Abstract
In human holoprosencephaly (HPE), the forebrain does not separate fully into two hemispheres. Further, the border between the telencephalon and diencephalon, the telencephalic/diencephalic junction (TDJ), is often indistinct, and the ventricular system can be blocked at the third ventricle, creating a forebrain ‘holosphere’. Mice deficient in Sonic Hedgehog (Shh) have previously been described to show HPE and associated cyclopia. Here we report that the third ventricle is blocked in Shh null mutants, similar to human HPE, and that characteristic telencephalic and diencephalic signaling centers, the cortical hem and zona limitans intrathalamica (ZLI), are merged, obliterating the TDJ. The resulting forebrain holosphere comprises Foxg1-positive telencephalic- and Foxg1-negative diencephalic territories. Loss of one functional copy of Gli3 in Shh nulls rescues ventricular collapse and substantially restores the TDJ. Characteristic regional gene expression patterns are rescued on the telencephalic side of the TDJ but not in the diencephalon.
Further analysis of compound Shh;Gli3 mutants revealed an unexpected type of signaling center deregulation. In Shh;Gli3 mutants, adjacent rings of Fgf8 and Wnt3a expression are induced in the diencephalon at the ZLI, reminiscent of the Fgf8/Wnt1-expressing isthmic organizer. Neither Shh nor Gli3 single mutants show this forebrain double ring of Fgf/Wnt expression; thus both Shh and Gli3 are independently required to suppress it. Adjacent tissue is not respecified to a midbrain/hindbrain fate, but shows overgrowth, consistent with ectopic mitogen expression.
Our observations indicate that the separation of the telencephalon and diencephalon depends on interactions between Shh and Gli3, and, moreover, demonstrate that both Shh and Gli3 suppress a potential Fgf/Wnt signaling source in the forebrain. That optional signaling centers are actively repressed in normal development is a striking new insight into the processes of vertebrate brain development.
Keywords: Sonic Hedgehog, Gli3, zona limitans, patterning, forebrain, holoprosencephaly
Introduction
Hallmark features of human holoprosencephaly (HPE) include loss of forebrain midline structures (Kinsman et al., 2000; Muenke and Beachy, 2000; Hayhurst and McConnell, 2003; Takahashi et al., 2004; Fernandes and Hebert, 2008). Most commonly the neocortex is conjoined to some degree, and deep gray nuclear defects affecting the striatum and thalamus are often observed (Oba and Barkovich, 1995; Simon et al., 2000; Takahashi et al., 2004; Hahn et al., 2006). For clinical purposes, the degree of cortical non-separation has been used to classify HPE into the DeMeyer subtypes: lobar, semilobar, or alobar (DeMeyer, 1977).
Analyses of the HPE spectrum revealed that in many of the more severe cases, loss of the telencephalic-diencephalic junction (TDJ) and physical obstruction of the diencephalic ventricle occurs (Yokota et al., 1998; Simon et al., 2001; Takahashi et al., 2004; Fernandes and Hebert, 2008). A striatothalamic eminence, comprising remnants of the rostrocaudally-conjoined telencephalon and diencephalon, has also been reported (Fertuzinhos et al., 2009). However the mechanisms underlying these deep gray nuclear defects are unknown, as is their connection with the more widely studied cortical phenotype.
It remains unclear which HPE defects arise due to problems in initial patterning and neural tube development or subsequent degenerative processes, however progress has been made recently in the Shh null mouse (for review see Fernandes and Hebert, 2008). In Shh null embryos, both the telencephalon and diencephalon are reduced in size and the rostral cortical primordium is continuous at the midline. However the dorsal telencephalic midline initially forms, including a robust cortical hem signaling center, a small choroid plexus, and a substantial interhemispheric fissure (Rash and Grove, 2007; Fernandes et al., 2007). Together these cortical defects qualify the Shh mouse mutant as a model of semilobar HPE. Indeed, mutations in human SHH have been associated in several studies with some forms of familial HPE (Muenke and Beachy, 2000). Since no human patients are found with homozygotic SHH mutations, probably because of early death, the Shh null mouse may represent an endpoint in the severity of the Shh-related HPE spectrum. The high variance in severity in human cases may be due to additional mutations or degenerative processes that are at present not understood (Fernandes and Hebert, 2008; Muenke and Beachy, 2000).
In the telencephalon, the dorsal midline secretes Bmp and Wnt proteins, the rostral telencephalic midline acts as a major source of Fgf8, 17, 18, and the ventral telencephalon secretes Shh, Fgf3, and Fgf15 (Shimamura and Rubenstein, 1997; Furuta et al., 1997; Grove et al., 1998; Ohkubo et al., 2002; Hebert et al., 2002; Shimogori et al., 2004; Kiecker and Lumsden, 2005; Rash and Grove, 2007; for review, see Hebert and Fishell, 2008). These signaling centers communicate extensively with one another, coordinately patterning the telencephalon (Shimamura and Rubenstein, 1997; Furuta et al., 1997; Grove et al., 1998; Theil et al., 1999; Ohkubo et al., 2002; Hebert et al., 2002; Shimogori et al., 2004; Storm et al., 2006), and defects in signaling center production and/or interaction may be a root cause of HPE (Monuki, 2007; Fernandes et al., 2007; Rash and Grove, 2007). Thus, in the Shh null, loss of both Shh and Fgf signaling leads to a failure of hemisphere separation at both the rostral and ventral midlines. Notably, removing a copy of Gli3, encoding a transcription factor downstream of Shh, partially rescues the telencephalic phenotype in Shh nulls in part by restoring Fgf expression (Rash and Grove, 2007, Rallu et al., 2002).
The developmental role of Shh in the mouse diencephalon is less well characterized than in other parts of the brain because of exceptionally severe diencephalic growth retardation in Shh mutant mice (Ishibashi and McMahon, 2002). Initially, at embryonic day (E) 8.5, dorsal structures of the diencephalon appear relatively normal in size in Shh mutants compared with control mice, but by E9.5 the Shh mutant diencephalon is much smaller (Ishibashi and McMahon, 2002). These data indicate generally normal patterning and growth of the dorsal brain in Shh mutants until just after neural tube closure when growth in the diencephalon is dramatically reduced by decreased proliferation and increased cell death. The latter are likely to be mediated by the disruption of dorsal Wnt, BMP and FGF signaling caused by loss of ventral Shh and increased levels of Gli3 repressor function (Ishibashi and McMahon, 2002; Litingtung and Chiang, 2000; Ulloa et al., 2007).
Shh is normally secreted from two sources in the diencephalon, the floor plate and the zona limitans intrathalamica (ZLI) (Echelard et al., 1993). The dorsal extension of Shh expression that fills the ZLI develops by E10.5, and loss of Shh protein secreted from the ZLI is likely to contribute significantly to the continued reduction of diencephalic growth (Chiang et al., 1996; Ishibashi and McMahon, 2002). Like the midbrain-hindbrain boundary, the ZLI acts as an important rostrocaudal signaling center that patterns the dorsal thalamus and prethalamus through interaction with other signaling molecules, including Wnt8b, Wnt3a, Fgf8, and Fgf15 (Kiecker and Lumsden, 2004, 2005; Scholpp et al., 2006). Consequently, in the Shh null diencephalon, gene expression of the transcription factors Gbx2 and Dlx2, marking the thalamus and prethalamus respectively, does not appear.
Due to the difficulties presented by such a severe growth phenotype, further analysis of the Shh null diencephalon has been hindered. We reasoned that Shh;Gli3 compound mutants with a partially rescued phenotype could be useful in investigating the diencephalic role of Shh, and in characterizing the genetic relationship between Shh and Gli3 in the diencephalon. We found that comparing single and compound mutants revealed roles for Shh and Gli3 mutations in generating defects similar to the conjoined thalamic nuclei, impairment of the TDJ, and occlusion of the third ventricle observed in humans. In the process, we uncovered an unexpected, covert signaling source, with the potential to perturb normal diencephalic development, which is suppressed by the combined function of Shh and Gli3.
Materials and Methods
Mouse lines
Animal protocols were approved by the University of Chicago’s IACUC, and mice were utilized according to National Institutes of Health guidelines. Noon of the day of vaginal plug detection was designated embryonic day (E) 0.5.
Shh mutant mice (gift of P. Beachy) were obtained in a mixed C57BL6/129 background utilized in the initial analysis of this mutant (Chiang et al., 1996) (C. Chiang, personal communication). A background strain characterization by Charles River Laboratories confirmed the mixed background. To decrease variability, and potentially reduce exencephaly in Shh/Gli3 compound mutants (see below), we increased the contribution of the C57BL/6 strain over 5 generations of further crossbreeding. All Shh nulls from each generation showed similar gross abnormalities, including cyclopia, a facial proboscis, reduction of limb and tail bud and loss of digits. Features of forebrain development, described here, were qualitatively indistinguishable across generations. Shh−/− embryos of a given age were therefore pooled for analysis. No Shh−/− mice were excluded.
In mice with a greater contribution of the C57Bl6 strain we found no evidence of reduction in the deficits caused by deletion of Shh. Indeed, older Shh−/− embryos were recovered at lower rates than previously (Chiang et al., 1996) suggesting greater earlier lethality. Between E9.5 and 12.5, however, Shh mutants were recovered at Mendelian ratios (at E10.5, 51 Shh+/+, 87 Shh+/−, and 51 Shh−/−).
Available evidence indicates that the extra-toesJ (XtJ) mutation is a loss-of-function mutation in the Gli transcription factor gene, Gli3 (Buscher et al., 1998). Gli3XtJ/XtJ embryos can have a high incidence of midbrain exencephaly, with gross morphological changes that confound forebrain analysis. As in previous studies (Theil et al., 1999; Tole et al., 2000; Rallu et al., 2002; Kuschel et al., 2003; Theil, 2005; Rash and Grove, 2007), we restricted forebrain analysis to non-exencephalic Gli3XtJ/XtJ embryos. To reduce the incidence of exencephaly, Gli3XtJ mice were maintained on a C57Bl6 background (gift of Y. Furuta); thus, we discarded only 1/34 Gli3XtJ/XtJ E10.5 embryos.
Mice heterozygous for both the Gli3XtJ and Shh mutations were intercrossed to obtain Shh; Gli3XtJ compound mutants. At E10.5, 194 embryos were recovered. Of 43 Shh−/−;Gli3+/XtJ mice, only 2 were exencephalic; by contrast, 12/16 Shh−/−;Gli3XtJ/XtJ mutants were exencephalic. PCR genotyping for Shh and Gli3XtJ mice was performed as described (Chiang et al., 1996; Maynard et al., 2002).
Ventricular analysis
E9.5 and E10.5 embryos were harvested and immediately placed into PBS for dye injection. A small window was cut in the wall of the mesencephalon to allow dye to flow freely through the ventricular system. Injections utilized a pulled glass capillary with an approximate 30µm interior tip diameter filled with a 0.02% solution of fast green in DEPC-PBS attached to a mouth pipette. Dye solution was injected into the right telencephalic hemisphere and pressure was sustained for at least 10 seconds to fill the ventricular system. Unfixed embryos were used to eliminate the possibility of ventricular deformity due to tissue shrinkage during fixation.
In situ hybridization, tissue processing and imaging
Embryos were fixed in 4% paraformaldehyde/PBS and processed for whole mount or section in situ hybridization as described previously (Grove et al., 1998). Brains were sectioned with a Leica EM200R sledge microtome. Processed tissue was photographed using a Leica dissecting microscope for wholemount embryos, or a Zeiss Axioskop for sections. Images were obtained with a Zeiss Axiovision camera and software, with image processing using Adobe Photoshop.
DiI axon tracing
Brains were dissected and fixed by immersion in 4% PFA overnight. A solution of 10% DiI (Molecular Probes D-282) in 100% dimethyl formamide was injected via a pulled glass capillary attached to a picospritzer into the cerebral cortex to label corticofugal projections. Injected brains were incubated at 37°C in the dark for 2–3 weeks before sectioning at 50µm on a Leica VT1000S vibrating microtome.
Results
The third ventricle is closed and the forebrain is a holosphere in Shh nulls
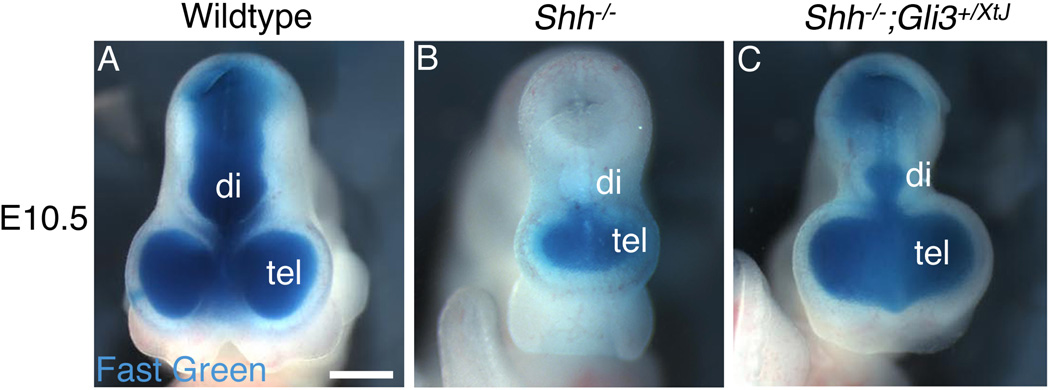
To investigate ventricular development in Shh nulls and Shh−/−;Gli3+/XtJ embryos, we injected fast green into the E9.5–E10.5 telencephalic lateral ventricles after cutting a hole in the wall of the mesencephalon. In wildtype embryos the dye solution passed freely through the third ventricle, filled the ventricular system, and exited the neural tube (Figure 1A). However, in Shh null embryos there was no fluid flow from the forebrain to the mesencephalon at E9.5 (n=3/3) or E10.5 (n=6/6). Indicating total occlusion in the diencephalon, dye filled only a monoventricle in the mutant forebrain (Figure 1B). In Shh−/−;Gli3+/XtJ embryos, the ventricular system was substantially rescued, displaying a small diencephalic ventricle (n>6) (Figure 1C).
Figure 1. Blockage of the third ventricle is a feature of HPE in Shh null embryos and is rescued by removing one functional copy of Gli3.
The telencephalic vesicle (tel) in E10.5 embryos was injected with a solution of fast green to fill the ventricular system, with a hole cut in the mesencephalon to allow the dye to exit. The lateral ventricles and third ventricle are filled with dye in a wildtype embryo (A). Ventricular flow is blocked at the level of the third ventricle in a Shh null embryo, so that the forebrain ventricular system forms a sealed monoventricle (B). In contrast, ventricular system continuity is restored in a Shh−/−;Gli3+/XtJ embryo (C), although the diencephalic ventricle (di) is reduced in size compared with wildtype. Scale bar is 700µm.
As previously noted, in Shh null mice at E8.5, just before neural tube closure, the dorsal diencephalon is normal in size (Ishibashi and McMahon, 2002). Only one day later, just after neural tube closure, the diencephalon is greatly reduced compared with control mice (Ishibashi and McMahon, 2002), suggesting that dramatic defects in the Shh null diencephalon begin around the time the tube forms. The closing of the neural tube in Shh mutants may therefore never leave space for the third ventricle. Alternatively, the third ventricle may be open immediately after the neural tube forms; if so, it is patent for a very short time, becoming occluded by E9.5.
Indicating that boundary disruption between the telencephalon and diencephalon is ongoing in the Shh mutant, we found that forebrain tissue rostral to the closed third ventricle (roughly at the level of the ZLI; see below) later separated from the remainder of the neural tube. By E17.5, no continuity of the neural tube could be detected at this site by analysis of tissue sections, and indeed during dissection, the forebrain became easily detached (n>8). Developing meninges and Reelin-expressing cells marking the cortical marginal zone were observed intervening at all points between the forebrain and the more caudal neural tube (n>3) (Supplemental Figure 1B,D). Not surprisingly, corticofugal axons were unable to exit the telencephalon to enter the vestigial dorsal thalamus, and instead of passing through an internal capsule, they were redirected medially to form an ectopic commissure (n=3/3; Supplemental Figure 1F).
Merged gene expression domains indicate a malformed TDJ in the Shh mutant forebrain
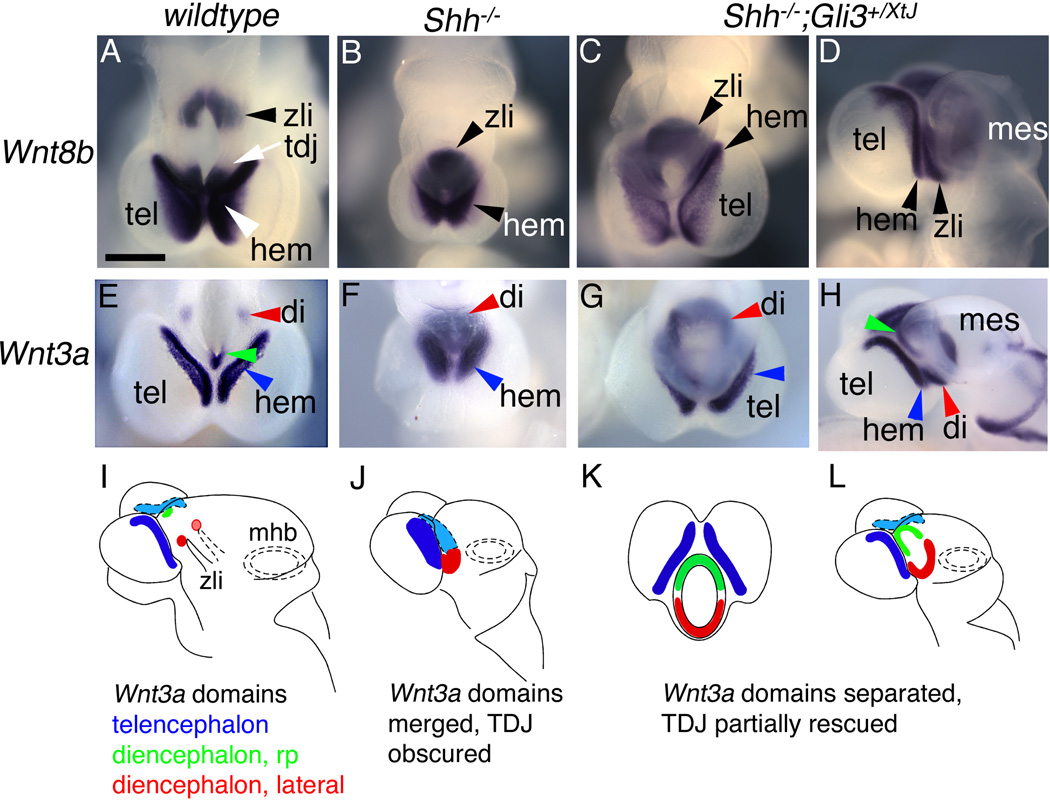
The telencephalic cortical hem expresses Wnt3a and Lmx1a, and both the hem and hippocampal primordium express Wnt8b. In Shh nulls, Wnt3a, Wnt8b, and Lmx1a expression formed a horseshoe-like shape in the prosencephalon rostral to the point of third ventricle closure, giving the initial impression that the cortical hem was fused caudoventrally with itself (Figure 2B,F; Rash and Grove, 2007). On closer inspection of Shh−/−;Gli3+/XtJ mice, which show partial but incomplete rescue of normal forebrain Wnt3a, Wnt8b, and Lmx1a expression, the horseshoe shape was revealed as a merger of telencephalic and diencephalic gene expression domains, representative of the lack of a clear TDJ.
Figure 2. Separate Wnt gene expression domains in the cortical hem and diencephalon are merged in the Shh mutant.
(A–H) Wholemount in situ hybridization for Wnt8b and Wnt3a in E10.5 control (A,E), Shh null (B,F), and Shh−/−;Gli3+/XtJ (C,D,G,H) embryos. Forebrains in (A–C, E–G) are shown in dorsal views, rostral is down; (D,H) are lateral views of the embryos in (C,G), rostral is to the left. (I–L) Schematics of Wnt3a expression in all three genotypes represent the data shown in (E–H). (A–D) In a wild type embryo, domains of Wnt8b in the hem and ZLI are separated by the TDJ (white arrow) and rostral diencephalon (A). A Shh null mouse has a single horseshoe-shaped domain of Wnt8b (B), whereas in a Shh−/−;Gli3+/XtJ mutant, distinct expression domains of Wnt8b in the hem and diencephalon are recovered (D, arrowheads); diencephalic Wnt8b expression remains expanded (arrowhead indicating the ZLI in C,D). (E–L) Wnt3a expression shows similar patterns (E–H), schematized in (I–L). In a wildtype forebrain (E), Wnt3a expression in the telencephalic hem (blue arrowhead) is distinct from Wnt3a expression in the diencephalic roofplate (rp) (green arrowhead), and two lateral diencephalic expression domains (red arrowhead). Schematic in (I) shows these domains in colors matching the arrowheads in (E). The lateral diencephalic domains (red) lie just dorsal to the tips of the forked ZLI (I). In a Shh null forebrain (F,J), Wnt3a expression in the hem (blue arrowhead in F, filled blue in J) merges with expanded diencephalic Wnt3a expression (red arrowhead in F, filled red in J) to form a horseshoe shape (F). In a Shh−/−;Gli3+/XtJ mutant (G,H,K,L), separate telencephalic and diencephalic domains of Wnt3a expression are recovered, but differences from wildtype brains remain. The lateral diencephalic Wnt3a domains are conjoined across the ventral midline in a ventral crescent (red arrowheads in G,H, filled red in K,L), and the roof plate Wnt3a domain expands ventrally, forming a dorsal crescent (green arrowhead in H, filled green in K,L) nearly meeting the ventral crescent. Hem, cortical hem; tel, telencephalon; tdj, telencephalic-diencephalic junction; di, diencephalon; mes, mesencephalon; zli, zona limitans intrathalamica. Scale bar is 540µm.
In wildtype mice, Wnt8b is robustly expressed in the diencephalic basal plate, partially overlapping the ZLI, as well as in the telencephalic cortical hem and hippocampal primordium at E10.5 (Figure 2A; Supplemental Figure 2; Grove et al., 1998; Zeltser, 2005; Kiecker and Lumsden, 2005; Scholpp et al., 2006). In Shh null embryos the telencephalic and ZLI expression domains of Wnt8b were merged (n=3; Figure 2B; Supplemental Figure 2). When one functional copy of Gli3 was removed in Shh−/−;Gli3+/XtJ mice, the cortical hem and ZLI Wnt8b expression domains were once more distinguishable, but still closely adjacent (n=3) (Figure 2C,D). Wnt3a is normally expressed in the telencephalic cortical hem and in the diencephalic roofplate and small bilateral regions near the dorsal tip of the ZLI (Figure 2E,I; Supplementary Figure 2; Braun et al., 2003; Louvi et al., 2007). In Shh nulls, these Wnt3a expression domains merged to form the horseshoe shape described above (Figure 2F,J). Comparing the expression of Wnt3a in Shh and Shh−/−;Gli3+/XtJ mutant mice revealed the same merger of telencephalic and diencephalic domains as seen previously for Wnt8b in the Shh null mouse, and the same partial rescue when one functional copy of Gli3 was removed (Figure 2E–L; Supplementary Figure 2). At E10.5, in Shh−/−;Gli3+/XtJ embryos, the telencephalic cortical hem domain of Wnt3a could be distinguished from the diencephalic domains, although the latter remained expanded, almost around the circumference of the diencephalon (n>3; Figure 2G,H,K,L).
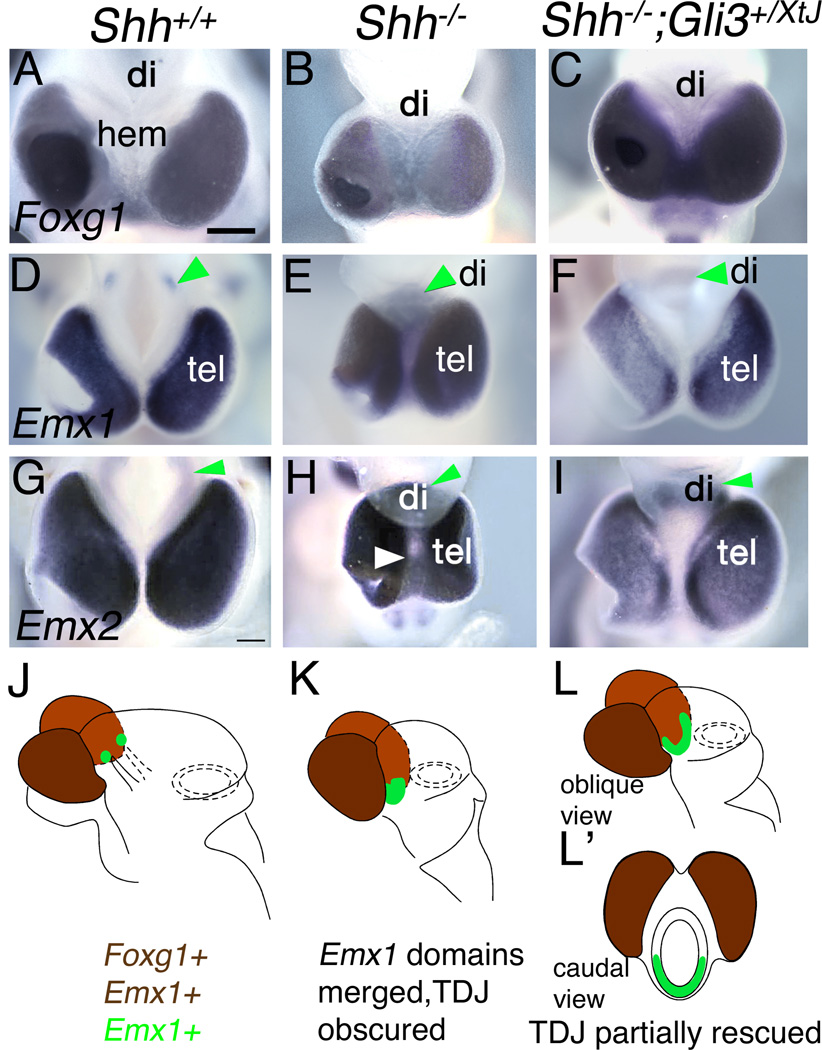
Importantly, the diencephalic and telencephalic contributions to the Shh null forebrain holosphere were distinguishable in all genotypes by differential expression of Foxg1, which in the wildtype mouse brain is a molecular marker of the telencephalon (Figure 3A,J; Shimamura and Rubenstein, 1997). In the control telencephalon, Emx1 and Emx2 expression roughly colocalizes with Foxg1 expression but also shows diencephalic domains (Figure 3A,D,G). In Shh nulls, Emx1 and Emx2 expression filled both the Foxg1-positive, telencephalic portion and the Foxg1-negative, diencephalic portion of the holosphere (Figure 3B,E,H,K), reflecting the expansion of the small, discrete domains of Emx gene expression in the wildtype diencephalon (Figure 3D,G; arrowheads), and a merger with telencephalic Emx gene expression domains, a conclusion again supported by comparing Shh and Shh−/−;Gli3+/XtJ mutant mice. As before, diencephalic and telencephalic domains of Emx gene expression could be distinguished in the compound mutants, although diencephalic Emx gene expression was still expanded (Figure 3F,I,L,L’; n>3 for both Emx1 and Emx2). In summary, diencephalic and telencephalic expression domains of Wnt3a, Wnt8b, Emx1 and Emx2 all merge in the Shh null mouse, indicating the lack of a normal TDJ. This merger was revealed by comparing gene expression patterns in Shh and Shh−/−;Gli3+/XtJ mutant mice. In the latter, Wnt3a, Wnt8b, Emx1 and Emx2 expression domains are distinguishable between the telencephalon and diencencephalon without completely regaining their wildtype restriction. We term this separation into clear telencephalic and diencephalic domains at least a partial restoration of the TDJ.
Figure 3. Molecular identification of telencephalic and diencephalic components of the holosphere.
Dorsal views of E10.5 wildtype (A,D,G), Shh null (B,E,H), and Shh−/−; Gli3+/XtJ (C,F,I) embryos processed for in situ hybridization for Foxg1, Emx1, and Emx2. Rostral is down. (J–L,L’) Schematics of all three genotypes based on Emx1 gene expression data in (D–F), but indicative of expression patterns of both Emx genes (D–I). Expression of both Foxg1 and Emx1 in the telencephalon is indicated by brown in (J–L,L’); diencephalic expression of Emx1 is green.
(A–C) Foxg1 is a marker of telencephalic but not diencephalic tissue at E10.5 (A). In a Shh null mouse (B) and a Shh−/−;Gli3+/XtJ mouse (C), Foxg1 is expressed appropriately in the telencephalon and not the diencephalon (also see filled brown in J–L,L’). (D–I) In a wildtype embryo, Emx1 and Emx2 are expressed in the telencephalon and discrete domains in the diencephalon (D,G, green arrowheads; also filled green in J). In a Shh mutant, diencephalic domains of Emx1 and Emx2 expression expand and merge with the telencephalic domain, so that the entire holosphere expresses Emx1 and Emx2 (E,H green arrowheads, and filled green in K). In Shh−/−;Gli3+/XtJ mice, separate Emx gene expression domains are partially restored, but Emx1 and Emx2 expression remains continuous across the Foxg1-negative ventral diencephalic midline (F,I, green arrowheads, filled green in L,L’). Tel, telencephalon; di, diencephalon; hem, cortical hem. Scale bar is 540µm.
Relationship between Shh and Gli3 in forebrain patterning
In the telencephalon, deletion of one copy of Gli3 in a Shh mutant substantially rescues dorsoventral patterning (Rash and Grove, 2007; Rallu et al., 2002). Consistent with this type of epistatic role for Gli3 over Shh in the division of the forebrain into diencephalon and telencephalon, we found that loss of one functional copy of Gli3 separated the conjoined telencephalic and diencephalic Wnt and Emx gene expression domains in Shh nulls, partially restoring the TDJ.
Nonetheless in Shh−/−;Gli3+/XtJ mutants, diencephalic Wnt and Emx expression domains extended abnormally across the ventral diencephalic midline, forming crescents of gene expression linking the two sides of the diencephalon (Figure 2C,D,G,H,K,L, and Figure 3 F,I,L,L’). We also noted that in Shh−/−;Gli3+/XtJ mutants, the eye field in the ventral diencephalon failed to develop bilaterally by E10.5 (see Figure 6C; white arrowhead), so that the two eyes subsequently connected across the midline (Supplementary Figure 3; arrowheads). These observations extend findings in the wild type spinal cord, where lateral gene expression domains of En1, Lhx3, and Pax2 link across the ventral midline of Shh mutants and continue to be linked in Shh;Gli3 compound mutants (Litingtung and Chiang, 2000).
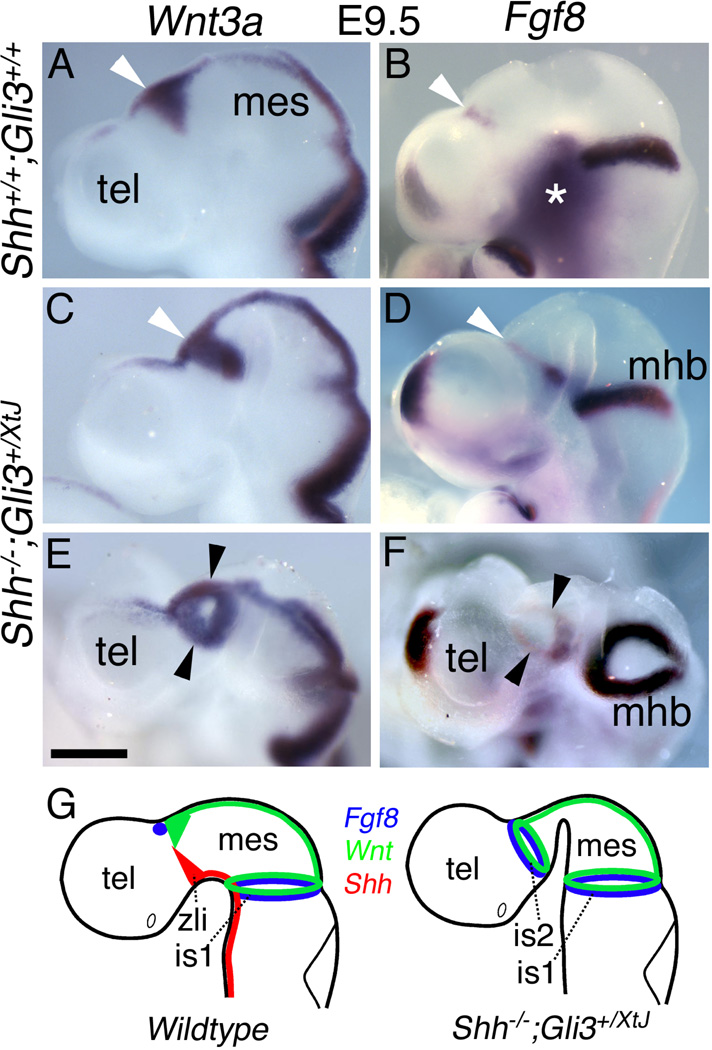
Figure 6. Appearance of a diencephalic ring of Fgf8 expression in Shh;Gli3 compound mutant embryos.
In situ hybridization for Fgf8 at E10.5 in wildtype (A,F), Gli3XtJ/XtJ (B,G), Shh null (C,H), Shh−/−;Gli3+/XtJ (D,I), and Shh;Gli3 double null (E,J) mutants. A–E are lateral views; F–J are dorsal views of the same embryos. (K–O) Schematics of Shh and Fgf8 expression represent the data shown in (A–J). (A–J) In a wildtype embryo, diencephalic expression is limited to the roof plate region (arrowheads in A,F) and floor plate (arrows in A and F). In a Gli3XtJ/XtJ embryo, the roof plate domain expands, extending towards the ZLI (arrowheads in B, G). In a Shh null mouse, no forebrain Fgf8 expression was detected except in the cyclopic eye primordium (white arrowhead in C), also visible in (H). However in both Shh−/−;Gli3+/XtJ and Shh;Gli3 double null embryos a nearly complete ring of Fgf8 encircles the diencephalon (arrowheads in D,I and E,J). Tel, telencephalon; hs, holosphere; di(rf), diencephalon (roof plate); di(fl), diencephalon (floor plate); zli, zona limitans intrathalamica; mhb, midbrain-hindbrain boundary. Scale bar is 1.2mm in A–E; 700µm in F–J.
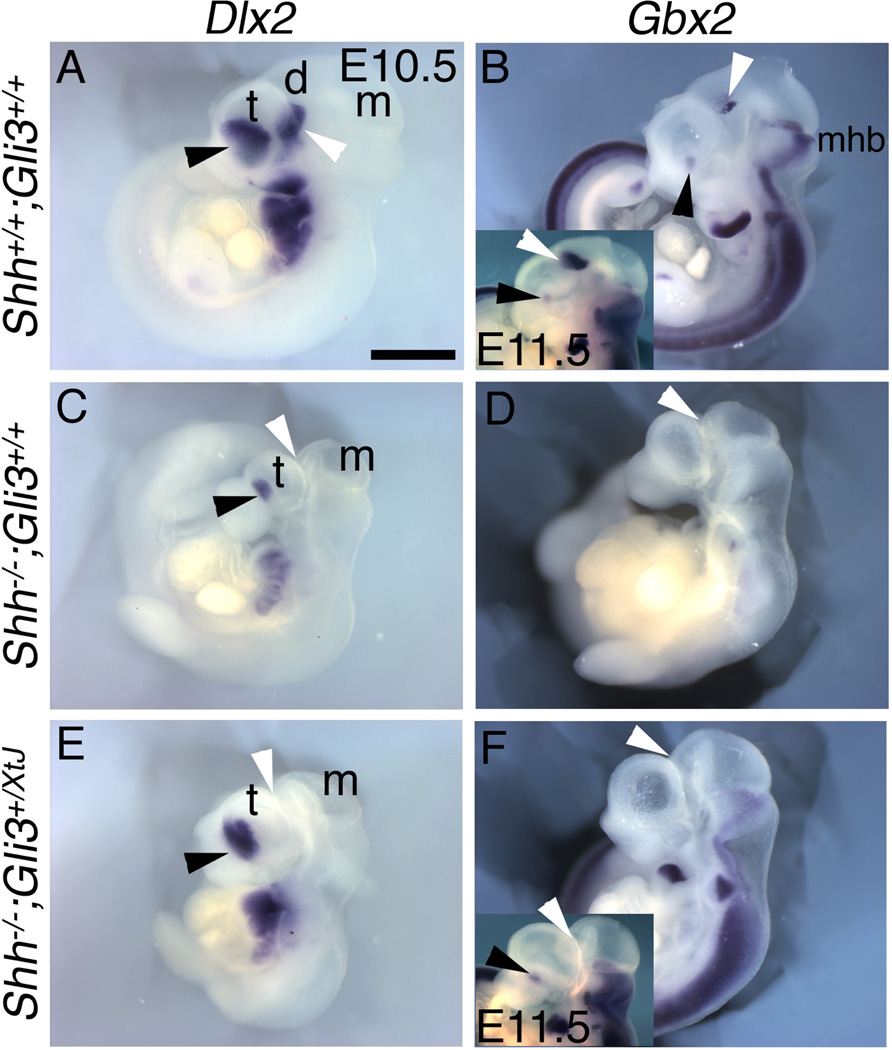
Most striking, the loss of patterned gene expression in the thalamus and prethalamus of the Shh mutant appears to differ from other phenotypic features, in that spinal cord patterning, digit number and telencephalic morphology are all at least partially rescued by reducing Gli3 (Litingtung and Chiang, 2002; Rallu et al., 2002; Rash and Grove, 2007). In the wildtype diencephalon, Gbx2 and Dlx2 mark the developing thalamus and prethalamus, respectively, at E10.5 (Figure 4A,B, white arrowheads) (Kiecker and Lumsden, 2004; Scholpp et al., 2006). Both Gbx2 and Dlx2 expression domains are lost in the Shh null diencephalon (Kiecker and Lumsden, 2004; Figure 4C,D), and after removing one or even both functional copies of Gli3 in the Shh null we found no evidence of any recovered expression of either Gbx2 or Dlx2 (n>4/4; Figure 4E,F). These observations suggest that patterned diencephalic expression of Gbx2 and Dlx2 depends on Shh signaling from the ZLI (Kiecker and Lumsden, 2004), and is not suppressed solely by Gli3 repressor activity. Fgf15, however, which is expressed in both thalamus and prethalamus in wildtype mice, is lost in the Shh null diencephalon, largely because the diencephalon itself appears vestigial, and restored in both thalamus and prethalamus in the double Shh;Gli3 null (Supplemental Figure 4).
Figure 4. Diencephalic Dlx2 and Gbx2 expression requires Shh function independent of Gli3.
Lateral views of E10.5 wildype (A,B), Shh null (C,D), or Shh−/−;Gli3+/XtJ (E,F), mutants processed for in situ hybridization. In control embryos Dlx2 marks rostral (prethalamus) regions of the diencephalon (white arrowheads) as well as domains in the ventral telencephalon (black arrowheads) (A). In Shh null embryos, Dlx2 is absent from the diencephalon and reduced in the telencephalon (C). In Shh−/−;Gli3+/XtJ mutants, Dlx2 expression recovered in the telencephalon, but not in the diencephalon (E). Gbx2 expression marking the thalamus (B; white arrowhead, inset is E11.5) is lost in Shh nulls (D) and not restored in Shh−/−;Gli3+/XtJ mutants (F), even at E11.5 (inset in F). Scale bar is 1.2mm (1.7mm in B,F insets).
Emergence of an isthmus-like source of Fgf8 and Wnt3a in the forebrain of Shh;Gli3 compound mutants
The isthmus at the midbrain-hindbrain boundary and the ZLI are signaling centers that direct patterning and morphogenesis of the neural tube (Ishibashi and McMahon, 2002; Kiecker and Lumsden, 2004, 2005; Scholpp et al., 2006; Wurst and Bally-Cuif, 2001; Martinez, 2001; Sato et al., 2004). Although the secreted signaling proteins normally generated by the isthmus and ZLI are different, the centers display similarities. Both are discrete transverse signaling centers that partially encircle the neural tube and pattern the brain along the rostrocaudal axis. Furthermore, their positioning is determined by transcription factor domains established just after neurulation (Ishibashi and McMahon, 2002; Kobayashi et al., 2002; Kiecker and Lumsden, 2004, 2005; Scholpp et al., 2006; Wurst and Bally-Cuif, 2001; Martinez, 2001). At the isthmus, a transverse source of Fgf8 and Wnt1 is critical for specifying the midbrain and cerebellum (Crossley et al., 1996; Wurst and Bally-Cuif, 2001; Martinez, 2001; Sato et al., 2004). The ZLI does not normally express Fgf8, but instead is a prominent source of Shh, directing diencephalic patterning (Ishibashi and McMahon, 2002; Kiecker and Lumsden, 2004, 2005).
Remarkably, in Shh;Gli3 compound mutants at E9.5–10.5, complete rings of Fgf8 and Wnt3a were evident around the diencephalon (n>9; Figures 5). Wnt3a and Wnt1 both initiate the canonical, β-catenin-mediated Wnt signaling pathway and have similar effects on neural tube patterning and growth (Megason and McMahon, 2002; Alvarez-Medina et al., 2008). Thus, ectopic adjacent rings of Fgf8 and Wnt3a expression in the Shh;Gli3 mutant diencephalon (Figure 5) strongly resemble Fgf8 and Wnt1 expression at the isthmus. Furthermore, these diencephalic rings of Fgf8 and Wnt3a appear only in brains deficient in both Shh and Gli3. In wildtype brains at E10.5, Fgf8 is expressed in the dorsal and ventral diencephalic midlines, near the ZLI (Figure 6A,F,K). Dorsally, the Fgf8 domain extends finger-like projections ventrally towards the Shh domain in the ZLI. These finger-like extensions of Fgf8 expression expanded in Gli3 mutants, but did not encircle the diencephalon (Figure 6B,G,L). In Shh nulls, no diencephalic Fgf8 could be detected (Figure 6C,H,M). In striking contrast, in Shh−/−;Gli3+/XtJ and Shh;Gli3 double null embryos two discrete rings of Fgf8 expression encircled the neural tube, one in the diencephalon, and the other at the isthmus (Figure 6D,E,I,J,N,N’,O). The diencephalic ring appeared to be located at the normal site of the ZLI, which is marked not only by a discrete band of Shh expression in wild type and Gli3 mutant embryos (Figure 7A–D), but also morphologically by an inflection of the neural tube visible in tissue sections (white arrowhead in Figure 7D,F). Shh;Gli3 double nulls showed a similarly positioned inflection, now expressing Fgf8 rather than Shh (Figure 7E,F). Potentially, the new Fgf8 ring could be generated by a merger of wild type dorsal and ventral Fgf8 domains (Figure 6A,F; arrow and arrowhead), but given that in a Shh null background ventral midline structures are typically lost and dorsal gene expression domains expanded, we suggest that the dorsal Fgf8 domain expands around the diencephalon to create the ring.
Figure 5. Appearance of an isthmus-like signaling center in the diencephalon of Shh−/−;Gli3+/XtJ mutants.
In situ hybridization in control embryos (A,B) shows dorsal Wnt3a (A) and Fgf8 domains (B). Shh−/−;Gli3+/XtJ embryos (C–F) show a complete ring of Wnt3a (C,E) and an adjacent, near-complete ring of Fgf8 (D,F) expression (arrowheads). Images in (E) and (F) are oblique views of the embryos in (C) and (D). In these mutants, the strongest Fgf8 expression is in the ventral diencephalon. As at the isthmus, Fgf8 expression is not detected at the diencephalic roof plate. Expression patterns of Wnt1/Wnt3a (green), Shh (red), and Fgf8 (blue) are schematized for wild type and Shh;Gli3 compound mutant embryos (G). The asterisk in (B) marks non-specific background. Tel, telencephalon; mes, mesencephalon; zli, zona limitans intrathalamica; mhb, midbrain-hindbrain boundary; is1, isthmus 1; is2, isthmus 2. Scale bar is 450µm.
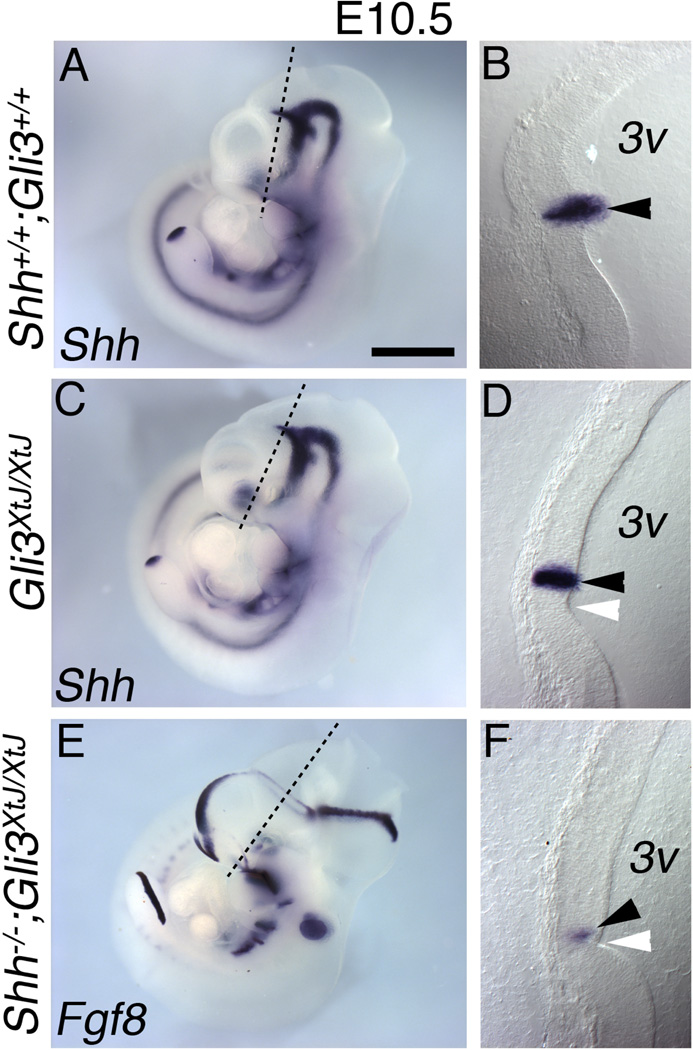
Figure 7. Identification of the ZLI as the site of the ectopic isthmus-like signaling source.
E10.5 wild type (A–B), Gli3XtJ/XtJ (C–D) and Shh;Gli3 double null (E–F) embryos processed for in situ hybridization for Shh (A–D) or Fgf8 (E–F). The embryos shown in A, C, and E were sectioned perpendicular to the ZLI at the position shown (black line) at 40µm, and sections are shown on the right (B,D,F). Note that panel E is the same as Figure 6E. In control embryos Shh expression appears near a morphological inflection in the interior wall of the third ventricle (3v) (B; arrowhead). In Gli3XtJ/XtJ and Shh;Gli3 double null embryos, this morphological feature remains (D,F; white arrowheads). In Gli3XtJ/XtJ embryos, Shh expression marks the ZLI (D; black arrowhead) as in wild type, but in Shh;Gli3 double nulls, a diencephalic Fgf8-expressing ring takes its place (F; black arrowhead). Scale bar is 1.2mm in A,C,E; 200µm in B,D,G.
Morphogenetic and gene expression changes in the diencephalon of Shh;Gli3 mutants
We hypothesized that the new Wnt/Fgf signaling source revealed in Shh;Gli3 compound mutants influences the development of the diencephalic vesicle. Consistent with this possibility, the diencephalon in Shh;Gli3 double null embryos was substantially larger at E10.5 than in Gli3 mutants (n=4/4) (Figure 8). We next investigated whether the Fgf8/Wnt3a rings respecified diencephalic tissue to an isthmic or midbrain/hindbrain-like identity in Shh−/−;Gli3+/XtJ embryos. Spry1, a downstream target of Fgf8, was upregulated in the Shh−/−;Gli3+/XtJ diencephalon compared with controls and Shh nulls (Supplemental Figure 5A,D,G), indicating that the ectopic ring of Fgf8 is successful in activating the Fgf signaling pathway. Although Wnt1 was not detected in this abnormal ZLI in compound mutants at E10.5 (data not shown), likely because of the continued presence of Emx2 (Ligon et al., 2003), Wnt3a showed robust expression. However, we did not observe diencephalic En1/2 gene expression, which is characteristic of the isthmus and activated by canonical Wnt signaling (McMahon et al., 1992), at E10.5–11.5 in Shh nulls and Shh−/−;Gli3+/XtJ mutants (n=4; Supplemental Figure 5B,C,E,F,H,I). Nor was diencephalic En1/2 expression activated in Shh;Gli3 double nulls (Supplemental Figure 5J,K; arrowheads), indicating that Gli3 gene dosage is not important. En1/2 was expressed at the isthmus itself in each genotype (Supplemental Figure 5B,C,E,F,H,I,J,K).
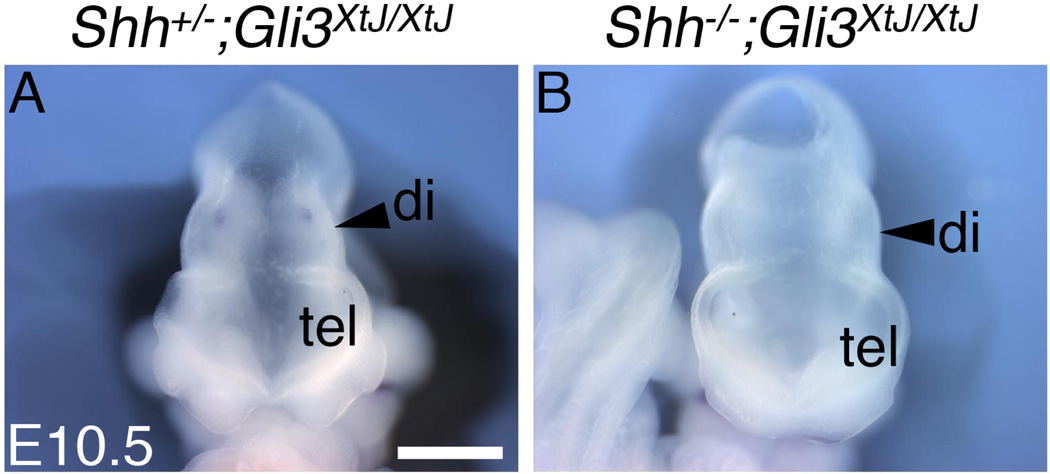
Figure 8. Increased diencephalic size in Shh;Gli3 double nulls.
Frontal views of Shh+/−;Gli3XtJ/XtJ (A) and Shh;Gli3 double null (B) embryos at E10.5. Double nulls (n=4) consistently showed larger relative diencephalic size compared with littermate Gli3 nulls or Gli3 nulls lacking one copy of Shh. Tel, telencephalon; di, diencephalon. Scale bar is 700µm.
Discussion
Our analysis of Shh and compound Shh;Gli3 mutants revealed interactions between Shh and Gli3 that underlie both normal diencephalic development as well as Shh-associated HPE in mice. Specifically, Shh is required for third ventricle formation and for production of an adequate TDJ to separate the telencephalon and diencephalon. These activities appear to be Gli3 dependent, given that removing a copy of Gli3 from Shh mutants partially rescues the TDJ. In contrast, Shh may function independently of Gli3 to maintain characteristic gene expression patterns in the thalamus and prethalamus. That is, removal of a copy of Gli3 fails to rescue diencephalic Dlx2- and Gbx2-positive territories in the Shh mutant. Strikingly, both Gli3 and Shh are required to suppress the emergence of an Fgf/Wnt source at the ZLI that resembles the isthmus. These findings clarify the interplay between Shh and Gli3 in diencephalic development. Moreover, they demonstrate a capacity for an isthmus-like signaling source to develop in the ZLI region that must be actively suppressed in normal brain development.
Formation of the holosphere in HPE
We propose a model whereby the holosphere in HPE arises as a result of three types of failure in neural tube development. In the Shh null mouse, which shows severe HPE, the rostral and ventral telencephalic midlines fail to develop, despite little initial change in dorsal midline formation (Fernandes and Hebert, 2008; Rash and Grove, 2007; Muenke and Beachy, 2000; Chiang et al., 1996). Second, the ventricular system is closed at the level of the diencephalon, apparently just caudal to the ZLI. Third, the TDJ fails to form normally, reflected by the merging of gene expression domains characteristic of the telencephalon and diencephalon. A single forebrain ‘holosphere’ forms with little morphological distinction between its telencephalic and diencephalic portions. A diencephalic remnant can be identified within the Shh null holosphere, however, by the absence of Foxg1 expression, providing a potential framework for understanding the severe deep gray nuclear defects of the thalamus in human HPE. For example, the appearance of bilaterally conjoined domains of Wnt3a (and Emx1) across the Foxg1-negative diencephalic midline in Shh nulls provides a plausible correlate to the observation of conjoined thalamic nuclei in human HPE, particularly considering that many neurons in the wildtype thalamus are derived from diencephalic Wnt3a-expressing progenitors (Louvi et al., 2007).
Capacity of the diencephalon to generate an isthmus-like signaling center
An important function of Shh and Gli3 in forebrain development appears to be the suppression of an isthmus-like organizer in the diencephalon. This process, which could only be discovered when both genes were deleted, reveals a striking degree of covert similarity between the ZLI and the isthmus. Not only is the ZLI a narrow, transverse signaling source partially encircling the neural tube, like the isthmus, it is also capable of expressing a canonical Wnt gene (Wnt3a, but not Wnt1) and Fgf8 in a double ring-like pattern. Both Shh and Gli3 are capable of repressing this diencephalic isthmus-like center in the absence of the other, given that this hidden signaling center is not seen in either single mutant. Upregulation of Fgf8 expression by loss of Gli3 function, independent of the status of Shh, has been characterized previously in the telencephalon and more generally in embryonic development (Theil et al., 1999; Grove et al., 1998; Aoto et al., 2002; Rash and Grove, 2007). Of particular interest, however, mice deficient in Shh and Gli3 do not simply show ectopic patches of upregulated Fgf8 and Wnt3a in the diencephalon, but well-formed rings of Fgf8 and Wnt3a expression. Further, these rings do not mimic the shape of the Shh expression domain in the normal ZLI. Rather, the forebrain appears to have a covert ability to generate ring patterns of Fgf8 and Wnt3a expression at the level of the ZLI, an ability that is actively suppressed in normal development. This type of prepattern in the neural tube at the level of the ZLI has not been previously reported.
Altered diencephalic patterning in Shh;Gli3 compound mutants
How does the diencephalon respond to the loss of Shh and the appearance of Fgf8/Wnt3a rings? Fgf8-containing beads can induce isthmic and midbrain/hindbrain fates in the caudal chick diencephalon (Martinez et al., 1999). We therefore investigated whether the ectopic Fgf8/Wnt3a ring in Shh;Gli3 compound mutants similarly reprograms the diencephalon. We found that Spry1 expression was upregulated, correlating with the appearance of the new ring of Fgf8 (Supplemental Figure 5Q), but that neither En1 nor En2 expression is induced, which would have been more indicative of a true midbrain/hindbrain fate. Nonetheless, the diencephalon appeared larger in Shh;Gli3 double null embryos than in either single mutant, perhaps because both Fgf8 and Wnt3a are mitogens (Storm et al., 2006; Megason and McMahon, 2002; Lee at al., 2000), and more than compensate for the loss of Shh proliferative activity. Our findings therefore support active Fgf8 signaling and increased growth in the diencephalon of double mutants, but not a transformation of diencephalic tissue to a midbrain-hindbrain fate. The reasons why Fgf8 beads, but not the ectopic ring of Fgf8 we observed, should induce a fate change, are unclear at this point, but could be as simple as a difference in levels of Fgf8. Fgf8 beads are likely to provide a high local concentration of Fgf8, which may be required to transform diencephalic tissue to a midbrain-hindbrain fate (Martinez et al., 1999), whereas the level of Fgf8 mRNA expression in the ectopic forebrain ring is noticeably lower than at the Fgf8 patterning sources at the isthmus, or in the rostral telencephalon (Figure 5F).
Our observations specifically indicate that the region of the ZLI has a versatile signaling capability. Normally the ZLI is marked by a fork-shaped domain of Shh expression, but in the absence of Shh and Gli3 function, rings of Fgf8 and Wnt3a expression surround the neural tube instead. Thus, perturbing genetic regulatory mechanisms responsible for a normal signaling center may result in the development of an entirely different but equally organized signaling source. An important process in vertebrate brain development, hitherto unappreciated, may be the suppression of such covert signaling centers. Together, our findings provide novel insights into the mechanisms of Shh and Gli3 function in forebrain development and disease.
Research Highlights.
HPE involves failed formation of the TDJ and ventricular collapse
Genetic relations of Shh and Gli3 differ in the telencephalon and diencephalon
Shh and Gli3 suppress an ‘isthmus-like organizer’ in the diencephalon
Ectopic rings of Fgf8 and Wnt3a correlate with increased growth
Ectopic Fgf8 and Wnt3a are unable to repattern the diencephalon
Supplementary Material
Acknowledgements
This work was supported by National Institute of Mental Health Grant R01 MH59962, by the Brain Research Foundation at the University of Chicago, and by a National Institutes of Health training grant administered by the Committee on Neurobiology. We thank Chip Ferguson, Vicky Prince, Chris Lowe, and Cliff Ragsdale for helpful discussions, Philip Beachy (Shh) and Yas Furuta (Gli3XtJ) for mice, and Stavroula Assimacopoulos and Albert Taylor for experimental and technical help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abzhanov A, Cordero DR, Sen J, Tabin CJ, Helms JA. Cross-regulatory interactions between Fgf8 and Shh in the avian frontonasal prominence. Congenit. Anom. 2007;47:136–148. doi: 10.1111/j.1741-4520.2007.00162.x. [DOI] [PubMed] [Google Scholar]
- Alvarez-Medina R, Cayuso J, Okubo T, Takada S, Martí E. Wnt canonical pathway restricts graded Shh/Gli patterning activity through the regulation of Gli3 expression. Development. 2008;135:237–247. doi: 10.1242/dev.012054. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Aoto K, Nishimura T, Eto K, Motoyama J. Mouse GLI3 regulates Fgf8 expression and apoptosis in the developing neural tube, face, and limb bud. Dev. Biol. 2002;251:320–332. doi: 10.1006/dbio.2002.0811. [DOI] [PubMed] [Google Scholar]
- Braun MM, Etheridge A, Bernard A, Robertson CP, Roelink H. Wnt signaling is required at distinct stages of development for the induction of the posterior forebrain. Development. 2003;130:5579–5587. doi: 10.1242/dev.00685. [DOI] [PubMed] [Google Scholar]
- Buscher D, Grotewold L, Ruther U. The XtJ allele generates a Gli3 fusion transcript. Mamm. Genome. 1998;9:676–678. doi: 10.1007/s003359900845. [DOI] [PubMed] [Google Scholar]
- Cheng X, Hsu CM, Currle DS, Hu JS, Barkovich AJ, Monuki ES. Central roles of the roof plate in telencephalic development and holoprosencephaly. J. Neurosci. 2006;26:7640–7649. doi: 10.1523/JNEUROSCI.0714-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martin GR. The mouse Fgf 8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martinez S, Martin GR. Midbrain development induced by FGF8 in the chick embryo. Nature. 1996;380:66–68. doi: 10.1038/380066a0. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martinez S, Ohkubo Y, Rubenstein JL. Coordinate expression of Fgf8, Otx2, Bmp4, and Shh in the rostral prosencephalon during development of the telencephalic and optic vesicles. Neuroscience. 2001;108:183–206. doi: 10.1016/s0306-4522(01)00411-0. [DOI] [PubMed] [Google Scholar]
- DeMyer W. Holoprosencephaly. In: Vinken PJ, Bruyn GW, editors. Handbook of clinical neurology. Vol. 30. Amsterdam: Elsevier; 1977. pp. 431–478. [Google Scholar]
- Dahmane N, Sanchez P, Gitton Y, Palma V, Sun T, Beyna M, Weiner H, Ruiz i Altaba A. The Sonic Hedgehog-Gli pathway regulates dorsal brain growth and tumorigenesis. Development. 2001;128:5201–5212. doi: 10.1242/dev.128.24.5201. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, Edlund T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995;81:747–756. doi: 10.1016/0092-8674(95)90536-7. [DOI] [PubMed] [Google Scholar]
- Fernandes M, Hebert JM. The ups and downs of holoprosencephaly: dorsal versus ventral patterning forces. Clin Genet. 2008;73:413–423. doi: 10.1111/j.1399-0004.2008.00994.x. [DOI] [PubMed] [Google Scholar]
- Fernandes M, Gutin G, Alcorn H, McConnell SK, Hébert JM. Mutations in the BMP pathway in mice support the existence of two molecular classes of holoprosencephaly. Development. 2007;134:3789–3794. doi: 10.1242/dev.004325. [DOI] [PubMed] [Google Scholar]
- Fertuzinhos S, Krsnikl Z, Kawasawa YI, Rasin MR, Kwan KY, Chen JG, Judas M, Hayashi M, Sestan N. Selective depletion of molecularly defined cortical interneurons in human holoprosencephaly with severe striatal hypoplasia. Cerebral Cortex. 2009;19:2196–2207. doi: 10.1093/cercor/bhp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Piston DW, Hogan BL. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–2212. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125:2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- Gutin G, Fernandes M, Palazzolo L, Paek H, Yu K, Ornitz DM, McConnell SK, Hebert JM. FGF signalling generates ventral telencephalic cells independently of SHH. Development. 2006;133:2937–2946. doi: 10.1242/dev.02465. [DOI] [PubMed] [Google Scholar]
- Hahn JS, Barkovich AJ, Stashinko EE, Kinsman SL, Delgado MR, Clegg NJ. Factor analysis of neuroanatomical and clinical characteristics of holoprosencephaly. Brain Dev. 2006;28:413–419. doi: 10.1016/j.braindev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Araki S, Kumada S, Itoh M, Morimatsu Y, Matsuyama H. Neuropathological evaluation of the diencephalon, basal ganglia and upper brainstem in alobar holoprosencephaly. Acta. Neuropathol. 2004;107:190–196. doi: 10.1007/s00401-003-0784-0. [DOI] [PubMed] [Google Scholar]
- Hayhurst M, McConnell SK. Mouse models of holoprosencephaly. Curr. Opin. Neurol. 2003;16:135–141. doi: 10.1097/01.wco.0000063761.15877.40. [DOI] [PubMed] [Google Scholar]
- Hebert JM, Mishina Y, McConnell SK. BMP Signaling Is Required Locally to Pattern the Dorsal Telencephalic Midline. Neuron. 2002;35:1029–1041. doi: 10.1016/s0896-6273(02)00900-5. [DOI] [PubMed] [Google Scholar]
- Hebert JM, Hayhurst M, Marks ME, Kulessa H, Hogan BL, McConnell SK. BMP ligands act redundantly to pattern the dorsal telencephalic midline. Genesis. 2003;35:214–219. doi: 10.1002/gene.10183. [DOI] [PubMed] [Google Scholar]
- Hébert JM, Fishell G. The genetics of early telencephalon patterning: some assembly required. Nat. Rev. Neurosci. 2008;9:678–685. doi: 10.1038/nrn2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M, McMahon AP. A sonic hedgehog-dependent signaling relay regulates growth of diencephalic and mesencephalic primordia in the early mouse embryo. Development. 2002;129:4807–4819. doi: 10.1242/dev.129.20.4807. [DOI] [PubMed] [Google Scholar]
- Khokha MK, Hsu D, Brunet LJ, Dionne MS, Harland RM. Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat. Genet. 2003;34:303–307. doi: 10.1038/ng1178. [DOI] [PubMed] [Google Scholar]
- Kiecker C, Lumsden A. Hedgehog signaling from the ZLI regulates diencephalic regional identity. Nat. Neurosci. 2004;7:1242–1249. doi: 10.1038/nn1338. [DOI] [PubMed] [Google Scholar]
- Kiecker C, Lumsden A. Compartments and their boundaries in vertebrate brain development. Nat. Rev. Neurosci. 2005;6:553–564. doi: 10.1038/nrn1702. [DOI] [PubMed] [Google Scholar]
- Kinsman SL, Plawner LL, Hahn JS. Holoprosencephaly: recent advances and new insights. Curr. Opin. Neurol. 2000;13:127–132. doi: 10.1097/00019052-200004000-00003. [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Kobayashi M, Matsumoto K, Ogura T, Nakafuku M, Shimamura K. Early subdivisions in the neural plate define distinct competence for inductive signals. Development. 2002;129:83–93. doi: 10.1242/dev.129.1.83. [DOI] [PubMed] [Google Scholar]
- Kuschel S, Ruther U, Theil T. A disrupted balance between Bmp/Wnt and Fgf signaling underlies the ventralization of the Gli3 mutant telencephalon. Dev. Biol. 2003;260:484–495. doi: 10.1016/s0012-1606(03)00252-5. [DOI] [PubMed] [Google Scholar]
- Larsen CW, Zeltser LM, Lumsden A. Boundary formation and compartition in the avian diencephalon. J. Neurosci. 2001;21:4699–4711. doi: 10.1523/JNEUROSCI.21-13-04699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- Li JY, Lao Z, Joyner AL. New regulatory interactions and cellular responses in the isthmic organizer region revealed by altering Gbx2 expression. Development. 2005;132:1971–1981. doi: 10.1242/dev.01727. [DOI] [PubMed] [Google Scholar]
- Li JY, Joyner AL. Otx2 and Gbx2 are required for refinement and not induction of mid-hindbrain gene expression. Development. 2001;128:4979–4991. doi: 10.1242/dev.128.24.4979. [DOI] [PubMed] [Google Scholar]
- Liem KF, Tremml G, Jessell TM. A role for the roof plate and its resident TGFβ-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91:127–138. doi: 10.1016/s0092-8674(01)80015-5. [DOI] [PubMed] [Google Scholar]
- Liem KF, Jr, Tremml G, Roelink H, Jessell TM. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82:969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Echelard Y, Assimacopoulos S, Danielian PS, Kaing S, Grove EA, McMahon AP, Rowitch DH. Loss of Emx2 function leads to ectopic expression of Wnt1 in the developing telencephalon and cortical dysplasia. Development. 2003;130:2275–2287. doi: 10.1242/dev.00421. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Chiang C. Specification of ventral neuron types is mediated by an antagonistic interaction between Shh and Gli3. Nat. Neurosci. 2000;3:979–985. doi: 10.1038/79916. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Dahn RD, Li Y, Fallon JF, Chiang C. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature. 2002;418:979–983. doi: 10.1038/nature01033. [DOI] [PubMed] [Google Scholar]
- Louvi A, Yoshida M, Grove EA. The derivatives of the Wnt3a lineage in the central nervous system. J. Comp. Neurol. 2007;504:550–569. doi: 10.1002/cne.21461. [DOI] [PubMed] [Google Scholar]
- Lumsden A, Krumlauf R. Patterning the vertebrate neuraxis. Science. 1996;274:1109–1115. doi: 10.1126/science.274.5290.1109. [DOI] [PubMed] [Google Scholar]
- Martinez S, Crossley PH, Cobos I, Rubenstein JL, Martin GR. FGF8 induces formation of an ectopic isthmic organizer and isthmocerebellar development via a repressive effect on Otx2 expression. Development. 1999;126:1189–1200. doi: 10.1242/dev.126.6.1189. [DOI] [PubMed] [Google Scholar]
- Martínez S. The isthmic organizer and brain regionalization. Int. J. Dev. Biol. 2001;45:367–371. [PubMed] [Google Scholar]
- Maynard TM, Jain MD, Balmer CW, LaMantia AS. High-resolution mapping of the Gli3 mutation extra-toes reveals a 51.5-kb deletion. Mamm. Genome. 2002;13:58–61. doi: 10.1007/s00335-001-2115-x. [DOI] [PubMed] [Google Scholar]
- Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Joyner AL, Bradley A, McMahon JA. The midbrain-hindbrain phenotype of Wnt-1-/Wnt-1- mice results from stepwise deletion of engrailed-expressing cells by 9.5 days postcoitum. Cell. 1992;69:581–595. doi: 10.1016/0092-8674(92)90222-x. [DOI] [PubMed] [Google Scholar]
- Metin C, Baudoin JP, Rakic S, Parnavelas JG. Cell and molecular mechanisms involved in the migration of cortical interneurons. Eur. J. Neurosci. 2006;23:894–900. doi: 10.1111/j.1460-9568.2006.04630.x. [DOI] [PubMed] [Google Scholar]
- Meyer NP, Roelink H. The amino-terminal region of Gli3 antagonizes the Shh response and acts in dorsoventral fate specification in the developing spinal cord. Dev. Biol. 2003;257:343–355. doi: 10.1016/s0012-1606(03)00065-4. [DOI] [PubMed] [Google Scholar]
- Monuki ES. The morphogen signaling network in forebrain development and holoprosencephaly. J. Neuropathol. Exp. Neurol. 2007;66:566–575. doi: 10.1097/nen.0b013e3180986e1b. [DOI] [PubMed] [Google Scholar]
- Muenke M, Beachy PA. Genetics of ventral forebrain development and holoprosencephaly. Curr. Opin. Genet. Dev. 2000;10:262–269. doi: 10.1016/s0959-437x(00)00084-8. [DOI] [PubMed] [Google Scholar]
- Oba H, Barkovich J. Holoprosencephaly: an analysis of callosal formation and its relation to development of the interhemispheric fissure. Am. J. Neuroradiol. 1995;16:453–460. [PMC free article] [PubMed] [Google Scholar]
- Ohkubo Y, Chiang C, Rubenstein JL. Coordinate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles. Neuroscience. 2002;111:1–17. doi: 10.1016/s0306-4522(01)00616-9. [DOI] [PubMed] [Google Scholar]
- Palma V, Ruiz i Altaba A. Hedgehog-GLI signaling regulates the behavior of cells with stem cell properties in the developing neocortex. Development. 2004;131:337–345. doi: 10.1242/dev.00930. [DOI] [PubMed] [Google Scholar]
- Persson M, Stamataki D, te Welscher P, Andersson E, Bose J, Ruther U, Ericson J, Briscoe J. Dorsal-ventral patterning of the spinal cord requires Gli3 transcriptional repressor activity. Genes Dev. 2002;16:2865–2878. doi: 10.1101/gad.243402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rallu M, Machold R, Gaiano N, Corbin JG, McMahon AP, Fishell G. Dorsoventral patterning is established in the telencephalon of mutants lacking both Gli3 and Hedgehog signaling. Development. 2002;129:4963–4974. doi: 10.1242/dev.129.21.4963. [DOI] [PubMed] [Google Scholar]
- Rash BG, Grove EA. Patterning the dorsal telencephalon: a role for sonic hedgehog? J. Neurosci. 2007;27:11595–11603. doi: 10.1523/JNEUROSCI.3204-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Joyner AL, Nakamura H. How does Fgf signaling from the isthmic organizer induce midbrain and cerebellum development? Dev. Growth. Differ. 2004;46:487–494. doi: 10.1111/j.1440-169x.2004.00769.x. [DOI] [PubMed] [Google Scholar]
- Scholpp S, Wolf O, Brand M, Lumsden A. Hedgehog signalling from the zona limitans intrathalamica orchestrates patterning of the zebrafish diencephalon. Development. 2006;133:855–864. doi: 10.1242/dev.02248. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- Shimogori T, Banuchi V, Ng HY, Strauss JB, Grove EA. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development. 2004;131:5639–5647. doi: 10.1242/dev.01428. [DOI] [PubMed] [Google Scholar]
- Simon EM, Hevner R, Pinter JD, Clegg NJ, Miller VS, Kinsman SL, Hahn JS, Barkovich AJ. Assessment of the deep gray nuclei in holoprosencephaly. AJNR Am. J. Neuroradiol. 2000;21:1955–1961. [PMC free article] [PubMed] [Google Scholar]
- Simon EM, Hevner RF, Pinter J, Clegg NJ, Delgado M, Kinsman SL, Hahn JS, Barkovich AJ. The dorsal cyst in holoprosencephaly and the role of the thalamus in its formation. Neuroradiology. 2001;43:787–791. doi: 10.1007/s002340100567. [DOI] [PubMed] [Google Scholar]
- Storm EE, Garel S, Borello U, Hebert JM, Martinez S, McConnell SK, Martin GR, Rubenstein JL. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kinsman S, Makris N, Grant E, Haselgrove C, McInerney S, Kennedy DN, Fredrickson K, Mori S, Caviness VS. Semilobar holoprosencephaly with midline 'seam': a topologic and morphogenetic model based upon MRI analysis. Cereb. Cortex. 2003;13:1299–1312. doi: 10.1093/cercor/bhg077. [DOI] [PubMed] [Google Scholar]
- Takahashi TS, Kinsman S, Makris N, Grant E, Haselgrove C, McInerney S, Kennedy DN, Takahashi TA, Fredrickson K, Mori S, Caviness VS. Holoprosencephaly-topologic variations in a liveborn series: a general model based upon MRI analysis. J. Neurocytol. 2004;33:23–35. doi: 10.1023/B:NEUR.0000029646.75645.9c. [DOI] [PubMed] [Google Scholar]
- Theil T, Alvarez-Bolado G, Walter A, Rüther U. Gli3 is required for Emx gene expression during dorsal telencephalon development. Development. 1999;126:3561–3571. doi: 10.1242/dev.126.16.3561. [DOI] [PubMed] [Google Scholar]
- Theil T. Gli3 is required for the specification and differentiation of preplate neurons. Dev Biol. 2005;286:559–571. doi: 10.1016/j.ydbio.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Tole S, Ragsdale CW, Grove EA. Dorsoventral patterning of the telencephalon is disrupted in the mouse mutant extra-toes. J. Dev. Biol. 2000;217:254–265. doi: 10.1006/dbio.1999.9509. [DOI] [PubMed] [Google Scholar]
- Ulloa F, Itasaki N, Briscoe J. Inhibitory Gli3 activity negatively regulates Wnt/beta-catenin signaling. Curr. Biol. 2007;17:545–550. doi: 10.1016/j.cub.2007.01.062. [DOI] [PubMed] [Google Scholar]
- Wang C, Ruther U, Wang B. The Shh-independent activator function of the full-length Gli3 protein and its role in vertebrate limb digit patterning. Dev. Biol. 2007;305:460–469. doi: 10.1016/j.ydbio.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurst W, Bally-Cuif L. Neural plate patterning: upstream and downstream of the isthmic organizer. Nat. Rev. Neurosci. 2001;2:99–108. doi: 10.1038/35053516. [DOI] [PubMed] [Google Scholar]
- Yokota A, Hamada T, Miyayama H. Morphological features of holoprosencephaly. Neuropathol. 1998;18:419–426. [Google Scholar]
- Zeltser LM. Shh-dependent formation of the ZLI is opposed by signals from the dorsal diencephalon. Development. 2005;132:2023–2033. doi: 10.1242/dev.01783. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.