Abstract
Purpose
We compared visual and refractive outcomes after implantation of Visian toric implantable collamer lenses (toric ICLs) and iris-fixated toric pIOLs (toric Artisans).
Patients and methods
A comparative retrospective analysis was performed. Toric ICLs were implanted into 30 eyes of 18 patients, and toric Artisans into 31 eyes of 22 recipients. We measured the logarithms of the minimum angle of resolution of uncorrected visual acuity (logMAR UCVA), logMAR of best spectacle-corrected corrected VA (logMAR BSCVA), MR, SE, and astigmatism (by the power vector method) before surgery and 1, 3, and 6 months thereafter. Differences between patients receiving each type of lens were compared by using a mixed model of repeated measures.
Results
Visual improvements were evident after operation in both groups. By comparing the attempted to the achieved SE values, we were able to confirm that correction of refractive error was similar in both groups. However, the logMAR UCVA was significantly higher in the toric ICL group at all postoperative time points. Although manifest cylinder power and astigmatism (calculated by using the power vector method) gradually decreased in the toric ICL group, cylinder power 1 month postoperatively increased from −2.62 to −2.75 D; astigmatism was also increased at this time in the toric Artisan group.
Conclusion
The two tested toric pIOLs were similar in terms of the ability to correct refractive error, as assessed 3 months postoperatively. However toric ICLs corrected astigmatism more rapidly and safely. Notably, the large difference in astigmatism level between the two groups 1 month postoperatively indicates that toric ICLs are more effective when used to correct astigmatism.
Keywords: Visian toric implantable collamer lens, iris-fixated toric phakic intraocular lens, Verisys intraocular lens, phakic intraocular lens, correction of astigmatism
Introduction
Surgical methods correcting astigmatism, including astigmatic keratectomy,1, 2 limbal-relaxing incision,3 and excimer laser ablation, assist patients with moderate myopic or hyperopic astigmatism.4 However, postoperative regression can occur, some procedures lack predictability and reliability, and outcomes may vary according to the experience of the surgeon and the wound-healing parameters.5
The toric phakic intraocular lens (pIOL) has been introduced recently for management of astigmatism. Toric pIOL implant patients do not suffer from the disadvantages associated with corneal refractive surgery, and ametropia combined with astigmatism can be successfully corrected.6, 7, 8, 9, 10, 11 Several types of pIOLs, including angle-supported pIOLs, iris-fixated pIOLs, and posterior chamber pIOLs, are available. Recently, the latter two types of pIOLs have become increasingly popular.
The iris-fixated phakic intraocular lens (Artisan Lens; Ophtec BV, Groningen, the Netherlands) and the Visian implantable collamer lens (ICL; STAAR Surgical, Monrovia, CA, USA) have both been approved by the US Food and Drug Administration (FDA) for treatment of moderate-to-severe myopia. Recently, a toric iris-fixated lens and the Visian toric implantable collamer lens have been modified for use in the correction of spherical and astigmatic refractive errors.
No previous study has compared the efficacy and safety of toric pIOLs used to correct myopia with astigmatism. In the present work, we compared surgical outcomes in patients receiving iris-fixated toric pIOLs and Visian toric implantable collamer lenses to treat astigmatic myopia.
Patients and methods
We reviewed the medical records of 61 eyes of 40 patients who underwent toric pIOL implantation in our Department between May 2005 and March 2009. This comparative and retrospective study was approved by our Institutional Review Board. All procedures were performed by two surgeons (T-I Kim and EK Kim). Both had at least 5 years of experience with implantation of both types of pIOLs. All patients were fully briefed on the risks and details of the surgical methods employed, and provided written informed consent in accordance with the Declaration of Helsinki.
All patients were aged at least 21 years, were in good general health, had demonstrated stable refraction for at least 1 year, had astigmatism equal to or greater than 1.5 D, showed no ocular pathology, had endothelial cell counts of more than 2000 cells/mm2, showed an anterior chamber depth (ACD) of more than 3.0 mm, had a mesopic pupil size equal to or less than 6.0 mm, and did not have a convex iris configuration. Exclusion criteria were anisometropia; anterior segment pathology; presence of abnormal endothelial cells, a shallow ACD, or an abnormal iris or pupil configuration; inadequate eyelid closure; cataracts; recurrent or chronic uveitis; a history of prior corneal or intraocular surgery; glaucoma; any fundus abnormality; retinal detachment; a pre-existing macular pathology; chronic treatment with corticosteroids; pregnancy; and/or systemic disease.
We compared pre- and postoperative values, and achieved to attempted refractive outcomes. All eyes were examined preoperatively, and at 1, 3, and 6 months after surgery. Each examination included slit-lamp assessment; measurement of uncorrected visual acuity (UCVA), best spectacle-corrected visual acuity (BSCVA), logarithm of the minimum angle of resolution of UCVA (logMAR UCVA), and logMAR BSCVA; applanation tonometry; manifest refraction (MR) assessment; measurement of the spherical equivalent (SE); and indirect ophthalmoscopy. Power vector analysis was used to analyze the spherocylindrical refractive error change.12 Refractive errors were converted to power vectors suitable for display in a three-dimensional dioptric space by using a Cartesian coordinate system (M, J0, J45). Each power vector represented a spherical lens of power M, a Jackson crossed cylinder of power J0 with axes at 90 and 180 degrees, and a Jackson crossed cylinder of power J45 with axes at 45 and 135 degrees.
Surgically induced astigmatism was calculated by using the polar value method.13 In the relevant equation, the polar value KP(ϕ) denotes the net refractive power acting along the plane ϕ. Thus, a positive KP(ϕ)SIA value indicates a surgically induced increase in power, whereas a negative value reflects a surgically induced decrease in the power of the meridian. We used KP(90)SIA values to evaluate surgically induced astigmatism triggered by use of different incision sites and sizes. A positive KP(90)SIA value indicated a with-the-rule change and a negative KP(90)SIA value an against-the-rule change, with net flattening of the 90 degree corneal meridian. Astigmatism induced by the surgical procedure was calculated as the difference between the postoperative and preoperative polar values. To obtain KP(90)SIA values at 1-month postoperative follow-up visits, we subtracted KP(90)Preop from KP(90)Postop1M. KP(90)Postop3M and KP(90)Postop6M values were calculated in the same manner.
The standardized format of Koch et al14 is used to report refractive surgery results.
Surgical procedure
Toric ICL lens. A Visian toric ICL was implanted into 30 eyes. The toric ICL is a posterior chamber pIOL designed to vault anteriorly to the crystalline lens, thus minimally contacting the natural lens. The haptic design of the toric ICL is identical to that of a spherical ICL in terms of size, thickness, and configuration, and the lens has a central convex/concave optical zone and a cylinder located on an axis specific to each patient, for correction of astigmatism. All lens power calculations were performed by the STAAR Surgical Company, using the astigmatic power calculations for IOLs derived by Sarver and Sanders.15
Within 2 weeks prior to surgery, bilateral iridotomy was performed by using a neodymium:yttrium–aluminum–garnet (Nd:YAG) laser to prevent possible postoperative pupillary block glaucoma. On the day of surgery, each surgeon indicated the zero horizontal axis on a slit lamp with the patient sitting upright (to prevent possible cyclotorsion in the supine position). The surgeon used a Mendez ring to assess the extent of required rotation from the horizontal during the operation. The toric ICL was injected through a 3-mm-sized horizontal temporal corneal incision, and the haptics were located behind the iris by using a manipulator. Positioning of the toric ICL in the centre of the pupillary zone was checked before an intraocular miotic was used to decrease pupil size. Any remaining viscoelastic material (Healon; AMO, Santa Ana, CA, USA) was washed out of the anterior chamber by using a balanced salt solution.
Toric Artisan IOL. A toric Artisan IOL was implanted into 31 eyes. The toric Artisan is an iris-fixated anterior chamber lens made of Perspex CQ-UV polymethyl methacrylate, and filters ultraviolet light. Model-A, with a 0-degree torus axis, is recommended for eyes in which the preoperative cylinder axis lies between 0 and 45 degrees or 135 and 180 degrees. Model-B, with a 90-degree torus axis, is recommended for eyes in which the preoperative cylinder axis lies between 45 and 135 degrees. Both models can be suitably enclavated. The power and enclavation axis of the toric Artisan IOL were calculated based on ACD, keratometric readings, and subjective refraction error assessment, by using the Van der Heijde formula.16
Within 2 weeks prior to surgery, bilateral iridotomy was performed by using a Nd:YAG laser to prevent possible postoperative papillary block glaucoma. Miotic drops (pilocarpine; 1–2% v/v) were administered to prepare the iris for lens enclavation, and a superior sclerocorneal incision 6.1–6.3 mm in length and two paracenteses were created for all eyes. The anterior chamber was filled with viscoelastic material (Healon). After delivery of the toric Artisan IOL into the anterior chamber by using holding forceps, the lens was positioned on the desired axis and next fixated to the mid-peripheral iris stroma by using a disposable enclavation needle. After correction of alignment, the lens was enclavated onto the iris. The incision site was closed by using three-bite interrupted 10-0 nylon sutures. Within the first 2 postoperative months, the sutures were removed at times dictated by the presence of residual refraction and/or keratometric astigmatism.
Statistical methods
Changes between the two pIOL groups were compared by using a mixed model of repeated measures. Paired t-tests were employed to analyze within-group changes. Intra-class correlation coefficients (ICCs) were calculated after the 6-month follow-up to assess the difference between attempted and achieved diopter values. To compare gain or loss of BSCVA, Fischer's exact test was used. A P-value <0.05 was considered significant.
Results
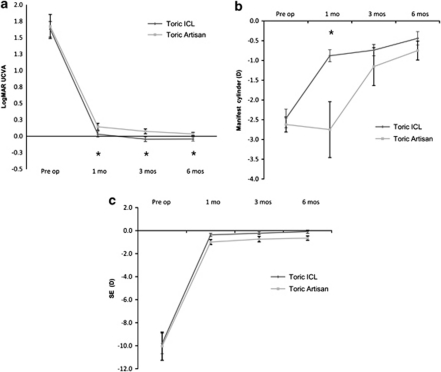
Table 1a summarizes the preoperative baseline characteristics of our patients. There were no significant differences between the two toric pIOL groups in any of preoperative logMAR UCVA, logMAR BSCVA, cylinder power, or SE values. We observed significant improvements in logMAR UCVA after surgery in both groups, but toric ICL patients showed better postoperative logMAR UCVA values at all follow-up visits (Table 1b and Figure 1a).
Table 1. Patient demographics and clinical outcomes.
| Characteristic | T-ICL | T-Artisan | P-value (CI)a |
|---|---|---|---|
| (a) Baseline demographic and clinical characteristics of the T-ICL and T-Artisan groups. | |||
| Number of eyes (OD/OS) | 30 (16/14) | 31 (13/18) | |
| Mean age: years (range) | 28.6 (21–38) | 29.0 (21–46) | |
| Gender | |||
| Male | 13 | 8 | |
| Female | 17 | 13 | |
| LogMAR UCVA | 1.67±0.33 | 1.65±0.52 | 0.940 (−0.231∼0.272) |
| LogMAR BSCVA | 0.02±0.05 | 0.03±0.06 | 0.732 (−0.021∼0.272) |
| Mean spherical equivalent (range) | −9.80±2.49 (−14.63 to −5.5) | −10.04±3.48 (−11.75 to −2.13) | 0.212 (−1.792∼1.317) |
| Mean manifest cylinder power (range) | −2.47±0.66 (−3.5 to −1.75) | −2.62±0.54 (−4.25 to −2.0) | 0.588 (−0.462∼0.154) |
| Pre-op. | Post-op. 1 month | Post-op. 3 months | Post-op. 6 months | |
|---|---|---|---|---|
| (b) Changes in visual acuity and refractive error in the T-ICL and T-Artisan groups. | ||||
| LogMAR UCVA | ||||
| T-ICL | 1.67±0.33 | 0.02±0.11 | −0.04±0.09 | −0.04±0.08 |
| T-Artisan | 1.65±0.52 | 0.13±0.15 | 0.06±0.10 | 0.03±0.06 |
| P-valuea | 0.940 | <0.001 | 0.026 | 0.021 |
| LogMAR BSCVA | ||||
| T-ICL | 0.02±0.05 | 0.02±0.08 | −0.07±0.04 | −0.09±0.03 |
| T-Artisan | 0.03±0.06 | 0.03±0.08 | −0.03±0.06 | −0.04±0.06 |
| P-valuea | 0.732 | 0.112 | 0.136 | 0.277 |
| SE | ||||
| T-ICL | −9.80±2.49 | −0.37±0.37 | −0.22±0.32 | −0.09±0.38 |
| T-Artisan | −10.04±3.48 | −1.00±0.62 | −0.75±0.62 | −0.65±0.50 |
| P-valuea | 0.212 | 0.20 | 0.266 | 0.245 |
| Manifest cylinder power | ||||
| T-ICL | −2.47±0.66 | −0.88±0.38 | −0.74±0.36 | −0.44±0.44 |
| T-Artisan | −2.62±0.54 | −2.75±1.97 | −1.17±1.29 | −0.75±0.64 |
| P-valuea | 0.588 | <0.001 | 0.162 | 0.330 |
Abbreviations: CI, 95% confidence interval; LogMAR BSCVA, logarithm of the minimum angle of resolution of best spectacle-corrected visual acuity; LogMAR UCVA, logarithm of the minimum angle of resolution of uncorrected visual acuity; op., operation; T-Artisan, iris-fixated toric phakic intraocular lens; T-ICL, Toric implantable collamer lens.
Mixed model allowing estimates of repeated measures.
Figure 1.
Mean changes in logMAR UCVA, SE, and manifest cylinder power in patients receiving the Visian toric ICL and the Iris-fixated toric pIOL (toric Artisan). The logMAR UCVA values improved in both groups, but the toric ICL group showed significantly better logMAR UCVA values at 1 month, 3 months, and 6 months after implantation (a). The manifest cylinder power improved in both groups, but a significant difference was evident between groups at the 1-month follow-up (b, P<0.001). The mean manifest cylinder power was −0.88 D in the toric ICL group and −2.75 D in the toric Artisan group at the 1-month follow-up. SE values improved in both groups, and no significant difference was evident between groups over the entire follow-up period (c). The vertical bars indicate 95% confidence intervals (*P<0.05).
SE improved in both groups (Table 1b and Figure 1c); no significant between-group difference was evident at any follow-up visit. The manifest cylinder power also improved in both groups by 6 months postoperatively (Table 1b and Figure 1b), but between-group differences were apparent. The toric ICL group attained −0.88 D at the 1-month follow-up, and this value decreased continuously thereafter. However, the toric Artisan group showed a definitive reduction in manifest cylinder power only at 3 months. Therefore, a significant difference in cylindrical power was evident only at the 1-month follow-up (Table 1b and Figure 1b).
In terms of gain or loss of BSCVA, the toric Artisan group showed a greater extent of loss of two or more lines of BSCVA at the 1-month follow-up visit (13.4% of toric Artisan patients; 4% of toric ICL patients; P=0.013), and loss of at least one line of BSCVA at all follow-up visits. The toric ICL group showed a better gain of one or more lines of BSCVA. During follow-up, the extent of gain of one or more lines of BSCVA changed from 72 to 92% in the toric ICL group and from 26.7 to 66.7% in the toric Artisan group. Improvement in gain of two or more lines of BSCVA was better in the toric ICL group at the 1- and 3-month follow-up visits (12 vs 3.3% at 1 month; 12 vs 10.7% at 3 months; 12 vs 14.8% at 6 months). The only statistically significant difference was noted at 1 month (P=0.003).
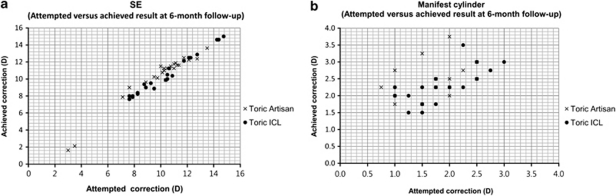
Scattergrams (Figure 2) depict predictability at the 6-month follow-up visit. The ICC of SE was 0.97 for the toric Artisan group and 0.98 for the toric ICL group. The ICC of manifest cylinder power was 0.53 for the toric Artisan group and 0.61 for the toric ICL group. These differences were not significant.
Figure 2.
Scattergrams of attempted vs achieved correction at the 6-month follow-up (a, SE; b, manifest cylinder power). The ICC of the SE was 0.98 in the toric ICL group and 0.97 in the toric Artisan group. The ICC of manifest cylinder power was 0.61 in the toric ICL group and 0.53 in the toric Artisan group.
However, in the toric Artisan group, the distribution of postoperative manifest cylinder power was wider and skewed more to larger values than was the case in the toric ICL group (Figure 3). Most manifest cylinder power values in toric ICL patients were between −1.50 and −0.25 D at the 1- and 3-month follow-up visits and between −0.25 and 0 D at the 6-month follow-up visits. Most patients in the toric Artisan group had manifest cylinder powers of more than −2.0 D at the 1-month follow-up visit, but this distribution pattern had changed by the 3-month follow-up. Most patients had manifest cylinder powers between −1.0 and 0 D at the 6-month follow-up.
Figure 3.
Distribution of manifest cylinder power 1 month and 6 months after surgery in the two groups, and distribution of manifest cylinder power in either toric pIOL implantation group after surgery.
For power vector analysis, we converted spherocylindrical refractive errors into three independent dioptric components, to allow changes in refraction caused by surgery to be calculated by simple vector subtraction. The vectors were expressed in a Cartesian coordinate system (M, J0, J45):
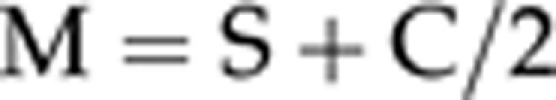 |
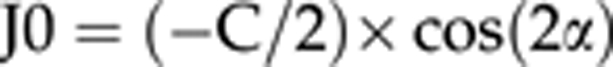 |
 |
where S is the spherical diopter value, C the cylinder diopter value, and α is an angle (in degrees).
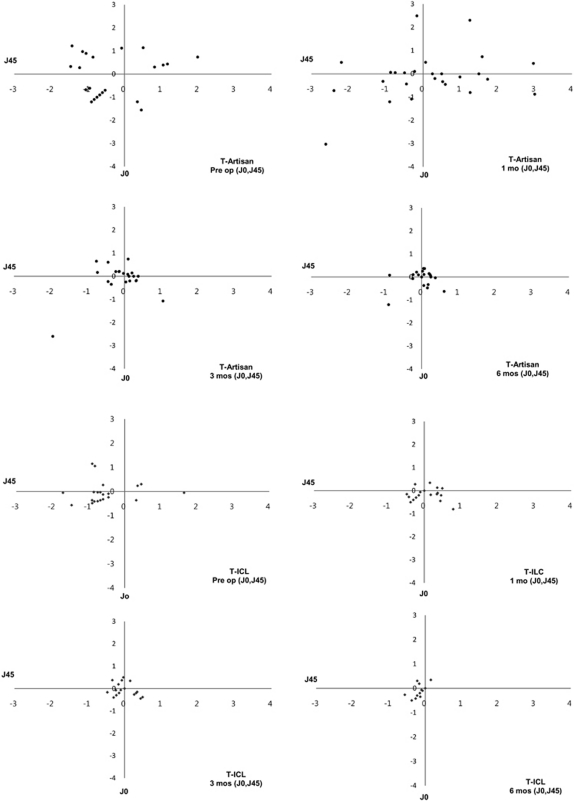
Manifest refraction is presented by using the conventional method (spherical diopter, and cylinder diopter × α). The converted cylinder values J0 and J45 reflect astigmatic components and were used to compare changes in astigmatism. The astigmatic component of the power vector was represented in a two-dimensional vector coordinate system (J0, J45), to assess changes in astigmatism. We calculated the magnitude of the astigmatism vector of each eye (J0, J45) and compared mean values between the two pIOL groups (Figure 4). The groups showed a significant difference in astigmatism at the 1-month follow-up visit. However, over time, each astigmatism vector approached zero (0, 0) in all patients of both groups. Point scattering began to close to zero after the 1-month follow-up visit in the toric ICL group and after the 3-month visit in the toric Artisan group.
Figure 4.
Distribution of the astigmatism vectors (J0, J45) of the toric Artisan and toric ICL groups using a two-dimensional vector coordinate system. The point scatter gradually approached zero, over time, in both groups. The astigmatism distribution in the toric Artisan group changed significantly between 1 month and 3 months after surgery (P<0.001); at 3 months after surgery, all points were close to the origin (0, 0). However, a significant difference was evident only between preoperative values and 1-month follow-up data in the toric ICL group (P<0.001).
The KP(90)SIA values in the toric Artisan group were 3.60±3.10, 0.22±0.76, and −0.2±0.53 D at the three follow-up visits (1, 3, and 6 months, respectively) and 0.18±0.23, 0.26±0.32, and 0.29±0.38 D in the toric ICL group. Significant changes in KP(90)SIA value were evident between every follow-up period in the toric Artisan group (P<0.001 between 1 month and 3 months; P=0.03 between 3 and 6 months). However, no significant difference in KP(90)SIA value was noted between visits in the toric ICL group. The KP(90)SIA values noted at 3 months postoperatively did not significantly differ between the two groups (P=0.43).
The safety index is defined as the ratio of mean postoperative BSCVA to mean preoperative BSCVA, and the efficacy index is the ratio of mean postoperative UCVA to mean preoperative BSCVA. The safety indices in the toric ICL group were 1.186, 1.228, and 1.278 at the three follow-up visits, and 1.003, 1.137, and 1.187 in the toric Artisan group. The efficacy indices were 1.016, 1.163, and 1.155 in the toric ICL group, and 0.827, 0.938, and 1.009 in the toric Artisan group. Both safety and efficacy index values were somewhat higher at all postoperative visits in the toric ICL group, but this was not statistically significant.
No patient experienced any pIOL-related complications, such as severe endothelial cell loss, a cataractous change, rotation of the pIOL, elevation of intraocular pressure, or inflammation, during follow-up.
Discussion
Correction of astigmatism is a current challenge in refractive ocular surgery. Laser surgery is limited in terms of correction of high myopia and high astigmatism,17, 18 and is also associated with the complications common to keratorefractive procedures (corneal ectasia, regression, poor quality of dark vision), likely caused by changes in corneal contours.19, 20
Toric pIOL implantation is not associated with such complications, and can correct high astigmatism regardless of corneal thickness. Several studies have found that the toric Artisan lens provides safe, effective, and reliable correction of high myopia and astigmatism.6, 10, 21 Alió et al7 previously described the ability of the toric Artisan lens to correct astigmatism and concluded that this approach offered a significant advantage over corneal refractive surgery when a high degree of astigmatism was present. A 6-month multicentre clinical trial in Europe demonstrated that the Artisan toric pIOL produced stable refractive effects, and predictably and effectively reduced astigmatism.6
A US FDA study of the toric ICL reported that use of this lens is a reliable, predictable, and effective option for correction of moderate-to-high myopic astigmatism.11 Another study, based on the US FDA data, reported similar visual and refractive outcomes, and predictabilities, of toric ICLs implanted in Asian and non-Asian populations.22 Schallhorn et al23 found that toric ICLs were better than was photorefractive keratectomy in terms of stability, predictability, safety, and efficacy.
Although several previous reports have compared visual outcomes between patients receiving ICL or Artisan implantation, and keratorefractive surgeries, the present study is the first to compare the toric ICL and toric Artisan lenses. We found no significant between-group difference in logMAR BSCVA at 1, 3, or 6 months after surgery. However, toric ICL patients experienced better outcomes in terms of logMAR UCVA at all postoperative follow-up visits. We are of the view that residual astigmatism might influence the statistically significant differences observed in logMAR UCVA. In other words, it is possible that a between-lens difference in astigmatism correction ability affected the logMAR UCVA values.
The toric ICL lens was associated with better outcomes at 1-month follow-up visits when cylinder power and vector astigmatism analysis were assessed (Figures 1c and 4). By contrast, the manifest cylinder power and astigmatism vector analysis of toric Artisan data 1 month after operation indicated an increase in the level of astigmatism, which we consider to be attributable to the incision length. Distribution analysis of manifest cylinder power also showed that the toric ICL lens afforded superior correction. After the 3-month follow-up visits, manifest cylinder power and astigmatism values stabilized.
We used ICC to evaluate the match between attempted and achieved correction at the 6-month follow-up visits. The two groups showed similar ICC values in terms of SE predictability, indicating that the SE correction ability of the two lenses was similar. Although the ICCs for predictability of cylinder power were low (0.53 in the toric Artisan group and 0.61 in the toric ICL group), the toric ICL group experienced superior outcomes in terms of predictability of cylinder power at the 6-month follow-up visits. The between-group differences in efficacy and safety indices were not statistically significant.
Vision stability after pIOL insertion is important. Despite the BSCVA decrease in both groups 1 month after pIOL insertion, almost all patients showed full recovery of BSCVA (one eye was an exception) after 6 months. As the pattern of BSCVA change indicates not only the extent of recovery after operation, but also the durability of the procedure per se, our results indicate that toric ICL patients experienced a faster recovery time and a greater operative stability than did the toric Artisan patients. Six months after pIOL insertion, only one eye in the toric Artisan group showed a BSCVA decrease of one line. The preoperative BSCVA of 20/16 became 20/20, which was not a negative outcome in terms of postoperative visual acuity, despite the fact that a recovery time of 6 months was insufficient.
Furthermore, no patient in either group had a BSCVA less than 20/20 at 6 months after operation. This shows that the two groups experienced similar levels of vision stability 6 months after lens insertion.
In comparing the two surgical methods it is necessary to consider incision location and size. The toric Artisan and toric ICL patients differed in terms of location and size; these factors can influence the extent of surgically induced astigmatism. In the present study, the toric Artisan patients showed astigmatism of 3.60±3.10 D KP(90)SIA at 1 month postoperatively. This value was sufficiently large to affect postoperative astigmatism and to decrease visual quality. Between-group differences in manifest cylinder power and the extent of astigmatism might also be affected by the nature of the surgical incision. Such factors can confuse the interpretation of surgical results. However, the most remarkable outcome was the change in the pattern of KP (90)SIA values from 3 months postoperatively. As mentioned above, a KP(90)SIA value measures the net refractive power acting along the axis of a plane at 90 degrees. Although KP(90)SIA was positive at all follow-up visits in patients in the toric ICL group, the variation in KP(90)SIA levels was not large in comparison with those of the toric Artisan group. This indicates that astigmatism induced in toric ICL patients was stable. When the two groups were compared, the KP(90)Postop3M values showed no statistically significant difference, whereas the KP(90)Postop6M value in the toric Artisan group was negative, indicating a flattening effect of the 90 degree axis. All patients in the toric Artisan group had with-the-rule astigmatism before surgery (average steep axis: 90.86±9.82 degrees in the toric Artisan group; 92.37±7.81 degrees in the toric ICL group). Therefore, the flattening effect of surgically induced astigmatism at 6 months of follow-up positively influenced the surgical results in the toric Artisan group. Obviously, longer postoperative follow-up is necessary to accurately evaluate the stability of surgically induced astigmatism. However, one can exclude the effect of incision location and size to some extent by calculating the level of surgically induced astigmatism. Such estimates revealed that the effect of incision location on induced astigmatism was not large; this was apparent when astigmatism levels in the two pIOL groups were compared 3 months after operation.
Based on our present results, we conclude that the two toric pIOLs are of similar effectiveness when correction of refractive error and astigmatism are assessed after 3 months. However, use of the toric ICL was associated with rapid clinical improvement and was superior in terms of predictability and safety. Although surgically induced astigmatism caused by incision was more effective in the toric Artisan group in terms of astigmatism correction, the better results in terms of cylinder power, as revealed by vector analysis, of the toric ICL group, indicate that the toric ICL better corrects astigmatism. Not only the difference in the extent of astigmatism correction 1 month after operation, but also the superior ability to correct astigmatism and the fast recovery, will greatly influence patient decision-making towards use of a toric ICL.

Acknowledgments
This work was partially supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST No. 2010-0022006) and Converging Research Center Program through the NRF funded by the Ministry of Education, Science, and Technology (2010K001134).
The authors declare no conflict of interest.
References
- Price FW, Grene RB, Marks RG, Gonzales JS. Astigmatism reduction clinical trial: a multicenter prospective evaluation of the predictability of arcuate keratotomy. Evaluation of surgical nomogram predictability. ARC-T Study Group. Arch Ophthalmol. 1995;113:277–282. doi: 10.1001/archopht.1995.01100030031017. [DOI] [PubMed] [Google Scholar]
- Kwitko ML, Jovkar S, Yan H, Rymer S. Arcuate keratotomy to correct naturally occurring astigmatism. J Cataract Refract Surg. 1996;22:1439–1442. doi: 10.1016/s0886-3350(96)80144-1. [DOI] [PubMed] [Google Scholar]
- Müller-Jensen K, Fischer P, Siepe U. Sutureless corneal cataract surgery. Limbal release incisions for correcting astigmatism. Ophthalmologe. 1999;96:432–436. doi: 10.1007/s003470050432. [DOI] [PubMed] [Google Scholar]
- Shah S. Photoastigmatic refractive keratectomy—the cure for astigmatism. Ophthalmology. 1999;106:2045–2046. doi: 10.1016/S0161-6420(99)90481-5. [DOI] [PubMed] [Google Scholar]
- Durrie DS, Lesher MP, Cavanaugh TB. Classification of variable clinical response after photorefractive keratectomy for myopia. J Refract Surg. 1995;11:339–340. doi: 10.3928/1081-597X-19950901-10. [DOI] [PubMed] [Google Scholar]
- Dick HB, Alió J, Bianchetti M, Budo C, Christiaans BJ, El-Danasoury MA, et al. Toric phakic intraocular lens. European multicenter study. Ophthalmology. 2003;110:150–162. doi: 10.1016/s0161-6420(02)01447-1. [DOI] [PubMed] [Google Scholar]
- Alió JL, Galal A, Mulet ME. Surgical correction of high degrees of astigmatism with a phakic toric-iris claw intraocularlens. Int Ophthalmol Clin. 2003;43:171–181. doi: 10.1097/00004397-200343030-00016. [DOI] [PubMed] [Google Scholar]
- Bartels MC, van Rij G, Luyten GP. Implantation of a toric phakic intraocular lens to correct high corneal astigmatism in a patient with bilateral marginal corneal degeneration. J Cataract Refract Surg. 2004;30:499–502. doi: 10.1016/j.jcrs.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Bartels MC, Santana NT, Budo C, van Rij G, Mulder PG, Luyten GP. Toric phakic intraocular lens for the correction of hyperopia and astigmatism. J Cataract Refract Surg. 2006;32:243–249. doi: 10.1016/j.jcrs.2005.12.083. [DOI] [PubMed] [Google Scholar]
- Tehrani M, Dick HB. Iris-fixated toric phakic intraocular lens: three-year follow-up. J Cataract Refract Surg. 2006;32:1301–1306. doi: 10.1016/j.jcrs.2006.02.058. [DOI] [PubMed] [Google Scholar]
- Sanders DR, Schneider D, Martin R, Brown D, Dulaney D, Vukich J, et al. Toric Implantable Collamer Lens for moderate to high myopic astigmatism. Ophthalmology. 2007;114:54–61. doi: 10.1016/j.ophtha.2006.08.049. [DOI] [PubMed] [Google Scholar]
- Thibos LN, Horner D. Power vector analysis of the optical outcome of refractive surgery. J Cataract Refract Surg. 2001;27:80–85. doi: 10.1016/s0886-3350(00)00797-5. [DOI] [PubMed] [Google Scholar]
- Naeser K. Assessment and statistics of surgically induced astigmatism. Acta Ophthalmol. 2008;86 (Suppl 1:5–28. doi: 10.1111/j.1755-3768.2008.01234.x. [DOI] [PubMed] [Google Scholar]
- Koch DD, Kohnen T, Obstbaum SA, Rosen ES. Format for reporting refractive surgical data. J Cataract Refract Surg. 1998;24:285–287. doi: 10.1016/s0886-3350(98)80305-2. [DOI] [PubMed] [Google Scholar]
- Sarver EJ, Sanders DR. Astigmatic power calculations for intraocular lenses in the phakic and aphakic eye. J Refract Surg. 2004;20:472–477. doi: 10.3928/1081-597X-20040901-10. [DOI] [PubMed] [Google Scholar]
- Van der Heijde GL, Fechner PU, Worst JG. Optical consequences of implantation of a negative intraocular lens in myopic patients. Klin Monatsbl Augenheilkd. 1988;193:99–102. doi: 10.1055/s-2008-1050231. [DOI] [PubMed] [Google Scholar]
- Danjoux JP, Fraenkel G, Lawless MA, Rogers C. Treatment of myopic astigmatism with the Summit Apex Plus excimer laser. J Cataract Refract Surg. 1997;23:1472–1479. doi: 10.1016/s0886-3350(97)80017-x. [DOI] [PubMed] [Google Scholar]
- Chavez S, Chayet A, Celikkol L, Parker J, Celikkol G, Feldman ST. Analysis of astigmatic keratotomy with a 5.0-mm optical clear zone. Am J Ophthalmol. 1996;121:65–76. doi: 10.1016/s0002-9394(14)70535-5. [DOI] [PubMed] [Google Scholar]
- Seiler T, Koufala K, Richter G. Iatrogenic keratectasia after laser in situ keratomileusis. J Refract Surg. 1998;14:312–317. doi: 10.3928/1081-597X-19980501-15. [DOI] [PubMed] [Google Scholar]
- Seiler T, Holschbach A, Derse M, Jean B, Genith U. Complications of myopic photorefractive keratectomy with the excimer laser. Ophthalmology. 1994;101:153–160. doi: 10.1016/s0161-6420(94)31371-6. [DOI] [PubMed] [Google Scholar]
- Güell JL, Vázquez M, Malecaze F, Manero F, Gris O, Velasco F, et al. Artisan toric phakic intraocular lens for the correction of high astigmatism. Am J Ophthalmol. 2003;136:442–447. doi: 10.1016/s0002-9394(03)00295-2. [DOI] [PubMed] [Google Scholar]
- Chang J, Lau S. Toric implantable collamer lens for high myopic astigmatic Asian eyes. Ophthalmology. 2009;116:2340–2347. doi: 10.1016/j.ophtha.2009.04.053. [DOI] [PubMed] [Google Scholar]
- Schallhorn S, Tanzer D, Sanders DR, Sanders ML. Randomized prospective comparison of visian toric implantable collamer lens and conventional photorefractive keratectomy for moderate to high myopic astigmatism. J Refract Surg. 2007;23:853–867. doi: 10.3928/1081-597X-20071101-01. [DOI] [PubMed] [Google Scholar]