Abstract
The title compound, C14H12O4, is an asymmetric substitution product of benzophenone. Both hydroxy groups are orientated towards the O atom of the keto group. Intramolecular as well as intermolecular O—H⋯O hydrogen bonds can be observed in the crystal structure, with the latter connecting the molecules into chains along the crystallographic b axis. C—H⋯O contacts [C⋯O = 3.3297 (18) Å] are also apparent. The closest centroid–centroid distance between two aromatic systems is 4.9186 (9) Å.
Related literature
For the crystal structure of benzophenone, see: Lobanova (1968 ▶); Kutzke et al. (2000 ▶); Fleischer et al. (1968 ▶); Bernstein et al. (2002 ▶); Moncol & Coppens (2004 ▶). For the crystal structure of bis(2-hydroxyphenyl)methanone, see: Betz et al. (2011 ▶). For details on graph-set analysis of hydrogen bonds, see: Etter et al. (1990 ▶); Bernstein et al. (1995 ▶). For a comparison of the thermodynamic stability of coordination compounds containing chelate ligands as opposed to monodentate ligands, see: Gade (1998 ▶).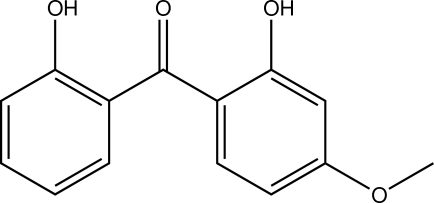
Experimental
Crystal data
C14H12O4
M r = 244.24
Orthorhombic,

a = 4.8582 (2) Å
b = 14.0236 (5) Å
c = 16.8636 (5) Å
V = 1148.91 (7) Å3
Z = 4
Mo Kα radiation
μ = 0.10 mm−1
T = 200 K
0.48 × 0.14 × 0.05 mm
Data collection
Bruker APEXII CCD diffractometer
6314 measured reflections
1683 independent reflections
1484 reflections with I > 2σ(I)
R int = 0.021
Refinement
R[F 2 > 2σ(F 2)] = 0.030
wR(F 2) = 0.083
S = 1.07
1683 reflections
166 parameters
H-atom parameters constrained
Δρmax = 0.18 e Å−3
Δρmin = −0.17 e Å−3
Data collection: APEX2 (Bruker, 2010 ▶); cell refinement: SAINT (Bruker, 2010 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶) and Mercury (Macrae et al., 2008 ▶); software used to prepare material for publication: SHELXL97 and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811030042/lw2071sup1.cif
Supplementary material file. DOI: 10.1107/S1600536811030042/lw2071Isup2.cdx
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811030042/lw2071Isup3.hkl
Supplementary material file. DOI: 10.1107/S1600536811030042/lw2071Isup4.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2—H2⋯O1 | 0.84 | 1.88 | 2.6058 (17) | 144 |
| O3—H3⋯O1 | 0.84 | 1.91 | 2.6267 (17) | 142 |
| O3—H3⋯O4i | 0.84 | 2.50 | 2.9306 (15) | 113 |
| C15—H15⋯O1ii | 0.95 | 2.57 | 3.3297 (18) | 137 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Acknowledgments
The authors thank Mrs Angelika Obermeyer for helpful discussions.
supplementary crystallographic information
Comment
Chelate ligands have found widespread use in coordination chemistry due to the enhanced thermodynamic stability of resultant coordination compounds in relation to coordination compounds exclusively applying comparable monodentate ligands (Gade, 1998). Combining two identical donor atoms in different states of hybridization seemed to be useful to us to accomodate a large variety of metal centers of variable Lewis acidity. To enable comparative studies in terms of bond lengths and angles in envisioned coordination compounds, we determined the molecular and crystal structure of the title compound. The crystal structure of benzophenone is apparent in the literature (Lobanova, 1968; Kutzke et al., 2000; Fleischer et al., 1968; Bernstein et al., 2002; Moncol & Coppens, 2004) as is the crystal structure of bis(2-hydroxyphenyl)methanone (Betz et al., 2011).
The title compound is an asymmetric substitution product of benzophenone. Both aromatic moieties adopt a conformation in which its hydroxyl group is orientated towards the central oxygen atom. The least-squares planes defined by the respective carbon atoms of both aromatic rings intersect at an angle of 42.11 (6) °. Intracyclic C–C–C angles hardly deviate from the ideal value of 120 °. The methoxy group is nearly in plane with its resident aromatic system, the respective C–O–C–C torsional angle is found at 4.9 (2) ° (Fig. 1).
In the crystal structure, intra- as well as intermolecular hydrogen bonds are observed. While the intramolecular hydrogen bonds are apparent between the hydroxyl groups as donors and the double-bonded oxygen atom as acceptor, the intermolecular hydrogen bond stems from the hydroxyl group on the otherwise unsubstituted phenyl ring and has the etheric oxygen atom as acceptor (Fig. 2). The latter hydrogen bond thus shows bifurcation. In addition, a C–H···O contact whose range falls by more than 0.1 Å below the sum of van-der-Waals radii is present in the crystal structure. The latter one is supported by one of the CH groups in ortho-position to the methoxy substituent and has the keto group's oxygen atom as acceptor. In terms of graph-set analysis (Etter et al., 1990; Bernstein et al., 1995), the descriptor for the hydrogen bonding system based on the hydroxyl groups on the unitary level is S(6)S(6)C(10) while the C–H···O contacts necessitate a C(6) descriptor on the same level. In total, the molecules are connected to undulated chains along the crystallographic b axis. The shortest intercentroid distance between two aromatic systems was measured to be at 4.9186 (9) Å and is apparent between the two different aromatic moieties.
The molecular packing of the title compound in the crystal structure is shown in Figure 3.
Experimental
The compound was obtained commercially (Aldrich). Crystals suitable for the X-ray diffraction study were taken directly from the provided product.
Refinement
Carbon-bound H atoms were placed in calculated positions (C—H 0.95 Å for aromatic carbon atoms) and were included in the refinement in the riding model approximation, with U(H) set to 1.2Ueq(C). The H atoms of the methyl group were allowed to rotate with a fixed angle around their respective C—O bond to best fit the experimental electron density (HFIX 137 in the SHELX program suite (Sheldrick, 2008)), with U(H) set to 1.5Ueq(C) and C—H set to 0.98 Å. The H atoms of the hydroxyl groups were allowed to rotate with a fixed angle around their respective C—O bond to best fit the experimental electron density (HFIX 147 in the SHELX program suite (Sheldrick, 2008)), with U(H) set to 1.5Ueq(O) and O—H set to 0.84 Å.
Figures
Fig. 1.
The molecular structure of the title compound, with atom labels and anisotropic displacement ellipsoids (drawn at 50% probability level).
Fig. 2.
Intermolecular contacts, viewed along [0 0 - 1]. Blue dashed lines indicate intramolecular hydrogen bonds, green dashed lines intermolecular hydrogen bonds and yellow dashed lines C–H···O contacts. Symmetry operators: i -x + 2, y + 1/2, -z + 1/2; ii -x + 2, y - 1/2, -z + 1/2.
Fig. 3.
Molecular packing of the title compound, viewed along [-1 0 0] (anisotropic displacement ellipsoids drawn at 50% probability level).
Crystal data
| C14H12O4 | F(000) = 512 |
| Mr = 244.24 | Dx = 1.412 Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 4306 reflections |
| a = 4.8582 (2) Å | θ = 2.8–28.2° |
| b = 14.0236 (5) Å | µ = 0.10 mm−1 |
| c = 16.8636 (5) Å | T = 200 K |
| V = 1148.91 (7) Å3 | Platelet, yellow |
| Z = 4 | 0.48 × 0.14 × 0.05 mm |
Data collection
| Bruker APEXII CCD diffractometer | 1484 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.021 |
| graphite | θmax = 28.3°, θmin = 1.9° |
| φ and ω scans | h = −4→6 |
| 6314 measured reflections | k = −18→17 |
| 1683 independent reflections | l = −21→22 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.030 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.083 | H-atom parameters constrained |
| S = 1.07 | w = 1/[σ2(Fo2) + (0.0509P)2 + 0.0857P] where P = (Fo2 + 2Fc2)/3 |
| 1683 reflections | (Δ/σ)max < 0.001 |
| 166 parameters | Δρmax = 0.18 e Å−3 |
| 0 restraints | Δρmin = −0.17 e Å−3 |
Special details
| Refinement. Due to the absence of a strong anomalous scatterer, the Flack parameter is meaningless. Thus, Friedel opposites (1966 pairs) have been merged and the item was removed from the CIF. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 1.0688 (3) | −0.07918 (7) | 0.34393 (6) | 0.0382 (3) | |
| O2 | 0.6572 (3) | −0.06852 (7) | 0.24525 (6) | 0.0374 (3) | |
| H2 | 0.7719 | −0.0968 | 0.2741 | 0.056* | |
| O3 | 1.4961 (3) | −0.08333 (8) | 0.43931 (7) | 0.0390 (3) | |
| H3 | 1.4061 | −0.1013 | 0.3995 | 0.058* | |
| O4 | 0.4289 (3) | 0.24323 (8) | 0.15520 (6) | 0.0405 (3) | |
| C1 | 1.0352 (3) | 0.00668 (10) | 0.36067 (8) | 0.0288 (3) | |
| C2 | 0.2546 (5) | 0.20454 (13) | 0.09489 (11) | 0.0447 (5) | |
| H2A | 0.3649 | 0.1660 | 0.0583 | 0.067* | |
| H2B | 0.1664 | 0.2567 | 0.0657 | 0.067* | |
| H2C | 0.1130 | 0.1644 | 0.1194 | 0.067* | |
| C11 | 0.8770 (3) | 0.06797 (10) | 0.30686 (8) | 0.0273 (3) | |
| C12 | 0.6941 (3) | 0.02686 (10) | 0.25159 (8) | 0.0279 (3) | |
| C13 | 0.5372 (4) | 0.08324 (11) | 0.20056 (8) | 0.0296 (3) | |
| H13 | 0.4108 | 0.0546 | 0.1648 | 0.036* | |
| C14 | 0.5676 (4) | 0.18145 (10) | 0.20262 (8) | 0.0309 (3) | |
| C15 | 0.7562 (4) | 0.22409 (10) | 0.25446 (8) | 0.0335 (4) | |
| H15 | 0.7797 | 0.2913 | 0.2545 | 0.040* | |
| C16 | 0.9063 (4) | 0.16846 (10) | 0.30496 (8) | 0.0309 (3) | |
| H16 | 1.0341 | 0.1980 | 0.3398 | 0.037* | |
| C21 | 1.1541 (4) | 0.04328 (10) | 0.43571 (8) | 0.0275 (3) | |
| C22 | 1.3752 (4) | −0.00534 (10) | 0.47108 (9) | 0.0297 (3) | |
| C23 | 1.4840 (4) | 0.02689 (11) | 0.54312 (9) | 0.0344 (4) | |
| H23 | 1.6380 | −0.0046 | 0.5659 | 0.041* | |
| C24 | 1.3687 (4) | 0.10414 (12) | 0.58103 (9) | 0.0373 (4) | |
| H24 | 1.4441 | 0.1256 | 0.6298 | 0.045* | |
| C25 | 1.1439 (4) | 0.15086 (11) | 0.54881 (9) | 0.0355 (4) | |
| H25 | 1.0621 | 0.2030 | 0.5760 | 0.043* | |
| C26 | 1.0397 (4) | 0.12097 (10) | 0.47670 (8) | 0.0310 (3) | |
| H26 | 0.8872 | 0.1537 | 0.4543 | 0.037* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0427 (7) | 0.0264 (5) | 0.0456 (6) | 0.0066 (6) | −0.0057 (6) | −0.0090 (4) |
| O2 | 0.0490 (7) | 0.0230 (5) | 0.0403 (5) | −0.0041 (5) | −0.0070 (6) | −0.0036 (4) |
| O3 | 0.0417 (7) | 0.0346 (6) | 0.0406 (6) | 0.0111 (6) | −0.0018 (6) | −0.0034 (5) |
| O4 | 0.0549 (8) | 0.0284 (5) | 0.0380 (5) | −0.0066 (6) | −0.0164 (7) | 0.0015 (4) |
| C1 | 0.0276 (8) | 0.0267 (6) | 0.0322 (6) | −0.0003 (6) | 0.0032 (7) | −0.0035 (5) |
| C2 | 0.0554 (12) | 0.0360 (8) | 0.0428 (8) | −0.0058 (9) | −0.0199 (10) | −0.0001 (7) |
| C11 | 0.0292 (8) | 0.0252 (7) | 0.0275 (6) | −0.0020 (7) | 0.0037 (7) | −0.0036 (5) |
| C12 | 0.0318 (8) | 0.0246 (7) | 0.0272 (6) | −0.0045 (6) | 0.0057 (7) | −0.0044 (5) |
| C13 | 0.0323 (8) | 0.0286 (7) | 0.0279 (6) | −0.0055 (7) | −0.0010 (7) | −0.0035 (5) |
| C14 | 0.0367 (9) | 0.0300 (7) | 0.0260 (6) | −0.0027 (7) | −0.0003 (8) | 0.0006 (5) |
| C15 | 0.0449 (9) | 0.0231 (6) | 0.0324 (7) | −0.0090 (7) | −0.0045 (9) | 0.0002 (5) |
| C16 | 0.0370 (9) | 0.0260 (7) | 0.0298 (6) | −0.0080 (7) | −0.0016 (8) | −0.0024 (5) |
| C21 | 0.0302 (8) | 0.0234 (6) | 0.0289 (6) | −0.0021 (6) | 0.0037 (7) | 0.0008 (5) |
| C22 | 0.0324 (8) | 0.0254 (7) | 0.0313 (6) | −0.0024 (7) | 0.0057 (7) | 0.0026 (5) |
| C23 | 0.0356 (9) | 0.0337 (8) | 0.0338 (7) | −0.0041 (7) | −0.0028 (8) | 0.0057 (6) |
| C24 | 0.0470 (10) | 0.0348 (8) | 0.0300 (7) | −0.0118 (8) | −0.0009 (8) | −0.0011 (6) |
| C25 | 0.0436 (10) | 0.0285 (7) | 0.0343 (7) | −0.0038 (7) | 0.0063 (8) | −0.0059 (6) |
| C26 | 0.0343 (9) | 0.0258 (7) | 0.0329 (7) | −0.0011 (7) | 0.0022 (7) | −0.0007 (5) |
Geometric parameters (Å, °)
| O1—C1 | 1.2475 (18) | C13—H13 | 0.9500 |
| O2—C12 | 1.3537 (16) | C14—C15 | 1.401 (2) |
| O2—H2 | 0.8400 | C15—C16 | 1.366 (2) |
| O3—C22 | 1.3522 (19) | C15—H15 | 0.9500 |
| O3—H3 | 0.8400 | C16—H16 | 0.9500 |
| O4—C14 | 1.3578 (19) | C21—C26 | 1.405 (2) |
| O4—C2 | 1.430 (2) | C21—C22 | 1.405 (2) |
| C1—C11 | 1.467 (2) | C22—C23 | 1.400 (2) |
| C1—C21 | 1.483 (2) | C23—C24 | 1.377 (2) |
| C2—H2A | 0.9800 | C23—H23 | 0.9500 |
| C2—H2B | 0.9800 | C24—C25 | 1.384 (3) |
| C2—H2C | 0.9800 | C24—H24 | 0.9500 |
| C11—C12 | 1.411 (2) | C25—C26 | 1.382 (2) |
| C11—C16 | 1.417 (2) | C25—H25 | 0.9500 |
| C12—C13 | 1.395 (2) | C26—H26 | 0.9500 |
| C13—C14 | 1.386 (2) | ||
| C12—O2—H2 | 109.5 | C16—C15—C14 | 119.64 (13) |
| C22—O3—H3 | 109.5 | C16—C15—H15 | 120.2 |
| C14—O4—C2 | 118.06 (12) | C14—C15—H15 | 120.2 |
| O1—C1—C11 | 119.60 (13) | C15—C16—C11 | 121.91 (15) |
| O1—C1—C21 | 118.44 (14) | C15—C16—H16 | 119.0 |
| C11—C1—C21 | 121.95 (12) | C11—C16—H16 | 119.0 |
| O4—C2—H2A | 109.5 | C26—C21—C22 | 118.02 (13) |
| O4—C2—H2B | 109.5 | C26—C21—C1 | 122.29 (15) |
| H2A—C2—H2B | 109.5 | C22—C21—C1 | 119.48 (13) |
| O4—C2—H2C | 109.5 | O3—C22—C23 | 116.15 (15) |
| H2A—C2—H2C | 109.5 | O3—C22—C21 | 123.81 (13) |
| H2B—C2—H2C | 109.5 | C23—C22—C21 | 120.04 (14) |
| C12—C11—C16 | 117.06 (14) | C24—C23—C22 | 120.20 (17) |
| C12—C11—C1 | 119.94 (12) | C24—C23—H23 | 119.9 |
| C16—C11—C1 | 122.95 (14) | C22—C23—H23 | 119.9 |
| O2—C12—C13 | 116.03 (13) | C23—C24—C25 | 120.74 (15) |
| O2—C12—C11 | 122.64 (13) | C23—C24—H24 | 119.6 |
| C13—C12—C11 | 121.33 (13) | C25—C24—H24 | 119.6 |
| C14—C13—C12 | 119.32 (14) | C26—C25—C24 | 119.38 (15) |
| C14—C13—H13 | 120.3 | C26—C25—H25 | 120.3 |
| C12—C13—H13 | 120.3 | C24—C25—H25 | 120.3 |
| O4—C14—C13 | 124.52 (14) | C25—C26—C21 | 121.54 (16) |
| O4—C14—C15 | 114.82 (13) | C25—C26—H26 | 119.2 |
| C13—C14—C15 | 120.64 (15) | C21—C26—H26 | 119.2 |
| O1—C1—C11—C12 | 20.0 (2) | C12—C11—C16—C15 | 2.8 (2) |
| C21—C1—C11—C12 | −159.02 (14) | C1—C11—C16—C15 | −179.82 (15) |
| O1—C1—C11—C16 | −157.31 (16) | O1—C1—C21—C26 | −152.15 (16) |
| C21—C1—C11—C16 | 23.7 (2) | C11—C1—C21—C26 | 26.9 (2) |
| C16—C11—C12—O2 | 177.13 (15) | O1—C1—C21—C22 | 22.6 (2) |
| C1—C11—C12—O2 | −0.3 (2) | C11—C1—C21—C22 | −158.41 (14) |
| C16—C11—C12—C13 | −3.7 (2) | C26—C21—C22—O3 | 177.31 (15) |
| C1—C11—C12—C13 | 178.86 (14) | C1—C21—C22—O3 | 2.4 (2) |
| O2—C12—C13—C14 | −178.84 (15) | C26—C21—C22—C23 | −3.2 (2) |
| C11—C12—C13—C14 | 1.9 (2) | C1—C21—C22—C23 | −178.13 (14) |
| C2—O4—C14—C13 | −4.9 (2) | O3—C22—C23—C24 | −178.06 (15) |
| C2—O4—C14—C15 | 173.44 (16) | C21—C22—C23—C24 | 2.4 (2) |
| C12—C13—C14—O4 | 179.11 (14) | C22—C23—C24—C25 | 0.1 (3) |
| C12—C13—C14—C15 | 0.9 (2) | C23—C24—C25—C26 | −1.8 (3) |
| O4—C14—C15—C16 | 179.84 (15) | C24—C25—C26—C21 | 0.9 (2) |
| C13—C14—C15—C16 | −1.8 (3) | C22—C21—C26—C25 | 1.6 (2) |
| C14—C15—C16—C11 | −0.1 (3) | C1—C21—C26—C25 | 176.35 (14) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H2···O1 | 0.84 | 1.88 | 2.6058 (17) | 144 |
| O3—H3···O1 | 0.84 | 1.91 | 2.6267 (17) | 142 |
| O3—H3···O4i | 0.84 | 2.50 | 2.9306 (15) | 113 |
| C15—H15···O1ii | 0.95 | 2.57 | 3.3297 (18) | 137 |
Symmetry codes: (i) −x+2, y−1/2, −z+1/2; (ii) −x+2, y+1/2, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LW2071).
References
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Bernstein, J., Ellern, A. & Henck, J.-O. (2002). Private communication (CCDC 118986, refcode BPHNO11). CCDC, Union Road, Cambridge, England.
- Betz, R., Gerber, T. & Schalekamp, H. (2011). Acta Cryst. E67, o1897. [DOI] [PMC free article] [PubMed]
- Bruker (2010). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Etter, M. C., MacDonald, J. C. & Bernstein, J. (1990). Acta Cryst. B46, 256–262. [DOI] [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Fleischer, E. B., Sung, N. & Hawkinson, S. (1968). J. Phys. Chem. 72, 4311–4312.
- Gade, L. H. (1998). Koordinationschemie, 1st ed. Weinheim: Wiley–VCH.
- Kutzke, H., Klapper, H., Hammond, R. B. & Roberts, K. J. (2000). Acta Cryst. B56, 486–496. [DOI] [PubMed]
- Lobanova, G. M. (1968). Kristallografiya, 13, 984–986.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Moncol, J. & Coppens, P. (2004). Private communication (CCDC 245188, refcode BPHNO12). CCDC, Union Road, Cambridge, England.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811030042/lw2071sup1.cif
Supplementary material file. DOI: 10.1107/S1600536811030042/lw2071Isup2.cdx
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811030042/lw2071Isup3.hkl
Supplementary material file. DOI: 10.1107/S1600536811030042/lw2071Isup4.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report





