Abstract
Virions of the type 1 human immunodeficiency virus (HIV-1) can enter target cells by fusion or endocytosis, with sharply different functional consequences. Fusion promotes productive infection of the target cell, while endocytosis generally leads to virion inactivation in acidified endosomes or degradation in lysosomes. Virion fusion and endocytosis occur equally in T cells, but these pathways have been regarded as independent because endocytosis of HIV virions requires neither CD4 nor CCR5/CXCR4 engagement in HeLa-CD4 cells. Using flow cytometric techniques to assess the binding and entry of green fluorescent protein (GFP)-Vpr-labeled HIV virions into primary peripheral blood mononuclear cells, we have found that HIV fusion and endocytosis are restricted to the CD4-expressing subset of cells and that both pathways commonly require the initial binding of HIV virions to surface CD4 receptors. Blockade of CXCR4-tropic HIV virion fusion with AMD3100, a CXCR4-specific entry inhibitor, increased virion entry via the endocytic pathway. Similarly, inhibition of endosome acidification with bafilomycin A1, concanamycin A, or NH4Cl enhanced entry via the fusion pathway. Although fusion remained dependent on CD4 and chemokine receptor binding, the endosome inhibitors did not alter surface expression of CD4 and CXCR4. These results suggest that fusion in the presence of the endosome inhibitors likely occurs within nonacidified endosomes. However, the ability of these inhibitors to impair vesicle trafficking from early to late endosomes in some cells could also increase the recycling of these virion-containing endosomes to the cell surface, where fusion occurs. In summary, our results reveal an unexpected, CD4-mediated reciprocal relationship between the pathways governing HIV virion fusion and endocytosis.
Human immunodeficiency virus type 1 (HIV-1) virions enter cells by fusion or endocytosis, with sharply different functional consequences. Fusion, which occurs at the plasma membrane and does not require pH-dependent changes in envelope conformation, promotes the insertion of HIV cores into the cytoplasm and often leads to productive infection (for a review, see references 3, 8, 11, and 13). The HIV-1 Nef protein accelerates viral pathogenesis and enhances viral infectivity, in part by increasing the cytoplasmic delivery of HIV cores through the fusion pathway (9, 17, 20, 25, 28, 32). In contrast, the endocytic pathway culminates in virion inactivation in acidified endosomes or degradation in lysosomes and, in most cells, nonproductive infection (19, 22, 27, 29, 30, 33).
In certain cell types, however, vesicular uptake of HIV virions may have important functional consequences. In dendritic cells, dendritic cell-specific ICAM-3-grabbing nonintegrin (DC-SIGN)-mediated capture and endocytosis of HIV virions may play a key role in HIV dissemination. Recycling of vesicles containing these virions to the cell surface likely facilitates productive infection of T cells interacting with the dendritic cell (4, 18). The spread of HIV seems to be enhanced by endocytosis, suggesting that either mild acidification of virions or trafficking of these virion-laden vesicles to the site of cell-cell contact facilitates virion transmission. In macrophages, HIV virions are actively internalized into large vesicles by a receptor-independent process called macropinocytosis. Although the majority of virions internalized in this manner are degraded, a few engage CD4 and chemokine receptors on the internal surface of the macropinosome, leading to HIV core release into the cytoplasm and thereby contributing to productive infection (23).
Endocytosis is an active pathway for virion uptake in epithelial cells. In HeLa-CD4 cells, 85 to 90% of the virions enter by endocytosis. This process, which does not require either CD4 or chemokine coreceptor engagement, promotes the passage of cargoes through a series of discrete vesicular compartments, including early and late endosomes, which differ in acidity (pH 6.2 versus 5.5, respectively) due to the action of a vacuolar proton ATPase. Within 1 to 2 min after internalization, endocytosed cargoes reach the early endosome, where a sorting reaction occurs (24). Certain cargoes are recycled back to the plasma membrane, whereas others destined for degradation are transported to perinuclear late endosomes before entering lysosomes. Endosomal carrier vesicles have been proposed to mediate microtubule-dependent transfer of cargoes from early to late endosomes. In some cells, potent inhibitors of the vacuolar proton ATPase, such as bafilomycin A1, not only block acidification of the early endosome but also impair transport of cargoes to the late endosomes (2).
Whether endocytosed HIV virions ever mediate productive infection of target cells has been a topic of considerable debate. Retroviruses generally infect cells by a receptor-dependent but pH-independent fusion reaction at the plasma membrane. However, a fraction of HIV virions entering the endocytic pathway might fuse to target cells within endosomes before inactivation. In support of this notion, NH4Cl, a lysomotropic weak base that impairs endosome and lysosome acidification, modestly enhanced HIV infectivity (1). More recently, potent inhibitors of endosome acidification, like bafilomycin A1 and concanamycin A, have been shown to produce a 2- to 15-fold enhancement of HIV-1NL4-3, HIV-1SF2, and HIV-1LAI infectivity in epithelial Magi cells (a HeLa cell derivative) (16). In another study, these agents had similar effects on infectivity with HIV-1NL4-3, but blockade of endosomal acidification with bafilomycin impaired rather than enhanced the infectivity of HIV-1SF2 (14).
However, several important questions remain unresolved. First, are there differences in the endocytic properties of biologically relevant cellular targets, (e.g., primary CD4 T lymphocytes versus epithelial HeLa CD4 cells)? Second, do endosome inhibitors similarly enhance HIV infectivity in CD4 T lymphocytes? Third, if HIV infectivity is enhanced by endosome inhibitors, does the fusion reaction require HIV Env engagement of CD4 and the appropriate chemokine coreceptor? Fourth, in view of the effects of bafilomycin on both endosome acidification and trafficking, does fusion occur within the endosome or at the plasma membrane?
In the present report, we investigate these key questions, revealing an intriguing compensatory relationship between HIV fusion and endocytosis governed by the common involvement of CD4 in both entry events.
MATERIALS AND METHODS
Reagents.
Bafilomycin A1, concanamycin A, and nocodazole (Sigma) were dissolved in dimethyl sulfoxide (DMSO) at concentrations of 10μM, 25μM, and 20 mM, respectively. After the drugs were diluted in cultures, the final concentration of DMSO never exceeded 1% (vol/vol). AMD3100 was a generous gift of D. Schols (University of Leuven, Leuven, Belgium) (5, 31).
Cells and viruses.
Human peripheral blood mononuclear cells (PBMCs) were isolated from the buffy coats of pooled blood from six healthy donors by Ficoll-Hypaque density gradient centrifugation. Cells were resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) and penicillin-streptomycin. PBMCs were activated with phytohemagglutinin (PHA) and cultured in medium containing human recombinant interleukin-2. Human SupT1 T cells were grown in RPMI 1640 supplemented with 10% heat-inactivated FBS and penicillin-streptomycin. MAGI (HeLa-CD4-LTR-LacZ) cells were cultured in Dulbecco's modified Eagle's medium containing the same supplements.
Molecular clones of the HIV-1NL4-3 and HIV-1SF2 viral strains containing or lacking a deletion in the nef open reading frame were transfected into 293T cells, and viruses were harvested from the supernatants 48 h later. An R5-tropic version of NL4-3 virus (30) was similarly prepared. Viruses lacking the HIV envelope (ΔEnv) were prepared by transfection of 293T cells with pNL4-3.Luc.R-E- expression vector DNA (National Institutes of Health AIDS Research Program, catalog number 3418, generously donated by N. Landau). In some experiments, these Env-deficient HIV-1 viruses were pseudotyped with the vesicular stomatitis virus (VSV)-G envelope (kindly provided by O. Keppler, Gladstone Institute of Virology and Immunology, San Francisco, Calif.). All transfections were performed with the calcium phosphate method. Viral stocks were normalized according to their p24 Gag content measured in an enzyme-linked immunosorbent assay (ELISA) (NEN Life Science Products).
Preparation of GFP-Vpr-labeled HIV-1 virions.
Virions were labeled with GFP by cotransfection of 293T cells with HIV-1 pNL4-3 proviral DNA and an expression vector encoding a GFP-Vpr fusion protein (30). After 48 h, the virus-containing supernatant was subjected to low-speed centrifugation to remove cells and debris, followed by ultracentrifugation at 20,000 rpm in an SW41 rotor (Beckman) for 2 h at 4°C to sediment viral particles. The virus-containing pellet was resuspended in complete medium (0.5 ml) and stored in aliquots at −70°C.
Preparation of beta-lactamase (BlaM)-Vpr-labeled HIV-1 virions.
To produce wild-type (WT) viral particles containing BlaM-Vpr for the virion-based fusion assay, 293T cells in a T175 flask were cotransfected with proviral NL4-3 (60 μg), pCMV-BlaM-Vpr (20 μg), and pAdvantage (10 μg). To generate VSV-G-pseudotyped Env-deficient viral particles containing BlaM-Vpr, 293T cells were cotransfected with proviral NL4-3Δenv (50 μg), pCMV-VSV-G (10 μg), pCMV-BlaM-Vpr (20 μg), and pAdvantage (10 μg). Viruses were harvested from the supernatant 48 h later, and cellular debris was removed by low-speed centrifugation. Viral particles were subsequently concentrated via ultracentrifugation at 20,000 rpm in an SW41 rotor (Beckman) for 2 h at 4°C, resuspended in 1 ml of Dulbecco's modified Eagle's medium and aliquoted for storage at −80°C. pCMV-BlaM-Vpr (generously provided by Marielle Cavrois, Gladstone Institute of Virology and Immunology) has been previously described (7), and the NL4-3Δenv clone was generated by end filling of an NheI digest of NL4-3 (kindly provided by C. de Noronha, Gladstone Institute of Virology and Immunology).
Flow cytometric analysis.
Human SupT1 T cells (105) or PHA-activated lymphoblasts (2 × 105) were resuspended in fresh medium in the presence or absence of AMD3100 (5 μg/ml) or with purified mouse anti-human monoclonal anti-CD4 antibodies (20 μg/ml; Pharmingen International) at 37°C for 30 min before exposure to virus. GFP-Vpr-labeled HIV-1 (150 to 300 ng of p24 Gag) was added for 3 h at 37°C or at 4°C. Cells were washed with phosphate-buffered saline (PBS) or treated with a trypsin-EDTA solution (1×; Sigma) for 2 min at room temperature to remove surface-bound virions, followed by additional washing and fixation in 1% paraformaldehyde-PBS solution. For quantitation of CD4-positive PHA-activated lymphoblasts, cells were resuspended in PBS-1% FBS (100 μl) and stained for 30 min at 4°C in the dark with a 1:50 dilution of phycoerythrin (PE)- or allophycocyanin (APC)-conjugated mouse monoclonal CD4 antibodies (Caltag Laboratories) or with immunoglobulin G (IgG) isotype control antibodies. After one additional wash, cells were resuspended in PBS.
SupT1 cells were either untreated or incubated with bafilomycin A1 (100 nM) or concanamycin A (0.6 nM) for 3 h at 37°C. Cells were washed with PBS-1% FBS and stained with PE-conjugated anti-CD4, anti-CXCR4 monoclonal antibody, or IgG2a isotype control (1:50 dilution; all from Pharmingen) for 30 min at 4°C in the dark. After one wash, cells were resuspended in PBS, and the fluorescence intensity was analyzed by flow cytometry (FACSCalibur; Becton Dickinson, San Jose, Calif.). For each condition, 2 × 105 events were acquired, and cells in the live cell gate were analyzed with CellQuest software.
Virion-based fusion assay.
The virion-based fusion assay was performed as described previously (7). Briefly, 250,000 SupT1 cells were pretreated with DMSO (0.1%; diluent control), AMD3100 (250 nM), bafilomycin A1 (10 nM), concanamycin A (1 nM), or NH4Cl (10 mM) for 1 h at 37°C. BlaM-Vpr-labeled virions (25 to 100 ng of p24 Gag) were then added and incubated for a further 3 h at 37°C in the continued presence of drugs as indicated. Virions were then removed by washing the cells once with CO2-independent medium (Gibco-BRL, Rockville, Md.), and the cells were loaded with CCF2/AM dye by incubation for 1 h at room temperature in CO2-independent medium supplemented with CCF2/AM and pluronic acid as suggested in the manufacturer's instructions (Aurora Biosciences). The cells were then washed twice with CO2-independent medium, and the BlaM reaction was allowed to incubate overnight in CO2-independent medium supplemented with 10% FBS and 2.5 mM probenecid (Sigma Pharmaceuticals, St. Louis, Mo.). The cells were washed once with PBS and fixed in 1.25% paraformaldehyde for 2 h. The change in emission fluorescence of CCF2 after cleavage by BlaM-Vpr was then monitored by flow cytometry using a three-laser Vantage DIVA (Becton Dickinson) as previously described (7). Data were collected with CellQuest and analyzed with FlowJo software (Treestar, San Carlos, Calif.).
Infectivity analysis.
Human SupT1 T cells (2 × 105) were pretreated at 37°C for 30 min with AMD3100, anti-CD4 antibodies, bafilomycin A1, concanamycin A, nocodazole, or NH4Cl at the indicated concentrations and infected with HIV-1 pNL4-3 or HIV/VSV-G pseudotypes (50 ng of p24 Gag) for 16 h. Excess free virus was removed by serial washing. HIV-1 replication was monitored by measuring p24 Gag levels in the culture supernatants during subsequent culture.
Single-cycle infectivity studies were performed with MAGI cells pretreated at 37°C for 30 min with either DMSO (diluent control), bafilomycin A1, concanamycin A, nocodazole, or NH4Cl followed by infection with HIV-1 (1 to 20 ng of p24 Gag) in 48-well plates for 16 h. Viral spread was prevented by addition of soluble CD4 (2 μg/ml) after 16 h. The cells were fixed 48 h after infection and incubated with the β-galactosidase substrate, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside. Blue-staining cells were enumerated by light microscopy.
RESULTS
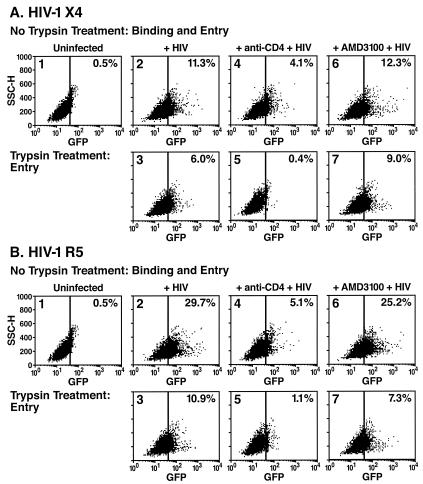
HIV-1 entry and replication in the presence of anti-CD4 blocking antibodies or AMD3100. HIV-1 virions labeled with GFP-Vpr fusion proteins were used to monitor virion entry into target cells. These fluorescent virions were prepared as described in Materials and Methods. X4- or R5-tropic virions were added to the different cellular targets for 3 h at 37 or 4°C in the presence or absence of blocking anti-CD4 antibodies or the AMD3100 bicyclam inhibitor of CXCR4-dependent fusion (31). Selected aliquots of these cells were treated with trypsin to remove noninternalized, surface-bound virions prior to analysis by flow cytometry (Fig. 1A, X4-tropic virions; Fig. 1B, R5-tropic virions).
FIG. 1.
Flow cytometric analysis of human SupT1 cells incubated with GFP-Vpr-labeled X4-tropic (A) or R5-tropic (B) HIV-1 virions in the presence of medium (panels 2 and 3), neutralizing anti-CD4 antibodies (panels 4 and 5), or AMD3100 (panels 6 and 7). SupT1 T cells were preincubated in the presence or absence of anti-CD4 antibodies or AMD3100 for 30 min at 37°C. Cells were then inoculated with GFP-Vpr-labeled X4-tropic HIV-1 or CCR5-tropic HIV-1 R5 (200 ng of p24 Gag) for 3 h at 37°C. The cells were subsequently washed with PBS (panels 1, 2, 4, and 6) or treated with a trypsin-EDTA solution (panels 3, 5, and 7) to remove surface-bound virions. Live cells were then analyzed by flow cytometry for GFP epifluorescence. The vertical bar indicates a gate established by analysis of uninfected cells (panels 1). The percentage of GFP-positive cells is indicated in the upper right hand corner of each panel. The anti-CD4 antibodies significantly inhibited the entry of both X4-tropic and R5-tropic virions, whereas AMD3100 unexpectedly failed to reduce the overall entry of X4-tropic virions.
After a 3-h incubation with a viral input of 200 ng of p24 Gag, approximately 11% of CD4-expressing human SupT1 T cells contained HIV-X4 virions and nearly 30% contained HIV-R5 virions (Fig. 1, panels 2). After trypsin treatment, 6% of the HIV-X4-infected cells and ∼11% of the HIV-R5-infected cells retained a fluorescent signal, consistent with internalization of virions into a trypsin-resistant compartment (Fig. 1, panels 3). Trypsin treatment of SupT1 cells incubated with virions at 4°C produced a near-complete loss of all associated fluorescence, validating this technique for removal of surface-bound virions (data not shown). Prior treatment of these cells with anti-CD4 antibodies that block gp120 engagement of CD4 reduced X4 virion binding from 11.3 to 4.1% (Fig. 1A, panel 4 versus panel 2) and reduced R5 virion binding from 29.7 to 5.1% (Fig. 1B, panel 4), suggesting that the CD4 receptor is a major but perhaps not exclusive site of attachment on the cell surface (10, 26). Treatment with anti-CD4 antibodies markedly reduced the entry of both X4 viruses (6 to 0.4%; Fig. 1A, panel 5) and R5 viruses (10.9 to 1%; Fig. 1B, panel 5) into these cells. Conversely, incubation of the cells with AMD3100 did not impair HIV virion internalization (6 versus 9% for HIV-X4 and 11 versus 7% for HIV-R5, compare panels 3 and 7 in Fig. 1A and 1B).
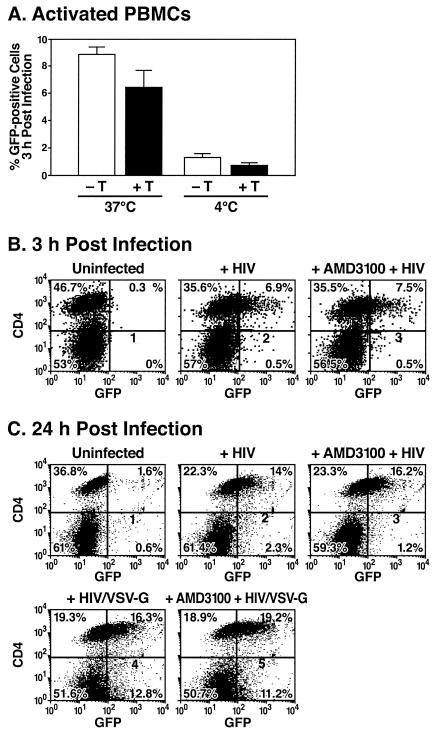
Similar studies were performed with biologically relevant primary human T lymphocytes as cellular targets in the X4-tropic HIV binding and entry assays (Fig. 2). With a viral input of 300 ng of p24 Gag, approximately 8.5% of these cells bound, and 6.3% internalized, the fluorescent HIV virions (Fig. 2A). Binding of virions at 4°C followed by trypsin treatment yielded ≤0.7% positive cells (Fig. 2A). Two-color immunofluorescence studies revealed that virion entry during the 3-h incubation was almost exclusively restricted to the CD4 subset of PHA-activated lymphoblasts. As observed with the SupT1 cells, total virion entry into these CD4 T lymphoblasts was not impaired by AMD3100 (Fig. 2B, compare panels 2 and 3). Even after a 24-h exposure to these HIV virions (Fig. 2C), the CD4-positive cells were the major cellular subset containing fluorescent virions (panel 2, 14% CD4-positive cells versus 2.3% CD4-negative cells). Further, entry of these virions into the CD4-positive PBMCs was not impaired by AMD3100 (Fig. 2C, panel 3). As expected, after a 24-h infection with VSV-G-pseudotyped HIV virions, which are internalized via endocytosis in a CD4-independent manner (1), both CD4-positive (16%) and CD4-negative (13%) cells contained fluorescently labeled virions (Fig. 2C, panel 4). These results suggest that virion engagement of CD4 is required not only for fusion but also for endocytosis in PBMCs.
FIG. 2.
Flow cytometric analysis of PHA-activated human lymphoblasts infected with GFP-Vpr-labeled HIV-1 virions. (A) PBMCs activated with PHA and cultured in interleukin-2 for 4 days were inoculated with GFP-Vpr-labeled X4-tropic HIV-1 (300 ng of p24 Gag) at 37 or 4°C as indicated. After 3 h, the cells were washed at room temperature with PBS (−T) or a trypsin-EDTA solution (+T) to remove surface-bound virions. Live cells were then analyzed by flow cytometry to detect GFP epifluorescence. Error bars indicate standard deviations of the mean derived from two independent experiments. (B and C) PHA-activated human lymphoblasts were pretreated for 30 min at 37°C with either medium (panels 2 and 4) or AMD3100 (panels 3 and 5). Cells were then incubated at 37°C for 3 or 24 h with GFP-Vpr-labeled X4-tropic HIV-1 or HIV virions pseudotyped with the VSV-G envelope (200 ng of p24 Gag) at 37°C. Cells were subsequently stained with APC-conjugated CD4 antibodies (B) or PE-conjugated CD4 antibodies (C). Cells were analyzed for GFP epifluorescence and APC or PE immunofluorescence. The percentage of cells in each quadrant is indicated. Data shown are from a representative experiment performed three times with comparable results. Note preferential entry of HIV into CD4 cells and the absence of inhibitory effects of AMD3100 measured either at 3 or 24 h.
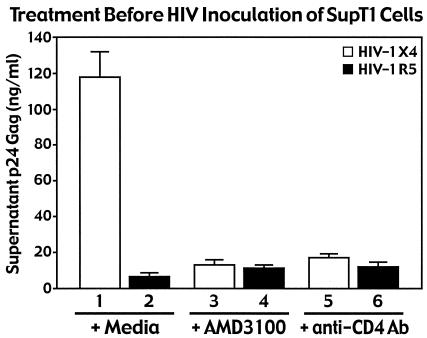
The efficiencies of the AMD3100 fusion inhibitor and the blocking anti-CD4 antibodies were assessed in viral replication assays in which SupT1 cells served as targets for HIV-1 infection with X4 or R5 strains of NL4-3 viruses (Fig. 3). Both AMD3100 and the anti-CD4 blocking antibodies markedly inhibited production of p24 Gag in the supernatants of the SupT1 cells cultures infected with X4 NL4-3 viruses. As expected, no productive viral infection occurred when R5 NL4-3 viruses were added, consistent with the absence of CCR5 receptors on the target cells. These findings confirmed that AMD3100 is biologically active in this culture system.
FIG. 3.
Neutralizing anti-CD4 antibodies and the X4-specific AMD3100 entry inhibitor efficiently block replication of X4-tropic HIV in SupT1 cells. HIV-1 NL4-3 X4 and R5 viruses (100 ng of p24 Gag) were incubated with SupT1 cells pretreated for 30 min at 37°C with AMD3100 or neutralizing anti-CD4 antibodies. After 3 h at 37°C, the cultures were extensively washed. After 2 days of culture, p24 Gag present in the cell medium was quantitated by ELISA. Data represent an average of three independent infections performed in duplicate. Error bars indicate standard deviations. AMD3100 did not impair overall entry of X4-tropic virions into SupT1 cells (Fig. 1A) but markedly impaired viral replication.
Through cell fractionation studies, it has been previously demonstrated by both our lab (30) and others (22) that approximately 50% of HIV-1 virions enter T cells by fusion and 50% enter by endocytosis. Thus, the finding in this study that AMD3100 effectively blocked viral replication (Fig. 3) but did not impair viral entry (Fig. 1A, panel 7, compare with panel 3) suggests that a compensatory increase in endocytosis occurred in the presence of this bicyclam inhibitor. The fact that HIV-1 virion entry by endocytosis does not generally lead to productive infection is consistent with the lack of viral replication observed in the presence of AMD3100 despite unaltered virion uptake. In contrast, the inhibitory effect of anti-CD4 antibodies on total virion entry (Fig. 1A, panel 5) suggests that CD4 is required for HIV virion entry via both fusion and endocytosis. The involvement of the CD4 receptor in HIV-1 endocytosis in these T cells contrasts with the high levels of CD4-independent endocytosis of HIV-1 previously observed in epithelial HeLa cells (30). Together, these results not only highlight important cell type differences in the receptor requirements for endocytosis of HIV but also reveal an intriguing interrelationship between entry by fusion and endocytosis in human T lymphocytes.
HIV-1 replication and inhibitors of endosomal acidification and transport.
Since inhibition of virion entry through the fusion pathway increased HIV-1 entry by endocytosis, we next investigated whether inhibition of the endocytic pathway would increase HIV entry by fusion. Two classes of endosome inhibitors, including lysosomotropic weak bases (NH4Cl) and inhibitors of the vacuolar proton ATPases (bafilomycin A1 and concanamycin A), were tested. Both impair the replication of viruses when fusion occurs within acidified endosomes and is associated with requisite pH-induced conformational changes in Env structure (1, 2, 22, 23). Of note, bafilomycin A1 and concanamycin A not only block endosome acidification but also may arrest vesicular trafficking from early to late endosomes (2). The microtubule-disrupting agent nocodazole does not affect endosomal pH but inhibits the microtubule-dependent transport of endosomal carrier vesicles to late endosomes (2).
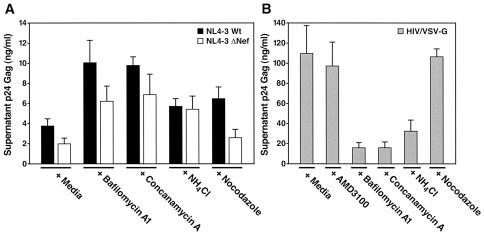
These endosome inhibitors were first tested for effects on the replication of the WT strain as well as on the ΔNef strain of HIV-1 in SupT1 cultures. Nef is an auxiliary protein of HIV that is required for maximal delivery of HIV cores into the cytoplasm and for full HIV replication. Interestingly, the Nef phenotype of infectivity can be overcome by pseudotyping of HIV virions with the VSV-G protein, which targets these viruses for entry into endosomes where fusion occurs following acidification (1). Accumulation of p24 Gag in the culture supernatant was measured 48 h after infection. Replication of the HIV-1 WT was increased 2.5-fold in the presence of bafilomycin A1 (100 nM) and concanamycin A (0.6 nM) and 1.5-fold in the presence of NH4Cl (10 mM) and nocodazole (Fig. 4A, solid bars). Even greater relative increases in viral replication were obtained when HIV-1NL4-3 ΔNef viruses were tested with these endosome inhibitors (Fig. 4A, open bars). Specifically, pretreatment with bafilomycin A1, concanamycin A, and NH4Cl produced three-, four- and twofold increases in HIV ΔNef viral replication, respectively (Fig. 4A). Conversely, pretreatment of SupT1 cells with comparable concentrations of these endosome acidification inhibitors markedly inhibited replication of HIV/VSV-G-pseudotyped virions (Fig. 4B).
FIG. 4.
Inhibitors of endosome acidification enhance HIV-1 replication but impair HIV-1/VSV-G pseudotype replication in SupT1 T cells. SupT1 cells were preincubated for 30 min at 37°C with DMSO (0.1%; diluent control), bafilomycin A1 (100 nM), concanamycin A (0.6 nM), NH4Cl (10 mM), or nocodazole (1 μM). Cells were infected with X4-tropic HIV WT (WT, closed bars) or HIVΔNef (open bars) (A) or HIV virions pseudotyped with the VSV-G envelope (50 ng of p24 Gag) (B). After incubation for 3 h at 37°C, the cells were washed. Two days later, p24 Gag in the culture medium was quantitated by ELISA. Data represent an average for three independent infections performed in duplicate. Error bars indicate standard deviations. The endosome inhibitors bafilomycin A1, concanamycin A, and NH4Cl enhanced the replication of both WT and ΔNef viruses but impaired replication of VSV-G pseudotypes of HIV where fusion is dependent on endosome acidification.
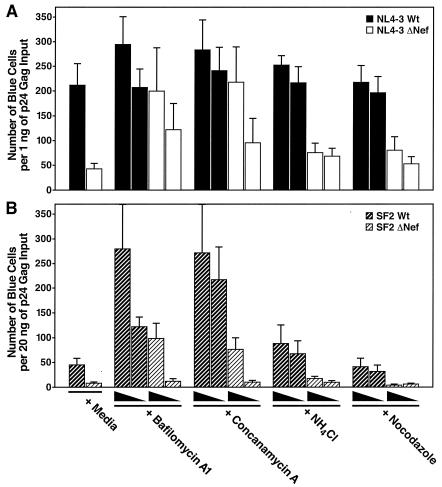
We next compared the effects of these endosomal acidification and trafficking inhibitors on a single cycle of HIV-1 replication in MAGI cells (HeLa cells expressing CD4 and a Tat-activated HIV-LTR-β-galactosidase reporter gene). The number of positive blue MAGI cells infected with NL4-3 WT increased 1.5- to 2-fold after 30 min of pretreatment with maximal concentrations of bafilomycin A1, concanamycin A, or NH4Cl that did not alter cell viability. These relatively modest increases are in agreement with a previous report (22). We next tested the effects of the inhibitors on HIV-1 viruses displaying lower infectivity, including NL4-3 ΔNef and SF2 viruses. After pretreatment with bafilomycin A1 or concanamycin A, the replication of ΔNef HIV-1 increased to levels comparable to that of WT HIV-1 in untreated cells (Fig. 5A), and the infectivity of SF2 increased roughly fivefold (Fig. 5B). In the presence of these endosome inhibitors, the expression of SF2 ΔNef viruses increased 12- to 15-fold, which surpassed replication of SF2 WT in untreated cells (Fig. 5B). Nocodazole did not significantly affect viral infectivity. These results demonstrate that inhibitors of endosome acidification enhance the replication of HIV both in T cells and in epithelial HeLa-CD4 cells. Even greater increases in the replication of viruses displaying intrinsically diminished infectivity (i.e., ΔNef mutants and SF2) are obtained in the presence of these inhibitors.
FIG. 5.
Endosome inhibitors enhance HIV-1 replication in MAGI cells. (A and B) MAGI cells were pretreated for 45 min at 37°C with DMSO (0.1%; diluent control), bafilomycin A1 (10 and 1 nM), concanamycin A (1.2 and 0.6 nM), NH4Cl (10 and 1 mM), or nocodazole (200 and 20 nM) and inoculated with HIV-1 NL4-3 WT or ΔNef viruses (1 ng of p24 Gag) (A) or HIV-SF2 WT and ΔNef viruses (20 ng of p24 Gag) (B) as indicated. Foci of blue cells reflecting Tat activation of the integrated HIV-LTR-β-galactosidase reporter gene were enumerated 48 h later. Values are representative of at least three independent experiments performed in duplicate. Error bars indicate standard deviations The endosome inhibitors enhanced the infectivity of WT HIV, but the greatest effects were obtained with viruses displaying intrinsically diminished infectivity (SF2 and ΔNef).
HIV-1 fusion is enhanced in the presence of endosome inhibitors.
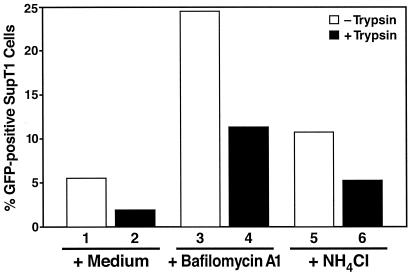
SupT1 cells preincubated with endosome inhibitors were inoculated with GFP-Vpr-labeled virions for 3 h at 37°C and then trypsinized to remove virions bound at the cell surface. Flow cytometric assays showed that bafilomycin A1 and NH4Cl produced fivefold and twofold increases, respectively, in the number of GFP-positive cells (Fig. 6). Both the overall levels of virion binding (measured in the absence of trypsin) and virions entering cells (measured in the presence of trypsin) were increased.
FIG. 6.
Both surface binding and intracellular entry by fusion of HIV-1 virions are enhanced by endosome inhibitors. SupT1 cells were pretreated for 30 min at 37°C with medium (columns 1 and 2), bafilomycin A1 (100 nM; columns 3 and 4), or NH4Cl (10 mM; columns 5 and 6). Cells were then incubated with GFP-Vpr-labeled HIV-1 virions (200 ng of p24 Gag) for 3 h at 37°C. Cells were either washed in PBS (columns 1, 3, and 5) or treated with a trypsin-EDTA solution to remove surface-bound virions (columns 2, 4, and 6) followed by flow cytometric analysis of living cells. The percentage of SupT1 cells displaying GFP epifluorescence is indicated on the ordinate. The data presented are from a representative experiment repeated three times with comparable results.
To examine whether HIV-1 entry by fusion is increased in the presence of endosome inhibitors, we employed a virion-based fusion assay we recently described (7) that measures cytosolic delivery of BlaM from virions into target cells. BlaM, fused to the viral protein Vpr, is packaged into virions, and delivery of BlaM-Vpr to cells can be subsequently detected by a change in fluorescence emission of target cells loaded with the fluorescent dye CCF2. This assay has been demonstrated to discriminate between fusion and endocytosis of virions due to the lack of CCF2 availability within endosomes (7).
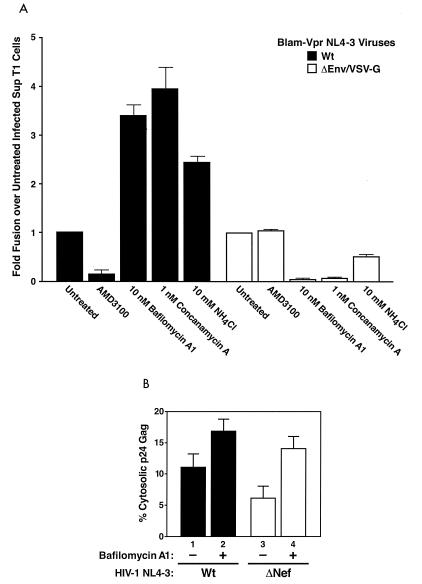
SupT1 cells pretreated with various HIV-1 entry or endosome inhibitors were inoculated with BlaM-Vpr-labeled virions for 3 h at 37°C. Cells were subsequently loaded with CCF2, and the fluorescence emission of the cells was analyzed by flow cytometry. Under these conditions, on average, after 3 h of incubation with virus in the absence of any inhibitors, 90% of cells fused with VSV-G-pseudotyped virions while 5% of cells fused with WT NL4-3 (data not shown). However, upon pretreatment of SupT1 cells with endosome inhibitors, WT NL4-3 virus entry was increased up to fourfold while VSV-G-pseudotyped NL4-3Δenv virus entry was fully inhibited (Fig. 7A). Entry specifically involved CXCR4, since AMD3100 treatment completely blocked WT NL4-3 virus entry while AMD3100 treatment slightly enhanced the already robust entry mediated by VSV-G-pseudotyped NL4-3Δenv. Thus, treatment of cells with endosome inhibitors led to an increase in virion fusion with SupT1 cells, suggesting that viruses present in neutralized endosomes are available for entry into the target cell through fusion mediated by the CXCR4 chemokine coreceptor. This increase in entry via fusion could thereby enhance productive infection of cells upon inhibition of endocytosis (Fig. 4), suggesting that inhibition of endocytosis results in either increased fusion from within neutralized endosomes or recycling of vesicles to the cell surface and enhanced fusion at the plasma membrane.
FIG. 7.
(A) Endosome inhibitors enhance HIV-1 fusion in SupT1 cells. SupT1 cells were pretreated for 1 h at 37°C with DMSO (0.1%; diluent control), AMD3100 (250 nM), bafilomycin A1 (10 nM), concanamycin A (1 nM), or NH4Cl (10 mM) and inoculated with BlaM-Vpr-containing viruses, either WT NL4-3 or VSV-G-pseudotyped NL4-3Δenv (25 ng or 100 ng of p24 Gag), as indicated, for 3 h. The fusion assay was performed as described in Materials and Methods, and the percentage of blue cells was determined. Shown is the fold increase in fusion, which is the ratio of percent blue cells upon treatment with indicated drug to percent blue cells without any treatment for the given virus. Thus, entry of virus into untreated cells is equal to 1. The values are representative of at least three independent experiments performed in triplicate. Error bars are indicated. The endosome inhibitors enhanced the entry of WT NL4-3 viruses while inhibiting the entry of the VSV-G-pseudotyped NL4-3Δenv viruses. In contrast, AMD3100 treatment inhibited entry of WT NL4-3 virus only and not the VSV-G-pseudotyped virus. (B) Cytosolic entry of HIV-1 particles is enhanced by bafilomycin A1. MAGI cells were treated with 0.1% DMS0 (diluent control) or bafilomycin A1 (20 nM) and infected with the indicated HIV-1-NL4-3 WT or HIV-NL4-3 ΔNef viruses (500 ng of p24 Gag). After a 1-h incubation of cells and viruses at 37°C, cytosolic fractions were prepared as described elsewhere (30). The level of p24 Gag present in each fraction was quantitated by ELISA. Values correspond to the percentage of p24 Gag present in the cytosolic fraction relative to the total intracellular p24 Gag. The total intracellular p24 Gag was approximately 1,000 pg, representing 0.2% of the input. Data are representative of seven experiments performed in duplicate. Error bars indicate standard deviations. Bafilomycin A1 enhanced cytoplasmic delivery of p24 Gag with both viruses, indicating increased fusion; however, greater relative effects were obtained with the less infectious HIV ΔNef virus.
To confirm that the enhancement of virion entry observed in the presence of the endosome inhibitors led to increased cytoplasmic delivery of viral cores, we performed subcellular fractionation studies. MAGI cells, which are more suitable than T cells for such fractionation studies, were pretreated with bafilomycin A1 or medium and incubated with NL4-3 WT or ΔNef virus for 1 h at 37°C. Cytosolic fractions were prepared and analyzed for p24 Gag content, which is expressed as a percentage of the whole-cell p24 Gag detected (Fig. 7B). After infection with NL4-3 WT virus, the cytosolic p24 Gag content was approximately 11%. Preincubation with bafilomycin A1 increased fusion-mediated virus entry 1.5-fold (lanes 1 and 2). Infection with ΔNef HIV-1 yielded lower levels of cytosolic p24 Gag (6%, lane 3). Bafilomycin A1 pretreatment of these ΔNef HIV-1-infected cells increased the cytosolic levels of p24 Gag 2.2-fold (lane 4) to approximately the levels in untreated cells incubated with WT virus. These entry findings coupled with the replication results indicate that treatment of target cells with endosome inhibitors enhances virion entry via the productive fusion pathway. The additional finding that these inhibitors increased the surface binding of virions raises the possibility that the endocytic process is altered in a manner that leads to increased surface virions. As such, a proportion of the increased cytosolic entry events could occur as a result of fusion at the cell surface.
Cell surface expression of CD4 and CXCR4 with endosome inhibitors.
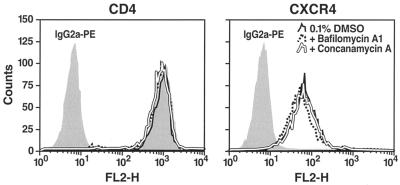
One possible mechanism to explain the increased surface virion binding in the presence of the endosome inhibitors would be an induced increase in surface CD4 or CXCR4 receptor expression. However, flow cytometric analyses of SupT1 cells treated with bafilomycin A1 or concanamycin revealed no effects on the levels of surface CD4 or CXCR4 receptors (Fig. 8). These findings in T cells are in agreement with a recent analysis of CD4 and CXCR4 receptor expression in MAGI cells (16) and would suggest that fusion in the presence of the endosome inhibitors likely occurs within nonacidified endosomes.
FIG. 8.
Bafilomycin A1 and concanamycin A treatment do not alter cell surface expression of either CD4 or CXCR4. SupT1 cells were incubated for 3 h at 37°C with medium containing or lacking 0.1% DMSO, bafilomycin A1 (100 nM), or concanamycin A (0.6 nM). Cells were then washed and immunostained at 4°C with PE-conjugated anti-CD4, anti-CXCR4, or isotype control IgG2a monoclonal antibodies. Immunofluorescence intensities were analyzed by flow cytometry.
DISCUSSION
Our studies have focused on HIV virion entry into the cell by two pathways, fusion and endocytosis. In general, virions entering by fusion support continuation of the HIV replicative life cycle, while endocytosis of virions leads to their inactivation in the acidified environment of endosomes and degradation in lysosomes. However, our studies have revealed an unexpected compensatory interplay between these two entry pathways, explained by the common involvement of CD4.
CD4 receptors are required not only for HIV fusion but also for endocytosis in T cells and PHA-activated lymphoblasts.
CD4 binding plays a key initial role in the fusion of HIV virions at the plasma membrane by promoting conformational changes in gp120 that facilitate subsequent engagement of specific chemokine coreceptors (CCR5 or CXCR4). Conversely, based on studies in epithelial HeLa cells, endocytosis of HIV virions requires neither CD4 nor chemokine coreceptor binding (22, 30). However, in human SupT1 T cells or PHA-activated lymphoblasts, CD4 receptors were required both for effective fusion and for endocytosis of HIV virions. The addition of neutralizing anti-CD4 antibodies impaired both the fusion and endocytosis of X4 and R5 HIV-1 virions. Further, the endocytosis of X4 HIV-1 virions in the presence of AMD3100 was almost entirely limited to a CD4-positive subset of cells. In contrast, the chemokine coreceptors were not required for virion endocytosis, since efficient endocytosis of R5 virions occurred in SupT1 cells lacking CCR5.
Endocytosis of HIV virions is upregulated when the HIV fusion pathway is inhibited.
HIV-1 fusion and endocytosis in human CEM (22) and Jurkat (30) T cells are balanced, with 40% of virions entering by fusion and 60% entering by endocytosis. However, when the fusion pathway was interrupted in SupT1 T cells or primary human T lymphoblasts by addition of the CXCR4 entry inhibitor AMD3100 (31), viral replication was inhibited, yet the overall entry of GFP-Vpr-labeled virions did not decline. In contrast, blocking anti-CD4 antibodies markedly diminished overall viral entry. These results suggest that inhibition of fusion is associated with a compensatory increase in endocytosis. Virion endocytosis in T cells is generally a “dead end” pathway leading to virion inactivation and degradation. However, as noted for other cell types, such as macrophages or dendritic cells, enhanced internalization of HIV virions by macropinocytosis (23) or DC-SIGN-mediated endocytosis (6, 15), respectively, plays a key role in the infection of these or neighboring cells. In macrophages, macropinocytosis of HIV virions contributes to productive infection of these cells (23). In dendritic cells, the binding of HIV virions to DC-SIGN followed by endocytosis of these virions into an acidified vesicular compartment and subsequent recycling of these vesicles to the cell surface appears to facilitate infection of interacting T cells (21). Thus, entry of HIV virions into either the macropinocytic or endocytic compartment does not uniformly lead to virion inactivation and degradation. In most cells, however, including CD4 T cells, virion endocytosis does not contribute in a major manner to productive infection.
HIV-1 binding and entry via the fusion pathway are enhanced when endosome function is inhibited.
We also explored whether enhanced entry of HIV virions by fusion occurs when the endocytotic pathway is inhibited. For these studies, cells were treated with two classes of endosome inhibitors, including bafilomycin A1 and concanamycin A, which block the vacuolar proton ATPase, and NH4Cl, a lysosomotropic weak base that neutralizes the endosome. The vacuolar proton ATPase inhibitors not only impair endosome acidification but, in some cell types, also impair trafficking from early to late endosomes (2). As such, results obtained with these inhibitors must be interpreted with both potential effects in mind. Our results indicate that treatment of human T cells with bafilomycin A1, concanamycin A, or NH4Cl leads to a twofold to fourfold increase in fusion of HIV virions with target cells (Fig. 7A) and a concomitant increase in cytosolic delivery of viral cores (Fig. 7B), culminating in higher levels of HIV replication in these cells. Consistent with these results, treatment of epithelial HeLa-CD4 cells with endosome inhibitors increased HIV infectivity 2- to 15-fold (16). We found that endosome inhibitors were most effective with viruses displaying relatively low infectivity, such as NL4-3ΔNef or SF2 viruses, which are likely impaired for entry via the fusion pathway (1) and, thus, are more susceptible to endocytosis-mediated entry than WT NL4-3 viruses under steady-state conditions (14, 30). It would be of interest in future studies to study the effects of endocytosis inhibitors on viruses with reduced entry due to compromised CD4 or chemokine coreceptor binding. Based on our observations of the ΔNef and SF2 virions in this study, we would predict that such viruses would also display enhanced replication in response to inhibition of endosome acidification. The compensatory changes between these two entry pathways when one is blocked reveal that inhibition of endocytosis may disproportionately increase virion entry by fusion, leading to increased infectivity.
Bafilomycin A1, concanamycin A, and, to a lesser extent, NH4Cl all enhanced entry of virions into target cells via fusion, ultimately leading to enhanced cytosolic delivery of HIV-1 cores. This increase cannot be explained by increased surface expression of CD4 or CXCR4. The most straightforward explanation is that these agents promote HIV fusion within endosomes. An alternative explanation for these results is that HIV virions trapped in early endosomes in the presence of bafilomycin A1 are transported back to the plasma membrane in recycling vesicles lacking CD4 and CXCR4. As such, productive fusion might occur at the cell surface as well as within the neutralized early endosomes.
HIV-1 infectivity reflects a dynamic balance between virion fusion and endocytosis.
The infectivity of an HIV-1 particle is linked to its ability to fuse to its target cells. This process is cooperative and depends on receptor density, conformation, and envelope-receptor affinities (12). Our findings help refine the understanding of the mechanism of HIV-1 entry into T cells and PBMCs. Overall entry reflects the amount of HIV-1 X4 or R5 virions bound to CD4 molecules. After the initial step of binding to CD4, viral entry involves a competition between the pathways of fusion and endocytosis, controlled by secondary interactions with specific coreceptors that trigger fusion or with other cell surface proteins that induce endocytosis. Inhibiting fusion increases endocytosis, while inhibiting endosome acidification and trafficking increases fusion. Thus, perturbations of the host cell that affect the balance between fusion and endocytosis could markedly influence viral infectivity. These findings also highlight how cell-cell fusion assays (34) do not fully recapitulate the dynamics of HIV virion entry, since the competition between fusion and endocytosis is lost. In contrast, the virion-based cell fusion assay appears to more faithfully recapitulate the cell surface dynamic competition between fusion and endocytosis, revealing the effect of endocytosis inhibitors on virion fusion. Our findings also agree with the conclusions of Fredericksen and colleagues (16) concerning the potential detrimental effects on HIV infectivity of administration of clinically approved drugs, such as amatadine and chloroquine, which impair endosomal acidification. In particular, these drugs may potentiate the effects of viruses displaying diminished infectivity.
Acknowledgments
We thank Carlos de Noronha for GFP-Vpr and NL4-3ΔEnv, Marielle Cavrois for BlaM-Vpr, Dominique Schols for AMD3100, and Oliver Keppler for helpful suggestions. We also thank John Carroll for graphics support, Gary Howard and Stephen Ordway for editorial assistance, and Robin Givens and Sue Cammack for administrative assistance.
This work was supported by NIH ROI (CA86814), California AIDS Research Center (CC99-SF001), and the UCSF-GIVI Center for AIDS Research (P30MH59037). V.B.S. is supported by a fellowship from amfAR (70571-31-RF).
REFERENCES
- 1.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71:5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayer, N., D. Schober, E. Prchla, R. F. Murphy, D. Blaas, and R. Fuchs. 1998. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J. Virol. 72:9645-9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binley, J., and J. P. Moore. 1997. HIV-cell fusion. The viral mousetrap. Nature 387:346-348. [DOI] [PubMed] [Google Scholar]
- 4.Blauvelt, A., H. Asada, M. W. Saville, V. Klaus-Kovtun, D. J. Altman, R. Yarchoan, and S. I. Katz. 1997. Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J. Clin. Investig. 100:2043-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridger, G. J., R. T. Skerlj, D. Thornton, S. Padmanabhan, S. A. Martellucci, G. W. Henson, M. J. Abrams, N. Yamamoto, K. De Vreese, R. Pauwels, et al. 1995. Synthesis and structure-activity relationships of phenylenebis(methylene)-linked bis-tetraazamacrocycles that inhibit HIV replication. Effects of macrocyclic ring size and substituents on the aromatic linker. J. Med. Chem. 38:366-378. [DOI] [PubMed] [Google Scholar]
- 6.Cameron, P. U., M. G. Lowe, S. M. Crowe, U. O'Doherty, M. Pope, S. Gezelter, and R. M. Steinman. 1994. Susceptibility of dendritic cells to HIV-1 infection in vitro. J. Leukoc. Biol. 56:257-265. [DOI] [PubMed] [Google Scholar]
- 7.Cavrois, M., C. De Noronha, and W. C. Greene. 2002. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 20:1151-1154. [DOI] [PubMed] [Google Scholar]
- 8.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. [DOI] [PubMed] [Google Scholar]
- 9.Chowers, M. Y., C. A. Spina, T. J. Kwoh, N. J. Fitch, D. D. Richman, and J. C. Guatelli. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J. Virol. 68:2906-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cladera, J., I. Martin, and P. O'Shea. 2001. The fusion domain of HIV gp41 interacts specifically with heparan sulfate on the T-lymphocyte cell surface. EMBO J. 20:19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimitrov, D. S. 2000. Cell biology of virus entry. Cell 101:697-702. [DOI] [PubMed] [Google Scholar]
- 12.Doms, R. W. 2000. Beyond receptor expression: the influence of receptor conformation, density, and affinity in HIV-1 infection. Virology 276:229-237. [DOI] [PubMed] [Google Scholar]
- 13.Doms, R. W., and D. Trono. 2000. The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 14:2677-2688. [DOI] [PubMed] [Google Scholar]
- 14.Fackler, O. T., and B. M. Peterlin. 2000. Endocytic entry of HIV-1. Curr. Biol. 10:1005-1008. [DOI] [PubMed] [Google Scholar]
- 15.Frank, I., M. Piatak, Jr., H. Stoessel, N. Romani, D. Bonnyay, J. D. Lifson, and M. Pope. 2002. Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): differential intracellular fate of virions in mature and immature DCs. J. Virol. 76:2936-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fredericksen, B. L., B. L. Wei, J. Yao, T. Luo, and J. V. Garcia. 2002. Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. J. Virol. 76:11440-11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geleziunas, R., M. D. Miller, and W. C. Greene. 1996. Unraveling the function of HIV type 1 Nef. AIDS Res. Hum. Retrovir. 12:1579-1582. [DOI] [PubMed] [Google Scholar]
- 18.Granelli-Piperno, A., E. Delgado, V. Finkel, W. Paxton, and R. M. Steinman. 1998. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J. Virol. 72:2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grewe, C., A. Beck, and H. R. Gelderblom. 1990. HIV: early virus-cell interactions. J. Acquir. Immune Defic. Syndr. 3:965-974. [PubMed] [Google Scholar]
- 20.Jamieson, B. D., G. M. Aldrovandi, V. Planelles, J. B. Jowett, L. Gao, L. M. Bloch, I. S. Chen, and J. A. Zack. 1994. Requirement of human immunodeficiency virus type 1 nef for in vivo replication and pathogenicity. J. Virol. 68:3478-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135-144. [DOI] [PubMed] [Google Scholar]
- 22.Marechal, V., F. Clavel, J. M. Heard, and O. Schwartz. 1998. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J. Virol. 72:2208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marechal, V., M. C. Prevost, C. Petit, E. Perret, J. M. Heard, and O. Schwartz. 2001. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J. Virol. 75:11166-11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mellman, I. 1996. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12:575-625. [DOI] [PubMed] [Google Scholar]
- 25.Miller, M. D., M. B. Feinberg, and W. C. Greene. 1994. The HIV-1 nef gene acts as a positive viral infectivity factor. Trends Microbiol. 2:294-298. [DOI] [PubMed] [Google Scholar]
- 26.Patel, M., M. Yanagishita, G. Roderiquez, D. C. Bou-Habib, T. Oravecz, V. C. Hascall, and M. A. Norcross. 1993. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res. Hum. Retrovir. 9:167-174. [DOI] [PubMed] [Google Scholar]
- 27.Pauza, C. D., and T. M. Price. 1988. Human immunodeficiency virus infection of T cells and monocytes proceeds via receptor-mediated endocytosis. J. Cell Biol. 107:959-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piguet, V., and D. Trono. 1999. The Nef protein of primate lentiviruses. Rev. Med. Virol. 9:111-120. [DOI] [PubMed] [Google Scholar]
- 29.Pudney, J., and M. J. Song. 1994. Electron microscopic analysis of HIV-host cell interactions. Tissue Cell 26:539-550. [DOI] [PubMed] [Google Scholar]
- 30.Schaeffer, E., R. Geleziunas, and W. C. Greene. 2001. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J. Virol. 75:2993-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schols, D., S. Struyf, J. Van Damme, J. A. Este, G. Henson, and E. De Clercq. 1997. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J. Exp. Med. 186:1383-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spina, C. A., T. J. Kwoh, M. Y. Chowers, J. C. Guatelli, and D. D. Richman. 1994. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J. Exp. Med. 179:115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein, B. S., S. D. Gowda, J. D. Lifson, R. C. Penhallow, K. G. Bensch, and E. G. Engleman. 1987. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell 49:659-668. [DOI] [PubMed] [Google Scholar]
- 34.Tobiume, M., K. Tokunaga, E. Kiyokawa, M. Takahoko, N. Mochizuki, M. Tatsumi, and M. Matsuda. 2001. Requirement of nef for HIV-1 infectivity is biased by the expression levels of Env in the virus-producing cells and CD4 in the target cells. Arch. Virol. 146:1739-1751. [DOI] [PubMed] [Google Scholar]