Abstract
Purpose
The recently published seminal dry eye workshop proceedings defined Lissamine Green (LG), an organic dye, as a gold standard for demonstrating ocular surface staining. The purpose of the current study was to determine the optimal parameters of 1% LG instillation for the ocular surface examination in dry eye patients.
Design
Prospective and observational quality improvement study.
Methods
A quality improvement study evaluated 16 eyes from eight dry eye patients with different levels of severity. LG (1%), in three volumes (5, 10, and 20 μl) was instilled into the conjunctival cul-de-sac, and four masked observers with different levels of clinical expertise examined the patients with and without red filter. The staining pattern of the conjunctiva and cornea was documented with the Oxford scale within 4 min of LG instillation. Optimal volume and inter-observer reliability were assessed.
Results
All dye volumes were tolerated well by all patients. Experienced observers preferred 10 μl volume because of the ease of examination and accuracy. Although instillation of 20 μl yielded similar scores as 10 μl, it resulted in overflow of the lid and facial skin staining. The use of red filter significantly improved reading scores (P<0.01). Inter-observer reliability was higher for conjunctival scores than for corneal scores for all patients. The highest reliability was demonstrated with 10 μl volume and increased with greater experience of the observer.
Conclusions
Ocular surface examination with instillation of 10 μl 1% LG has good inter-observer reliability and is well tolerated. Observation through a red filter facilitates the examination.
Keywords: Lissamine Green, corneal staining, dry eye, ocular surface, conjunctiva, vital dye
Introduction
Lissamine Green (LG) B is a synthetically produced organic acid dye with two aminophenyl groups. Synonyms include acid green S, wool green S or C, and fast light green.1 Previous carcinogenicity and toxicity studies have been shown to be unremarkable and have demonstrated an excellent safety profile.1 The staining profile of LG has been demonstrated to be comparable to that of Rose Bengal. However, LG is less irritating and is better tolerated by patients.1, 2, 3
Lissamine Green stains ocular surface epithelial cells that are unprotected by mucin or glycocalyx, as well as cells that have been damaged. However, unlike Rose Bengal, it does not inhibit viral replication in vivo.1, 3, 4, 5 In addition, although Rose Bengal stains both proliferating corneal epithelial cells and affects their viability,6 LG does not. Given the better patient tolerance and non-toxic effect of LG, it has been recognized as a better dye than Rose Bengal in evaluating ocular surface disorders, and has been shown to be highly sensitive and specific.7, 8 Ocular surface staining is a critical variable in clinical trials for evaluation of dry eye disease. As new modalities for treatment of dry eye disease are being introduced, evaluation of the ocular surface is critical, as surface staining is an important endpoint, reflecting ocular surface integrity.9, 10 Lissamine Green is now being increasingly used to assess conjunctival staining for these trials.9 Unlike Rose Bengal staining, which is recommended to be used in small volumes (25 μl of a 1% solution),9 the application parameters and the method of evaluation for LG have, to date, neither been validated nor standardized. The purpose of this study was to determine the optimal parameters for ocular surface staining by 1% LG in dry eye disease in order to improve quality of care.
Materials and methods
This prospective study was performed to compare different volumes of 1% LG dye in patients with various degrees of dry eye disease. Further, the effect of using a red filter in observing the ocular surface of dry eye patients with different volumes of 1% LG dye was evaluated.
Sixteen eyes from eight subjects were evaluated in this study. The age and gender demographics are summarized in Table 1. All patients were Caucasians. Twelve eyes had mild to moderate dry eye disease, and four eyes suffered from severe dry eye. The diagnosis of dry eye and the degree of severity were made on previous visits by the attending cornea specialist, based on the following criteria: Schirmer's test without anesthesia: mild <10 mm; moderate <7 mm; severe <5 mm; tear break-up time (TBUT): mild <10 mm; moderate <7 mm; severe <5 mm; corneal staining with fluorescein: mild (none to 5 microdots); moderate (6–15 microdots); severe (greater than 16 microdots or 1 or more macrodots). The results must have been noted on at least two clinic visits.
Table 1. Demographic data.
| Mild to moderate | Severe | Total | |
|---|---|---|---|
| Gender | |||
| Female | 4 | 2 | 6 |
| Male | 2 | 0 | 2 |
| Age, mean (range) (in years) | 70.33 (65–82) | 54 (44–64) | 66.25 (44–82) |
| Diagnosis (number) | Aqueous tear deficiency (6) | Sjögren's syndrome (2) | Sjögren's syndrome (2) Aqueous tear deficiency (6) |
All subjects signed an informed consent after explanation of the details of the study and before enrollment in the study. The ocular surface staining in these subjects was evaluated sequentially after application of one of the three different volumes of topical 1% LG (Leiter's Pharmacy, San Jose, CA, USA) stain (5, 10, and 20 μl) with pipette in the lower conjunctival cul-de-sac, in order to compare scoring patterns of conjunctival and corneal staining, as well as inter-observer variability. Four observers, with varying degrees of clinical experience (one cornea attending, one senior ophthalmology resident, and two junior ophthalmology residents), examined the cornea and conjunctiva of all patients with each volume of LG applied. Staining scores were recorded according to the Oxford scheme,9, 10 evaluating the cornea, temporal, and nasal conjunctiva, separately in each case.
Examination started 2 min after the application of dye. The degree of LG staining fades variably after 4 min, such that reading between 2 and 4 min is optimal.11 Each observer was unaware of other examiners' results and was masked to the volume of dye used in each examination. After instillation of dye at a given volume, each observer, in turn, examined the patient and scored staining using white light and then a Hoya 25A red barrier filter (Tokina Co., Ltd, Tokyo, Japan) during slit-lamp biomicroscopy illumination with white light. Visualization of the ocular surface staining by LG can be enhanced by the use of a red transmitting filter that highlights the absorption of LG (634–567 nm wavelength). Following instillation of a given volume, and after all four observers had scored staining, eyes were washed with normal saline solution and 5 min was allowed for fading of previous staining and the next volume of dye was instilled. The observer sequence remained the same for each volume. Further, after each exam, observers rated the relative ease of examination, according to the staining pattern. The scoring of the attending cornea physician with 10 μl volume was considered gold standard and other readings were compared with this, based on the initial data analysis on agreement, 10 μl seemed to be the most accurate one (underreporting with 5 μl and pooling and overestimation with 20 μl).
SPSS 16 software (SPSS, Chicago, IL, USA) was used to analyze the data. To compare mean of readings between two groups, Student's t-test was used. In order to compare three volume groups, analysis of variance (ANOVA) was used. For agreement between the cornea specialist and other observers, Spearman's correlation coefficient was used. P-values of <0.05 were considered statistically significant. Simple κ statistics were calculated to describe the inter-observer reliability. κ Statistics were calculated for each area individually with and without scoring with red filter. The κ statistic is a measure of agreement between multiple raters, and represents the percent agreement among the raters beyond the agreement that would happen by chance alone. We certify that all applicable institutional regulations concerning the ethical use of human volunteers were followed during this quality improvement study. The study was HIPAA-compliant and adhered to the tenets of the Declaration of Helsinki.
Results
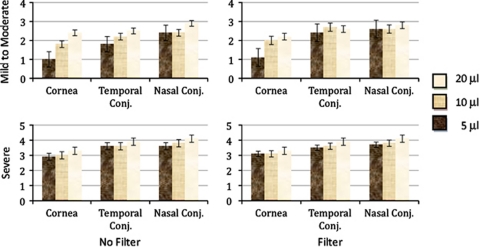
Demographic data are shown in Table 1. The mean of readings of all observers, based on the severity, use of the red filter, and region examined is shown in Figure 1. The readings show a significantly increasing score by increasing volume and use of red filter. Overall, use of red barrier filter increased the mean reading scores from 2.53 to 2.84, which was statistically significant (P<0.01). Comparing reading scores for each observer individually, red filter use improved readings by more experienced observers (1 and 2) significantly (P=0.02 for both observers) and closer to the assessment by the cornea specialist, but not those of less experienced readers 3 and 4 (P=0.23 and P=0.09, respectively). Stratifying the effect of red filter on readings by applied volumes demonstrated that red filter significantly increased mean reading scores for 5 and 10 μl volumes, but not for 20 μl. Mean (SD) reading scores with and without filter were 2.16 (1.38) and 2.60 (1.29), respectively, (P-value=0.01) for 5 μl, 2.53 (1.28), and 2.89 (1.00) (P-value=0.01) for 10 μl, and 2.99 (0.98) and 3.03 (0.98) (P-value=0.69) for 20 μl LG application. Observers commented that the red filter was valuable in improving and facilitating the detection of corneal staining with LG.
Figure 1.
Mean and SE bars of readings for different volumes and severity of dry eye disease, pooled from all four observers, using filter and regions subgroups.
The correlations between the cornea specialist and all other observers in different anatomical regions and volumes for the mild to moderate dry eye group are shown in Table 2. Table 3 shows the same findings for the severe dry eye group. As there was no significant difference between temporal and nasal conjunctival readings for all observers, the results of conjunctival readings have been pooled. As these tables demonstrate, correlations between other observers and the cornea specialist decreased from observer 1 to observer 4. The exception is for the 20 μl volume, in which observer 4 shows better correlation than observers 2 and 3 in scoring the conjunctival regions for mild to moderate group only. Interestingly, for the severe dry eye group, most of the correlations were statistically significant. Nonsignificant correlations were mainly in the corneal region and for observer 4. Overall for all patients, using 5, 10, or 20 μl volumes, correlation in corneal reading was less than conjunctival regions. All observers commented that the 5 μl volume had resulted in faint staining, making it difficult to appropriately score that staining. Further, they had noted that pooling, which usually occurred after use of 20 μl volume, was a potential source of confounding error in scoring. In addition, κ statistics demonstrated that the overall agreement of all regions between multiple observers was comparable with 5 (κ of 0.35), 10 (κ of 0.3), or 20 μl (κ of 0.36) volumes. The use of filter (κ of 0.32) vs no filter (κ of 0.36) did not change this agreement significantly.
Table 2. Mild to moderate dry eye group: correlations (r (P-value)) between the assessment of the cornea specialist and observers in different volumes and regions.
| Volume |
Cornea |
Conjunctiva |
||||
|---|---|---|---|---|---|---|
| 5 μl | 10 μl | 20 μl | 5 μl | 10 μl | 20 μl | |
| Observer 1 | 0.86 (<0.01) | 1.00 | 0.96 (<0.01) | 0.75 (<0.01) | 1.00 | 0.78 (<0.01) |
| Observer 2 | 0.77 (<0.01) | 0.89 (<0.01) | 0.77 (<0.01) | 0.60 (<0.01) | 0.87 (<0.01) | 0.58 (<0.01) |
| Observer 3 | 0.76 (<0.01) | 0.72 (<0.01) | 0.65 (0.02) | 0.75 (<0.01) | 0.50 (<0.01) | 0.18 (0.35) |
| Observer 4 | 0.52 (0.07) | 0.76 (<0.01) | 0.89 (<0.01) | 0.68 (<0.01) | 0.38 (0.04) | 0.62 (<0.01) |
Italic values indicate significant correlations.
Table 3. Severe dry eye group: correlations (r (P-value)) between the assessment of the cornea specialist and observers in different volumes and regions.
| Volume |
Cornea |
Conjunctiva |
||||
|---|---|---|---|---|---|---|
| 5 μl | 10 μl | 20 μl | 5 μl | 10 μl | 20 μl | |
| Observer 1 | 0.35 (0.40) | 1.00 | 0.26 (0.54) | 0.99 (<0.01) | 1.00 | 0.77 (<0.01) |
| Observer 2 | 0.81 (0.02) | 0.89 (<0.01) | 0.89 (<0.01) | 0.56 (<0.01) | 0.77 (<0.01) | 0.54 (0.01) |
| Observer 3 | 0.32 (0.45) | 0.78 (0.02) | 0.89 (<0.01) | 0.41 (0.04) | 0.66 (<0.01) | 0.73 (<0.01) |
| Observer 4 | 0.25 (0.55) | 0.10 (0.81) | 0.32 (0.45) | 0.53 (0.01) | 0.37 (0.07) | 0.45 (0.03) |
Italic values indicate significant correlations.
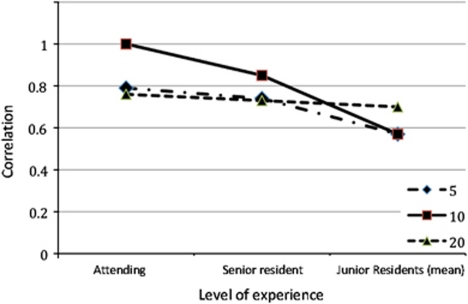
In summary, as shown in Figure 2, best results were obtained with 10 μl volume, demonstrating the highest correlation between observers and the attending physician. However, with decrease in experience, there was decrease in correlation between scores. Although the results for 20 μl seemed more uniform between all observers, regardless of level of experience, the staining scores were likely overreported. Early observation (at 2 min) and using a volume of 20 μl could be confounded by residual pooling that made the evaluation more difficult, particularly in patients who had prior punctal occlusion.
Figure 2.
Comparing correlations with the assessment of the cornea specialist based on observers' experience level. Summed (corneal/conjunctival) scores are compared, for different volume instillations between observers and the cornea specialist.
Discussion
According to the International Dry Eye Workshop, the definition for dry eye is ‘a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface'.12 Lissamine Green staining of the conjunctiva complements fluorescein staining of the cornea and is the method of choice for examining conjunctival staining in some countries.9 The application of ocular surface staining dates back to 1882.13 As the search for new treatment modalities intensifies for ocular surface disorders,14, 15, 16, 17 the stringent evaluation of endpoints (eg, conjunctival staining) has gained importance. Using a standard protocol for tests, including application of known quantities for LG solution, may produce more reliable staining scores.
In order to determine the optimal parameters for a test, it is important to ascertain that inter-observer reliability and variability have been studied. Inter-observer reliability should not be considered the same as repeatability (intra-observer agreement), which means stability of staining pattern in consecutive examination sessions by the same observer.18 Although intra-observer studies are more complex, because of the requirement of a return visit, they do provide a statement about the precision of the method. A limitation of the current study is that patients were not re-assessed on a return visit and thus, intra-observer agreement could not be analyzed. Nichols et al19 reported repeatability of corneal and conjunctival staining, with weighted κ of 0.69 and 0.33 for fluorescein and Rose Bengal staining, respectively.9 In their study, repeatability of inferior corneal fluorescein staining (κ=0.25), and inferior conjunctival Rose Bengal staining (κ=0.21) were poor.19 Although many details of ocular surface examination—especially in dry eye—have been addressed in the International Dry Eye Workshop,9, 12, 18, 20 the specific and optimal parameters for LG application were yet to be determined. To the best of our knowledge, this is the first quality improvement study evaluating the inter-observer reliability in the literature. This study was designed to determine the optimal volume of instilled LG dye, by studying variation between observers with different levels of clinical expertise.
Use of a red filter significantly improved readings with increased staining scores. This finding is further supported by the subjective opinion of observers with respect to perceived ease of assessment. However, owing to the fact that red filter assessments were performed immediately after white light assessments, a bias may have been created by the expectation of a higher score or even by the recollection of the previous scores that were obtained by white light. Although increased scores were obtained with the red filter, this increase was only significant for the more experienced observers. The results further demonstrate that conjunctival readings (both nasal and temporal) showed significant correlation with the assessment of the cornea specialist for all volumes. Overall, best results were obtained with 10 μl volume. Although more experienced observers (observers 1 and 2) had the highest correlation with 10 μl, scoring with 5 μl was comparable. However, the least experienced observer (observer 4) had inconsistent results and had better readings with 20 μl volume. Unlike conjunctival readings, corneal readings showed significantly less correlation, regardless of the volume used, which could, at least in part, relate to the influence of iris color on contrast and hence detection. It is likely that fluorescein might yield less variability between observers than LG for corneal staining in dry eye patients. However, a previous study did show low repeatability for corneal fluorescein staining.1
In conclusion, on the basis of this quality improvement study, we recommend a volume of 10 μl as the optimally instilled volume of 1% LG for ocular surface examination in dry eye patients. Although we acknowledge that the number of patients in this study was limited, the comparison of staining scores with different volumes in each patient in the same session has decreased the fluctuations that exist between patient visits. In addition, the range of observers with varying clinical experience was very important, confirming that the staining scores with 10 μl lead to the highest overall correlation with an experienced corneal specialist. Studying repeatability with a larger sample size and a single observer in the future, comparing LG with other dyes directly would aid in refined selection of dyes for scoring surface staining.

Acknowledgments
Financial support was provided by NIH K08-EY020575 (PH), New England Corneal Transplant Research Fund (PH) and RPB Department Grant (GF).
The authors declare no conflict of interest.
References
- Kim J. The use of vital dyes in corneal disease. Curr Opin Ophthalmol. 2000;11:241–247. doi: 10.1097/00055735-200008000-00005. [DOI] [PubMed] [Google Scholar]
- Chodosh J, Dix RD, Howell RC, Stroop WG, Tseng SC. Staining characteristics and antiviral activity of sulforhodamine B and lissamine green B. Invest Ophthalmol Vis Sci. 1994;35:1046–1058. [PubMed] [Google Scholar]
- Manning FJ, Wehrly SR, Foulks GN. Patient tolerance and ocular surface staining characteristics of lissamine green versus rose bengal. Ophthalmology. 1995;102:1953–1957. doi: 10.1016/s0161-6420(95)30769-5. [DOI] [PubMed] [Google Scholar]
- Brooks SE, Kaza V, Nakamura T, Trousdale MD. Photoinactivation of herpes simplex virus by rose bengal and fluorescein. In vitro and in vivo studies. Cornea. 1994;13:43–50. doi: 10.1097/00003226-199401000-00008. [DOI] [PubMed] [Google Scholar]
- Feenstra RP, Tseng SC. What is actually stained by rose bengal. Arch Ophthalmol. 1992;110:984–993. doi: 10.1001/archopht.1992.01080190090035. [DOI] [PubMed] [Google Scholar]
- Lee YC, Park CK, Kim MS, Kim JH. In vitro study for staining and toxicity of rose bengal on cultured bovine corneal endothelial cells. Cornea. 1996;15:376–385. doi: 10.1097/00003226-199607000-00008. [DOI] [PubMed] [Google Scholar]
- Lemp MA, Bron AJ, Baudouin C, Del Castillo JM, Geffen D, Tauber J, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;1 (5:792–798.e1. doi: 10.1016/j.ajo.2010.10.032. [DOI] [PubMed] [Google Scholar]
- Versura P, Frigato M, Cellini M, Mule R, Malavolta N, Campos EC. Diagnostic performance of tear function tests in Sjogren′s syndrome patients. Eye (Lond) 2007;21 (2:229–237. doi: 10.1038/sj.eye.6702204. [DOI] [PubMed] [Google Scholar]
- Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye Workshop (2007) Ocul Surf. 2007;5:108–152. doi: 10.1016/s1542-0124(12)70083-6. [DOI] [PubMed] [Google Scholar]
- Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–650. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- Foulks GN. Challenges and pitfalls in clinical trials of therapy of dry eye. Ocul Surf. 2003;1:20–30. doi: 10.1016/s1542-0124(12)70004-6. [DOI] [PubMed] [Google Scholar]
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007) Ocul Surf. 2007. pp. 575–592. [DOI] [PubMed]
- Conn HJ. Biological Stains. Williams & Wilkins, Co.: Baltimore; 1961. [Google Scholar]
- Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126:219–225. doi: 10.1001/archophthalmol.2007.61. [DOI] [PubMed] [Google Scholar]
- Weber J, Keating GM. Cevimeline. Drugs. 2008;68:1691–1698. doi: 10.2165/00003495-200868120-00006. [DOI] [PubMed] [Google Scholar]
- Peral A, Dominguez-Godinez CO, Carracedo G, Pintor J. Therapeutic targets in dry eye syndrome. Drug News Perspect. 2008;21:166–176. [PubMed] [Google Scholar]
- Pinheiro MN, Jr, dos Santos PM, dos Santos RC, Barros Jde N, Passos LF, Cardoso Neto J. Oral flaxseed oil (Linumusitatissimum) in the treatment for dry-eye Sjögren′s syndrome patients. Arq Bras Oftalmol. 2007;70:649–655. doi: 10.1590/s0004-27492007000400016. [DOI] [PubMed] [Google Scholar]
- Research in dry eye: report of the Research Subcommittee of the International Dry Eye Workshop (2007) Ocul Surf. 2007;5:179–193. doi: 10.1016/s1542-0124(12)70086-1. [DOI] [PubMed] [Google Scholar]
- Nichols KK, Mitchell GL, Zadnik K. The repeatability of clinical measurements of dry eye. Cornea. 2004;23:272–285. doi: 10.1097/00003226-200404000-00010. [DOI] [PubMed] [Google Scholar]
- Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye Workshop (2007) Ocul Surf. 2007;5:163–178. doi: 10.1016/s1542-0124(12)70085-x. [DOI] [PubMed] [Google Scholar]