Abstract
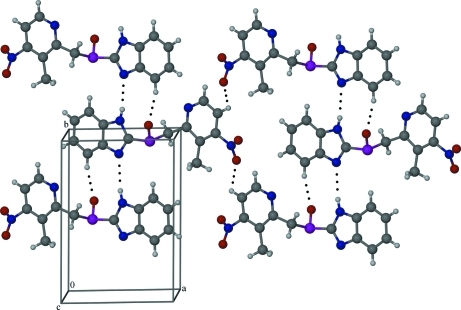
The title compound, C14H12N4O3S, is an intermediate of Dexlansoprazole, a proton pump inhibitor (PPI) mainly developed for anti-ulcer activity. The absolute configuration of the title compound was determined as R. The crystal structure reveals that the molecules form chains along the b axis through N—H⋯N and C—H⋯O hydrogen-bonded dimers. These chains are connected via weak C—H⋯O hydrogen bonds.
Related literature
For the synthesis of the title compound, see: Kumar et al. (2009 ▶). For background to this class of anti-ulcer drugs, see: Arimori et al. (1998 ▶); Masa et al. (2001 ▶). For a related structure, see: Fujishima et al. (2002 ▶).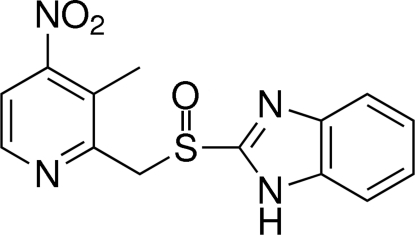
Experimental
Crystal data
C14H12N4O3S
M r = 316.33
Monoclinic,
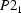
a = 7.7422 (13) Å
b = 11.0505 (15) Å
c = 8.2318 (13) Å
β = 103.697 (7)°
V = 684.24 (18) Å3
Z = 2
Mo Kα radiation
μ = 0.26 mm−1
T = 298 K
0.22 × 0.20 × 0.18 mm
Data collection
Rigaku Mercury diffractometer
Absorption correction: multi-scan (REQAB; Jacobson, 1998 ▶) T min = 0.942, T max = 0.950
7636 measured reflections
2752 independent reflections
2601 reflections with F 2 > 2σ(F 2)
R int = 0.025
Refinement
R[F 2 > 2σ(F 2)] = 0.037
wR(F 2) = 0.038
S = 1.25
2752 reflections
215 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.48 e Å−3
Δρmin = −0.37 e Å−3
Absolute structure: Flack (1983 ▶), with 1292 Friedel pairs
Flack parameter: −0.02 (4)
Data collection: CrystalClear (Rigaku, 2005 ▶); cell refinement: CrystalClear; data reduction: CrystalStructure (Molecular Structure Corporation & Rigaku, 2006 ▶); program(s) used to solve structure: SIR2004 (Burla et al. 2005 ▶); program(s) used to refine structure: CRYSTALS (Betteridge et al. 2003 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: CrystalStructure.
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811029990/gw2104sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811029990/gw2104Isup3.hkl
Supplementary material file. DOI: 10.1107/S1600536811029990/gw2104Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1⋯N2i | 0.881 (17) | 2.553 (18) | 3.425 (2) | 170.5 (13) |
| C2—H2⋯O1ii | 0.95 | 2.33 | 3.251 (2) | 164 |
| C12—H12⋯O2iii | 0.95 | 2.55 | 3.164 (2) | 122 |
Symmetry codes: (i) 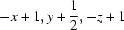 ; (ii)
; (ii)  ; (iii)
; (iii) 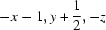 .
.
Acknowledgments
The authors are grateful to the management of IPDO-API and Dr Reddy’s Laboratories Ltd for encouragement. Many thanks to our colleagues Srinivas Gangula, Naredla Anitha and Baddam Sudhakar Reddy for their support in the overall process development.
supplementary crystallographic information
Comment
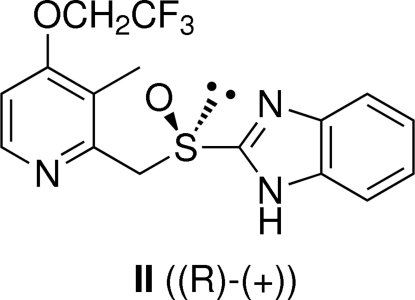
Dexlansoprazole II ((R)-(+)) (Fig. 1), (R)-enantiomer of Lansoprazole, is a proton pump inhibitor (PPI) mainly developed for anti-ulcer activity by TAP Pharmaceuticals Ltd., employing new modified-release technology (Arimori et al. 1998; Masa et al. 2001). Dexlansoprazole II ((R)-(+)) was first approved by United States Food and Drug Administration (US-FDA) in the form of 30 and 60 mg capsules for the management of patients with erosive oesophagitis and non-erosive reflux disease (GERD or GORD), under the brand name of DEXILANT.
An alternative and large-scale synthetic method for II ((R)-(+)) was developed in our laboratory by employing asymmetric oxidation conditions on prochiral nitrosulfide intermediate to yield enantiomerically enriched nitro sulphoxide derivative of the title compound I ((R)-(+)) as first stage intermediate (>90% ee) (Kumar et al. 2009). Titanium derived chiral complex (2.2:1.1:0.6 ratio of Titanium (IV)-i-propoxide:(+)-Diethyl L-tartrate:Water) was used in the reaction to induce the chirality. The enantiomerically enriched title compound I ((R)-(+)) as a resultant was subjected to acetone mediated crystallization to yield enantiopure I ((R)-(+)) (>97% ee) which on treatment with potassium salt of 2,2,2-triflouroethanol in dimethylformamide (DMF) yielded Dexlansoprazole II ((R)-(+)) with ICH quality having >99.8% ee.
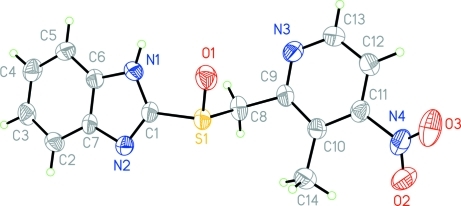
The structure and stereochemistry of Dexlansoprazole II ((R)-(+)) was well established in the literature with various spectroscopic and single-crystal X-ray diffraction (Fujishima et al. 2002). Herein we have determined the absolute configuration of the title compound I as `R' by anomalous dispersion (Fig. 2). The Flack parameter value, -0.02 (4) for the assigned absolute configuration, suggest that it is correct with high accuracy. The title compound is enantipure sulphoxide containing substituents of benzimidazole and 2-(3-methyl-4-nitro-pyridin-2-yl) methane moieties with dextro (d)- optical configuration. The crystal structure reveals that title molecules are forming chains along the b axis through N1—H···N2 and C2—H···O1 hydrogen-bonded dimers. Such chains are connected via weak C12—H···O2 hydrogen bonds (Fig. 3).
Experimental
A mixture of enantiomerically enriched title compound I ((R)-(+)) (12 g, 0.038 mol) and acetone (264 ml) were heated to 45–50 °C until clear solution obtained. The resulting clear solution was cooled to -5 to 0 °C and stirred for 1.0–1.5 h. The precipitated I (RS)-(±) was filtered and the filtrate was evaporated under vacuum at below 45 °C to obtain thick residue of the title compound I ((R)-(+)). The resulting thick residue of the title compound I ((R)-(+)) was dissolved in dichloromethane and kept for slow solvent evaporation to grow single crystals.
Refinement
The C-bound H atoms were geometrically placed (C—H = 0.95 Å) and refined as riding with Uiso(H) = 1.2Ueq(parent atom).
Figures
Fig. 1.
Schematic diagram of Dexlansoprazole (II) ((R)-(+)).
Fig. 2.
Molecular structure of (I), showing the atom numbering scheme. The displacement ellipsoids are drawn at the 50% probability level. H atoms are shown by small circles of arbitrary radii.
Fig. 3.
Crystal packing of (I).
Crystal data
| C14H12N4O3S | F(000) = 328.00 |
| Mr = 316.33 | Dx = 1.535 Mg m−3 |
| Monoclinic, P21 | Mo Kα radiation, λ = 0.71070 Å |
| Hall symbol: P 2yb | Cell parameters from 4183 reflections |
| a = 7.7422 (13) Å | θ = 1.8–27.5° |
| b = 11.0505 (15) Å | µ = 0.26 mm−1 |
| c = 8.2318 (13) Å | T = 298 K |
| β = 103.697 (7)° | Prism, colourless |
| V = 684.24 (18) Å3 | 0.22 × 0.20 × 0.18 mm |
| Z = 2 |
Data collection
| Rigaku Mercury diffractometer | 2601 reflections with F2 > 2σ(F2) |
| Detector resolution: 7.31 pixels mm-1 | Rint = 0.025 |
| ω scans | θmax = 27.5° |
| Absorption correction: multi-scan (REQAB; Jacobson, 1998) | h = −10→10 |
| Tmin = 0.942, Tmax = 0.950 | k = −13→13 |
| 7636 measured reflections | l = −7→10 |
| 2752 independent reflections |
Refinement
| Refinement on F2 | w = 1/[σ2(Fo2) + (0.1P)2] where P = (Fo2 + 2Fc2)/3 |
| R[F2 > 2σ(F2)] = 0.037 | (Δ/σ)max < 0.001 |
| wR(F2) = 0.038 | Δρmax = 0.48 e Å−3 |
| S = 1.25 | Δρmin = −0.37 e Å−3 |
| 2752 reflections | Absolute structure: Flack (1983), with 1292 Friedel pairs |
| 215 parameters | Flack parameter: −0.02 (4) |
| H atoms treated by a mixture of independent and constrained refinement |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement was performed using all reflections. The weighted R-factor (wR) and goodness of fit (S) are based on F2. R-factor (gt) are based on F. The threshold expression of F2 > 2.0 σ(F2) is used only for calculating R-factor (gt). |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.23549 (4) | 0.46611 (4) | 0.34638 (4) | 0.0355 (1) | |
| O1 | 0.24188 (14) | 0.57292 (10) | 0.23887 (14) | 0.0447 (3) | |
| O2 | −0.55199 (16) | 0.35772 (11) | 0.11414 (16) | 0.0604 (4) | |
| O3 | −0.57873 (16) | 0.49507 (15) | −0.07757 (16) | 0.0692 (5) | |
| N1 | 0.51652 (18) | 0.57978 (12) | 0.55851 (18) | 0.0374 (4) | |
| N2 | 0.52568 (17) | 0.37538 (12) | 0.56996 (17) | 0.0364 (4) | |
| N3 | −0.10635 (16) | 0.66905 (11) | 0.35720 (17) | 0.0430 (4) | |
| N4 | −0.50763 (15) | 0.45265 (15) | 0.05912 (16) | 0.0469 (4) | |
| C1 | 0.43749 (15) | 0.47231 (17) | 0.50384 (15) | 0.0352 (3) | |
| C2 | 0.8180 (2) | 0.36409 (15) | 0.7887 (2) | 0.0410 (5) | |
| C3 | 0.9495 (2) | 0.43573 (13) | 0.8856 (2) | 0.0438 (5) | |
| C4 | 0.9417 (2) | 0.56196 (15) | 0.8789 (2) | 0.0461 (5) | |
| C5 | 0.8050 (2) | 0.62249 (14) | 0.7738 (2) | 0.0417 (5) | |
| C6 | 0.6711 (2) | 0.55050 (13) | 0.67435 (19) | 0.0343 (5) | |
| C7 | 0.6765 (2) | 0.42432 (13) | 0.6819 (2) | 0.0336 (4) | |
| C8 | 0.08069 (19) | 0.50405 (15) | 0.47829 (19) | 0.0446 (5) | |
| C9 | −0.09062 (19) | 0.54887 (13) | 0.36425 (19) | 0.0371 (4) | |
| C10 | −0.21640 (16) | 0.46761 (16) | 0.27169 (16) | 0.0369 (3) | |
| C11 | −0.36204 (19) | 0.52325 (14) | 0.1662 (2) | 0.0383 (4) | |
| C12 | −0.3806 (2) | 0.64707 (13) | 0.1538 (2) | 0.0434 (5) | |
| C13 | −0.2491 (2) | 0.71654 (15) | 0.2537 (2) | 0.0454 (5) | |
| C14 | −0.1877 (2) | 0.33262 (15) | 0.2842 (2) | 0.0542 (6) | |
| H1 | 0.494 (2) | 0.6529 (16) | 0.516 (2) | 0.055 (5)* | |
| H2 | 0.82390 | 0.27830 | 0.79360 | 0.0480* | |
| H3 | 1.04750 | 0.39780 | 0.95950 | 0.0500* | |
| H4 | 1.03430 | 0.60730 | 0.94910 | 0.0530* | |
| H5 | 0.80050 | 0.70840 | 0.76880 | 0.0490* | |
| H12 | −0.48000 | 0.68360 | 0.08020 | 0.0510* | |
| H13 | −0.26100 | 0.80210 | 0.24930 | 0.0540* | |
| H81 | 0.05890 | 0.43470 | 0.53870 | 0.0520* | |
| H82 | 0.13040 | 0.56640 | 0.55450 | 0.0530* | |
| H141 | −0.29960 | 0.29300 | 0.26470 | 0.0660* | |
| H142 | −0.12570 | 0.30650 | 0.20380 | 0.0660* | |
| H143 | −0.12000 | 0.31340 | 0.39320 | 0.0660* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0266 (1) | 0.0382 (2) | 0.0387 (2) | 0.0014 (2) | 0.0019 (1) | −0.0015 (2) |
| O1 | 0.0386 (5) | 0.0509 (6) | 0.0422 (6) | 0.0037 (4) | 0.0051 (4) | 0.0085 (4) |
| O2 | 0.0521 (7) | 0.0478 (7) | 0.0793 (9) | −0.0140 (6) | 0.0116 (6) | −0.0158 (6) |
| O3 | 0.0467 (6) | 0.1104 (13) | 0.0441 (6) | −0.0083 (7) | −0.0019 (5) | −0.0079 (7) |
| N1 | 0.0350 (7) | 0.0330 (6) | 0.0412 (7) | 0.0040 (5) | 0.0029 (6) | 0.0029 (6) |
| N2 | 0.0302 (6) | 0.0355 (6) | 0.0401 (7) | −0.0010 (5) | 0.0015 (5) | 0.0009 (6) |
| N3 | 0.0369 (7) | 0.0406 (7) | 0.0516 (8) | −0.0051 (5) | 0.0110 (6) | −0.0030 (5) |
| N4 | 0.0320 (6) | 0.0581 (9) | 0.0502 (7) | −0.0062 (7) | 0.0088 (5) | −0.0179 (8) |
| C1 | 0.0258 (5) | 0.0395 (7) | 0.0381 (6) | −0.0008 (7) | 0.0030 (4) | −0.0012 (8) |
| C2 | 0.0389 (8) | 0.0372 (8) | 0.0435 (8) | 0.0058 (7) | 0.0028 (7) | 0.0037 (7) |
| C3 | 0.0322 (7) | 0.0528 (10) | 0.0399 (8) | 0.0058 (6) | −0.0043 (6) | 0.0029 (7) |
| C4 | 0.0360 (8) | 0.0552 (9) | 0.0414 (9) | −0.0054 (7) | −0.0019 (7) | −0.0057 (8) |
| C5 | 0.0401 (8) | 0.0343 (7) | 0.0480 (9) | −0.0037 (6) | 0.0053 (7) | −0.0025 (7) |
| C6 | 0.0311 (8) | 0.0373 (8) | 0.0350 (8) | 0.0025 (6) | 0.0089 (6) | 0.0032 (6) |
| C7 | 0.0226 (7) | 0.0407 (8) | 0.0355 (8) | −0.0005 (5) | 0.0032 (6) | 0.0015 (5) |
| C8 | 0.0312 (7) | 0.0580 (10) | 0.0420 (8) | 0.0063 (6) | 0.0038 (6) | 0.0046 (6) |
| C9 | 0.0280 (7) | 0.0433 (8) | 0.0399 (8) | 0.0042 (5) | 0.0079 (6) | 0.0028 (6) |
| C10 | 0.0304 (5) | 0.0385 (6) | 0.0433 (6) | 0.0012 (8) | 0.0116 (5) | 0.0006 (9) |
| C11 | 0.0290 (7) | 0.0430 (8) | 0.0449 (8) | −0.0024 (5) | 0.0126 (7) | −0.0055 (6) |
| C12 | 0.0366 (8) | 0.0427 (8) | 0.0490 (9) | 0.0051 (6) | 0.0066 (6) | 0.0053 (7) |
| C13 | 0.0431 (9) | 0.0355 (7) | 0.0566 (9) | 0.0007 (6) | 0.0097 (7) | 0.0000 (7) |
| C14 | 0.0431 (9) | 0.0394 (8) | 0.0812 (13) | 0.0029 (7) | 0.0172 (9) | 0.0010 (8) |
Geometric parameters (Å, °)
| S1—O1 | 1.4831 (12) | C6—C7 | 1.396 (2) |
| S1—C1 | 1.7806 (13) | C8—C9 | 1.515 (2) |
| S1—C8 | 1.8460 (16) | C9—C10 | 1.409 (2) |
| O2—N4 | 1.224 (2) | C10—C11 | 1.393 (2) |
| O3—N4 | 1.2229 (19) | C10—C14 | 1.508 (2) |
| N1—C1 | 1.363 (2) | C11—C12 | 1.377 (2) |
| N1—C6 | 1.381 (2) | C12—C13 | 1.380 (2) |
| N2—C1 | 1.317 (2) | C2—H2 | 0.9500 |
| N2—C7 | 1.413 (2) | C3—H3 | 0.9500 |
| N3—C9 | 1.3336 (19) | C4—H4 | 0.9500 |
| N3—C13 | 1.333 (2) | C5—H5 | 0.9500 |
| N4—C11 | 1.478 (2) | C8—H81 | 0.9500 |
| N1—H1 | 0.881 (17) | C8—H82 | 0.9500 |
| C2—C3 | 1.384 (2) | C12—H12 | 0.9500 |
| C2—C7 | 1.400 (2) | C13—H13 | 0.9500 |
| C3—C4 | 1.397 (2) | C14—H141 | 0.9500 |
| C4—C5 | 1.372 (2) | C14—H142 | 0.9500 |
| C5—C6 | 1.406 (2) | C14—H143 | 0.9500 |
| O1—S1—C1 | 104.84 (7) | C9—C10—C14 | 121.46 (13) |
| O1—S1—C8 | 106.76 (7) | N4—C11—C12 | 115.39 (14) |
| C1—S1—C8 | 98.26 (6) | N4—C11—C10 | 121.95 (14) |
| C1—N1—C6 | 105.78 (12) | C10—C11—C12 | 122.67 (15) |
| C1—N2—C7 | 103.05 (12) | C11—C12—C13 | 117.33 (15) |
| C9—N3—C13 | 118.24 (14) | N3—C13—C12 | 123.01 (15) |
| O2—N4—O3 | 124.37 (15) | C3—C2—H2 | 122.00 |
| O2—N4—C11 | 118.23 (13) | C7—C2—H2 | 122.00 |
| O3—N4—C11 | 117.38 (15) | C2—C3—H3 | 119.00 |
| C1—N1—H1 | 129.6 (11) | C4—C3—H3 | 119.00 |
| C6—N1—H1 | 123.1 (11) | C3—C4—H4 | 119.00 |
| N1—C1—N2 | 115.09 (12) | C5—C4—H4 | 119.00 |
| S1—C1—N1 | 121.48 (13) | C4—C5—H5 | 122.00 |
| S1—C1—N2 | 123.35 (13) | C6—C5—H5 | 122.00 |
| C3—C2—C7 | 116.71 (14) | S1—C8—H81 | 110.00 |
| C2—C3—C4 | 121.98 (15) | S1—C8—H82 | 110.00 |
| C3—C4—C5 | 122.11 (15) | C9—C8—H81 | 110.00 |
| C4—C5—C6 | 116.34 (14) | C9—C8—H82 | 109.00 |
| C5—C6—C7 | 121.95 (14) | H81—C8—H82 | 109.00 |
| N1—C6—C5 | 131.98 (14) | C11—C12—H12 | 122.00 |
| N1—C6—C7 | 106.07 (13) | C13—C12—H12 | 121.00 |
| C2—C7—C6 | 120.90 (14) | N3—C13—H13 | 118.00 |
| N2—C7—C6 | 110.00 (13) | C12—C13—H13 | 119.00 |
| N2—C7—C2 | 129.10 (14) | C10—C14—H141 | 109.00 |
| S1—C8—C9 | 107.75 (10) | C10—C14—H142 | 110.00 |
| N3—C9—C8 | 114.20 (13) | C10—C14—H143 | 109.00 |
| N3—C9—C10 | 124.54 (14) | H141—C14—H142 | 109.00 |
| C8—C9—C10 | 121.22 (13) | H141—C14—H143 | 109.00 |
| C9—C10—C11 | 114.19 (15) | H142—C14—H143 | 109.00 |
| C11—C10—C14 | 124.31 (14) | ||
| O1—S1—C1—N1 | 31.07 (13) | C3—C2—C7—C6 | 0.4 (2) |
| O1—S1—C1—N2 | −145.56 (12) | C2—C3—C4—C5 | −1.2 (3) |
| C8—S1—C1—N1 | −78.80 (12) | C3—C4—C5—C6 | 0.9 (2) |
| C8—S1—C1—N2 | 104.57 (12) | C4—C5—C6—N1 | 179.22 (16) |
| O1—S1—C8—C9 | 51.34 (12) | C4—C5—C6—C7 | 0.0 (2) |
| C1—S1—C8—C9 | 159.64 (11) | N1—C6—C7—N2 | 0.34 (18) |
| C6—N1—C1—S1 | −177.91 (10) | N1—C6—C7—C2 | 179.94 (14) |
| C6—N1—C1—N2 | −1.02 (17) | C5—C6—C7—N2 | 179.71 (14) |
| C1—N1—C6—C5 | −178.93 (16) | C5—C6—C7—C2 | −0.7 (2) |
| C1—N1—C6—C7 | 0.35 (17) | S1—C8—C9—N3 | −99.34 (14) |
| C7—N2—C1—S1 | 178.01 (10) | S1—C8—C9—C10 | 78.74 (15) |
| C7—N2—C1—N1 | 1.18 (16) | N3—C9—C10—C11 | 1.2 (2) |
| C1—N2—C7—C2 | 179.54 (16) | N3—C9—C10—C14 | 178.94 (14) |
| C1—N2—C7—C6 | −0.90 (17) | C8—C9—C10—C11 | −176.73 (13) |
| C13—N3—C9—C8 | 176.84 (14) | C8—C9—C10—C14 | 1.1 (2) |
| C13—N3—C9—C10 | −1.2 (2) | C9—C10—C11—N4 | 179.93 (14) |
| C9—N3—C13—C12 | −0.1 (2) | C9—C10—C11—C12 | 0.1 (2) |
| O2—N4—C11—C10 | 36.3 (2) | C14—C10—C11—N4 | 2.2 (2) |
| O2—N4—C11—C12 | −143.88 (15) | C14—C10—C11—C12 | −177.62 (15) |
| O3—N4—C11—C10 | −145.52 (15) | N4—C11—C12—C13 | 178.95 (14) |
| O3—N4—C11—C12 | 34.3 (2) | C10—C11—C12—C13 | −1.2 (2) |
| C7—C2—C3—C4 | 0.5 (2) | C11—C12—C13—N3 | 1.2 (2) |
| C3—C2—C7—N2 | 179.93 (17) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1···N2i | 0.881 (17) | 2.553 (18) | 3.425 (2) | 170.5 (13) |
| C2—H2···O1ii | 0.95 | 2.33 | 3.251 (2) | 164. |
| C12—H12···O2iii | 0.95 | 2.55 | 3.164 (2) | 122. |
Symmetry codes: (i) −x+1, y+1/2, −z+1; (ii) −x+1, y−1/2, −z+1; (iii) −x−1, y+1/2, −z.
Footnotes
DRL-IPD Communication No. IPDO-IPM-00262.
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: GW2104).
References
- Arimori, K., Yasuda, K., Katsuki, H. & Nakana, M. (1998). J. Pharm. Pharmacol. 50, 1241–1245. [DOI] [PubMed]
- Barbour, L. J. (2001). J. Supramol. Chem. 1, 189–191.
- Betteridge, P. W., Carruthers, J. R., Cooper, R. I., Prout, K. & Watkin, D. J. (2003). J. Appl. Cryst. 36, 1487.
- Burla, M. C., Caliandro, R., Camalli, M., Carrozzini, B., Cascarano, G. L., De Caro, L., Giacovazzo, C., Polidori, G. & Spagna, R. (2005). J. Appl. Cryst. 38, 381–388.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Fujishima, A., Aoki, I. & Kamiyama, K. (2002). US Patent No. 6462058B1.
- Jacobson, R. (1998). REQAB Private communication to Rigaku Corporation, Tokyo, Japan.
- Kumar, K. N., Nagaraju, M., Srinivas, G., Kumar, N. U., Anitha, N., Reddy, B. S., Vishwasrao, P. S., Kumar, T. A., Reddy, P. S., Gulabrao, S. S., Ashok, S. & Varma, M. S. (2009). Patent WO 2009/117489 A1.
- Masa, K., Hamada, A., Arimori, K., Fujii, J. & Nakano, M. (2001). Biol. Pharm. Bull. 24, 274–277. [DOI] [PubMed]
- Molecular Structure Corporation & Rigaku (2006). CrystalStructure MSC, The Woodlands, Texas, USA, and Rigaku Corporation, Tokyo, Japan.
- Rigaku (2005). CrystalClear Rigaku Corporation, Tokyo, Japan.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811029990/gw2104sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811029990/gw2104Isup3.hkl
Supplementary material file. DOI: 10.1107/S1600536811029990/gw2104Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report