation at 35 °C. The remaining aqueous phase was freeze dried. The crude brittle solid was extracted with acetone (6 times, 500 ml acetone, 40 °C, stirring for at least 15 min). Insoluble potassium salts were filtered off and the acetone was removed by rotary evaporation at 35 °C. 22.7 g (85%) of crude product were obtained. The crude product was dissolved in an eluent mixture of methanol and dichloromethane (v/v = 1/4, 600 ml) by heating to reflux once. The solution was filtered to remove polymeric impurities and purified by column chromatography (d = 9 cm, 900 g silica, porosity 60 Å, 0.063–0.2 mm mesh size, TLC: Rf(N-acryloyl glycinamide) = 0.40) to obtain 21.3 g (80%) of product which was recrystallized from 240 ml of a mixture of methanol and acetone (v/v = 1/2) to yield 15.3 g (57%) of colorless needle-like crystals.
For obtaining crystals that are suitable for X-ray analysis a small fraction of purified N-acryloyl glycinamide was again recrystallized from a dilute solution in 2-propanol.
Refinement
All H atoms were located in a difference Fourier map and refined isotropically. The C—H bond distances vary from 0.937 (18) to 0.981 (18), the N—H bond lenghts from 0.850 (19) to 0.927 (18) Å.
Figures
Fig. 1.
View of the crystal structure of the title compund. The thermal ellipsoids are drawn at 50% probability level. Dashed lines indicate hydrogen bonding contacts.
Fig. 2.
Two dimensional hydrogen bond network with plane direction (1 0 0). Dashed lines indicate hydrogen bonds.
Crystal data
| C5H8N2O2 | F(000) = 272 |
| Mr = 128.13 | Dx = 1.322 Mg m−3 |
| Monoclinic, P21/c | Melting point: 143 K |
| Hall symbol: -P 2ybc | Mo Kα radiation, λ = 0.71073 Å |
| a = 15.938 (2) Å | Cell parameters from 7769 reflections |
| b = 4.8055 (4) Å | θ = 2.6–27° |
| c = 8.4920 (12) Å | µ = 0.10 mm−1 |
| β = 98.109 (11)° | T = 100 K |
| V = 643.91 (14) Å3 | Plate, colourless |
| Z = 4 | 0.23 × 0.19 × 0.09 mm |
Data collection
| Stoe IPDS 2T diffractometer | 1362 independent reflections |
| Radiation source: fine-focus sealed tube | 1065 reflections with I > 2σ(I) |
| graphite | Rint = 0.049 |
| Detector resolution: 6.67 pixels mm-1 | θmax = 26.8°, θmin = 2.6° |
| rotation method scans | h = −20→17 |
| Absorption correction: integration (X-RED; Stoe & Cie, 2006) | k = −6→6 |
| Tmin = 0.991, Tmax = 0.997 | l = −10→10 |
| 6001 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.032 | All H-atom parameters refined |
| wR(F2) = 0.085 | w = 1/[σ2(Fo2) + (0.0548P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.97 | (Δ/σ)max < 0.001 |
| 1362 reflections | Δρmax = 0.18 e Å−3 |
| 115 parameters | Δρmin = −0.15 e Å−3 |
| 0 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.032 (7) |
Special details
| Experimental. DSC (rate of heating = 10 K min-1): Tm = 143 °C. IR (ATR): ν= 3380 (m, NH), 3312 (s, NH), 3187 (m, NH), 1652 (vs, C=O), 1621 (vs, C=O), 1551 (vs, NH) cm-1. 1H NMR (300 MHz, D2O): δ = 3.93 (s, 2H, N–CH2–CONH2), 5.77 [dd, J(doublet 1) = 2.0 Hz, J(doublet 2) = 9.5 Hz, 1H, Holef.], 6.20 [dd, J(doublet 1) = 2.0 Hz, J(doublet 2) = 17.1 Hz, 1H, Holef.], 6.29 [dd, J(doublet 1) = 9.5 Hz, J(doublet 2) = 17.2 Hz, 1H, Holef.]. 13C NMR (75 MHz, D2O): δ = 42.7 (–N—CH2–), 128.8 (Colef.), 130.0 (Colef.), 169.6 (–CO–), 174.8 (CO–). Flame emission spectroscopy: potassium content 5 p.p.m. |
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.91738 (9) | 0.4644 (3) | 0.66968 (16) | 0.0334 (3) | |
| C2 | 0.87077 (8) | 0.6065 (3) | 0.75755 (15) | 0.0269 (3) | |
| C3 | 0.80276 (7) | 0.4732 (3) | 0.83438 (13) | 0.0218 (3) | |
| C6 | 0.68733 (7) | 0.5421 (3) | 0.98560 (13) | 0.0229 (3) | |
| C7 | 0.61296 (7) | 0.4355 (3) | 0.86952 (13) | 0.0209 (3) | |
| N5 | 0.75513 (6) | 0.6473 (2) | 0.90665 (12) | 0.0221 (3) | |
| N8 | 0.56460 (7) | 0.2477 (2) | 0.92691 (12) | 0.0271 (3) | |
| O4 | 0.79110 (6) | 0.21891 (18) | 0.83202 (10) | 0.0280 (2) | |
| O9 | 0.59828 (5) | 0.52628 (19) | 0.73164 (9) | 0.0248 (2) | |
| H1A | 0.9069 (10) | 0.273 (4) | 0.6562 (19) | 0.038 (4)* | |
| H1B | 0.9623 (10) | 0.553 (4) | 0.620 (2) | 0.041 (4)* | |
| H2 | 0.8770 (10) | 0.802 (4) | 0.7774 (18) | 0.036 (4)* | |
| H5 | 0.7628 (10) | 0.822 (4) | 0.8996 (19) | 0.034 (4)* | |
| H6A | 0.7081 (8) | 0.395 (3) | 1.0551 (17) | 0.023 (3)* | |
| H6B | 0.6661 (9) | 0.697 (3) | 1.0471 (17) | 0.025 (3)* | |
| H8A | 0.5799 (10) | 0.181 (4) | 1.029 (2) | 0.042 (4)* | |
| H8B | 0.5176 (11) | 0.194 (4) | 0.867 (2) | 0.040 (4)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0316 (7) | 0.0359 (8) | 0.0336 (7) | 0.0022 (6) | 0.0078 (5) | 0.0017 (6) |
| C2 | 0.0260 (6) | 0.0247 (7) | 0.0300 (6) | −0.0003 (5) | 0.0039 (5) | 0.0015 (5) |
| C3 | 0.0246 (6) | 0.0198 (6) | 0.0201 (5) | 0.0000 (5) | 0.0001 (4) | 0.0001 (4) |
| C6 | 0.0261 (6) | 0.0237 (6) | 0.0189 (6) | −0.0002 (5) | 0.0028 (4) | −0.0010 (5) |
| C7 | 0.0231 (6) | 0.0211 (6) | 0.0193 (5) | 0.0030 (5) | 0.0058 (4) | −0.0008 (4) |
| N5 | 0.0244 (5) | 0.0177 (6) | 0.0246 (5) | −0.0012 (4) | 0.0040 (4) | −0.0010 (4) |
| N8 | 0.0286 (5) | 0.0318 (7) | 0.0204 (5) | −0.0076 (4) | 0.0020 (4) | 0.0030 (4) |
| O4 | 0.0361 (5) | 0.0180 (5) | 0.0304 (5) | −0.0008 (4) | 0.0065 (4) | −0.0011 (4) |
| O9 | 0.0273 (4) | 0.0291 (5) | 0.0178 (4) | 0.0006 (4) | 0.0028 (3) | 0.0025 (3) |
Geometric parameters (Å, °)
| C1—C2 | 1.3160 (19) | C6—C7 | 1.5198 (16) |
| C1—H1A | 0.937 (18) | C6—H6A | 0.950 (15) |
| C1—H1B | 0.981 (18) | C6—H6B | 0.994 (15) |
| C2—C3 | 1.4867 (17) | C7—O9 | 1.2405 (13) |
| C2—H2 | 0.956 (18) | C7—N8 | 1.3235 (16) |
| C3—O4 | 1.2360 (15) | N5—H5 | 0.850 (19) |
| C3—N5 | 1.3356 (16) | N8—H8A | 0.927 (18) |
| C6—N5 | 1.4412 (15) | N8—H8B | 0.881 (17) |
| C2—C1—H1A | 118.2 (10) | N5—C6—H6B | 108.4 (8) |
| C2—C1—H1B | 121.6 (10) | C7—C6—H6B | 107.4 (8) |
| H1A—C1—H1B | 120.2 (15) | H6A—C6—H6B | 110.1 (11) |
| C1—C2—C3 | 122.01 (13) | O9—C7—N8 | 123.05 (11) |
| C1—C2—H2 | 123.8 (10) | O9—C7—C6 | 121.30 (11) |
| C3—C2—H2 | 114.2 (10) | N8—C7—C6 | 115.62 (10) |
| O4—C3—N5 | 122.19 (11) | C3—N5—C6 | 120.42 (11) |
| O4—C3—C2 | 122.42 (11) | C3—N5—H5 | 119.2 (11) |
| N5—C3—C2 | 115.39 (11) | C6—N5—H5 | 120.2 (11) |
| N5—C6—C7 | 112.57 (9) | C7—N8—H8A | 119.6 (11) |
| N5—C6—H6A | 109.3 (8) | C7—N8—H8B | 118.6 (11) |
| C7—C6—H6A | 108.9 (8) | H8A—N8—H8B | 121.8 (15) |
| C1—C2—C3—O4 | 6.58 (19) | O4—C3—N5—C6 | −0.03 (16) |
| C1—C2—C3—N5 | −173.59 (12) | C2—C3—N5—C6 | −179.87 (10) |
| N5—C6—C7—O9 | −26.19 (17) | C7—C6—N5—C3 | −70.81 (14) |
| N5—C6—C7—N8 | 155.59 (11) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N5—H5···O4i | 0.850 (19) | 2.062 (19) | 2.8946 (14) | 166.3 (15) |
| N8—H8B···O9ii | 0.881 (17) | 2.081 (17) | 2.9494 (14) | 168.2 (15) |
| N8—H8A···O9iii | 0.927 (18) | 1.971 (18) | 2.8855 (14) | 168.6 (16) |
Symmetry codes: (i) x, y+1, z; (ii) −x+1, y−1/2, −z+3/2; (iii) x, −y+1/2, z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SJ5179).
References
- Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C., Polidori, G. & Camalli, M. (1994). J. Appl. Cryst. 27, 435.
- Brandenburg, K. (2007). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Farrugia, L. J. (1999). J. Appl. Cryst. 32, 837–838.
- Gao, X., Wu, C., Wang, H. & Wang, J. (2007). Acta Cryst. E63, o4580.
- Glatzel, S., Badi, N., Päch, M., Laschewsky, A. & Lutz, J.-F. (2010). Chem. Commun. 46, 4517–4519. [DOI] [PubMed]
- Haas, H. C., Chiklis, C. K. & Moreau, R. D. (1970a). J. Polym. Sci. Part A Polym. Chem. 8, 1131–1145.
- Haas, H. C., MacDonald, R. L. & Schuler, A. N. (1970b). J. Polym. Sci. Part A Polym. Chem. 8, 3405–3415.
- Haas, H. C., MacDonald, R. L. & Schuler, A. N. (1970c). J. Polym. Sci. Part A Polym. Chem, 8, 1213–1226.
- Haas, H. C., Manning, M. J. & Mach, M. H. (1970d). J. Polym. Sci. Part A Polym. Chem. 8, 1725–1730.
- Haas, H. C., Moreau, R. D. & Schuler, N. W. (1967). J. Polym. Sci. Part B Polym. Phys. 5, 915–927.
- Haas, H. C. & Schuler, N. W. (1964). J. Polym. Sci. Part B Polym. Lett. 2, 1095–1096.
- Marstokk, O. B., Nyström, B. & Roots, J. (1998). Macromolecules, 31, 4205–4212.
- Nagaoka, H., Ohnishi, N. & Eguchi, M. (2007). US Patent No. 0203313 A1.
- Ohnishi, N., Furukawa, H., Kataoka, K. & Ueno, K. (2007). US Patent No. 7,195,925 B2.
- Seuring, J. & Agarwal, S. (2010). Macromol. Chem. Phys. 211, 2109–2117.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Stoe & Cie (2006). X-AREA and X-RED32 Stoe & Cie, Darmstadt, Germany.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811029758/sj5179sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811029758/sj5179Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811029758/sj5179Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report