Abstract
Conventional method of cell culture studies has been performed on two-dimensional substrates. Recently, three-dimensional (3D) cell culture platforms have been a subject of interest as cells in 3D has significant differences in cell differentiation and behavior. Here we report a novel approach of 3D cell culture using a nylon micro mesh (NMM) as a cell culture scaffold. NMM is commonly used in cell culture laboratory, which eliminates the requirement of special technicality for biological laboratories. Furthermore, it is made of a micro-meter thick nylon fibers, which was adequate to engineer in cellular scales. We demonstrate the feasibility of the NMM as a 3D scaffold using E18 rat hippocampal neurons. NMM could be coated with cell adhesive coatings (polylysine or polyelectrolyte) and neurons showed good viability. Cells were also encapsulated in an agarose hydrogel and cultured in 3D using NMM. In addition, the 3D pattern of NMM could be used as a guidance cue for neurite outgrowth. The flexible and elastic properties of NMMs made it easier to handle the scaffold and also readily applicable for large-scale tissue engineering applications.
Keywords: 3D culture, scaffold, micro mesh, neuronal culture, 3D cell patterning
INTRODUCTION
Conventional method of cell culture studies has been performed on 2-dimensional (2D) surfaces such as glass, well plate and Petri dishes. Theses 2D cell culture platforms are widely used because of the easy of handling, its convenience and bio-compatibility. Although the understanding of basic biology has been improved with these advantages of 2D systems, many scientists give careful attention to 3-dimensional (3D) cell culture system due to the limitation of 2D environment (Lund et al., 2009).
Two-dimensional substrates have a limitation in the replication of 3D cellular environments and the absence of 3D factors in cellular developments could limit the biological outcome in the culture (Zhang, 2004). Moreover, cells cultured on the 2D substrate have to adapt to rigid and flat surfaces while natural in vivo environments have large influences from microenvironments derived from extracellular matrix (ECM) and cell-cell interactions. Recent studies showed that the elements of 2D environment that were different from 3D environment could lead to change from gene expression to cell functionality (Zahir and Weaver, 2004; Birgersdotter et al., 2005).
There have been active pursuits in developing new biomaterials for 3D cell culture scaffolds (Shin and Mikos, 2003). For example, polymer based biomaterials (Cheung et al., 2007), protein based biomaterials (Stegemann et al., 2007) and nanofiber (Yoshimoto et al., 2003) have been utilized as a 3D cell culture scaffold. And with these biomaterials, there have been many applications of 3D cell culture scaffolds (Dutta and Dutta, 2009). For example, there were clinical trials that artificial tissues which were made by 3D scaffolds used for regenerative medicine (Karageorgiou and Kaplan, 2005) and in vitro 3D tissue modeling (Yamada and Cukierman, 2007). However, most of the 3D cell culture scaffolds require complex fabrication process and expensive equipment that is usually not accessible for biologists, which limits the extended usage of 3D scaffold systems in cell culture.
Here we propose a nylon micro-mesh (NMM) as a new 3D cell culture scaffold based on its mechanical flexibility, biocompatibility, and practicality. And we show that NMM is suitable for 3D cell culture scaffold and has some potential for 3D tissue engineering applications.
MATERIALS AND METHODS
Substrate preparation
Three kinds of Nylon micro meshs (NMMs) which had 20, 10, 8 µm pore size were purchased from Spectrum Laboratories (Spectrum, CA, USA). And NMMs with 40 µm pores were taken from a cell strainer (BD Falcon, NJ, USA).
NMMs were sonicated with acetone, isopropylalcohol (IPA) and DI water for 5min per each and dried by air stream. NMMs were coated with Poly-D-Lysine (PDL, Sigma, MO, USA) by soaking the NMMs in PDL solution (0.1 mg/ml in borate buffer, pH 8.5) at 37℃. After 6 hour, PDL solution was aspirated and NMMs were immersed in 70% ethanol for sterilization. After the sterilization, NMMs were rinsed with DI water. The NMMs were finally dried in clean-bench or incubator.
Polyelectrolyte multilayer (PEL) were coated using layer-by-layer method. The cleaned NMMs were immerged in the solution of Poly allylamine hydrochloride (PAH, 20 mM, pH 9.0, Sigma, MO, USA) for 1 min, followed by five times washing with DI water. The PAH coated NMMs were immerged in the solution of Poly sodium 4-stlrensulfonate (PSS, 60 mM, pH 9.0, Sigma, MO, USA) for 1 min, followed by five times washing with DI water. The process of PEL coating were repeated ten times and finished with the PAH coating. NMMs were sterilized by UV for 5 min and dried in clean-bench or incubator.
Agarose gel (gelling range: 36~39℃, Amresco Inc., Ohio, USA) was dissolved in 100℃ DI water and 0.7% (w/v) agarose hydrogel was formed. After cooling the agarose gel to 36℃, cell suspension was mixed with the agarose and plated on PDL coated NMMs.
Cell culture
Hippocampus tissues were dissected from E18 Sprague/Dawley rat embryo. The tissues were washed three times with Hank's Balanced Salt Solution (HBSS) and mechanically dissociated using 1 ml pipette tips. Cell suspensions were centrifuged for 3 min at 1,000 rpm and the cell pellets were suspended again in plating medium which was composed of serum free Neurobasal/B27 (Invitrogen, CA), 12.5 µM L glutamate (sigma, MO), 2 mM L glutamine (Gibco) and 1% penicillin streptomysin (invtrogen, CA). Finally, the cell suspend plated onto the coated NMMs at the density of 200 to 300 cells/mm2. Cultures were maintained in an incubator (5% CO2, 37℃). After three days from cell plating, half of the medium was replaced with fresh culture medium without L glutamine every 3 days.
Live-dead staining
Neurons were stained by Live-Dead viability/cytotoxicity kit (Invitrogen, CA, USA). 10 µl of ethidium homodimer and 2.5 µl calcein acetocymethyl ester were mixed in 5 ml of phosphate buffered saline (PBS). 200 µl of the dye solution was added in 1 ml sample and covered with aluminum foil to protect the dye from photobleaching. After the incubation (5% CO2, 37℃) of the sample for 20 min, the solution in the sample was removed and NMMs washed with PBS.
RESULTS
Mechanical properties of nylon micro-mesh as a three dimensional cell culture scaffold
Appropriate mechanical properties of 3D scaffold are important in 3D tissue engineering (Lee et al., 2008). First, flexibility is required to maintain the shape of scaffold and handling. Second, micro and nano-scale structural architecture is desirable to mimic in vivo microenvironments (Curtis and Wilkinson, 1998; Bayliss et al., 1999). Third, some porosity is required for the diffusion of nutrients, metabolic wastes and interconnectivity of cells.
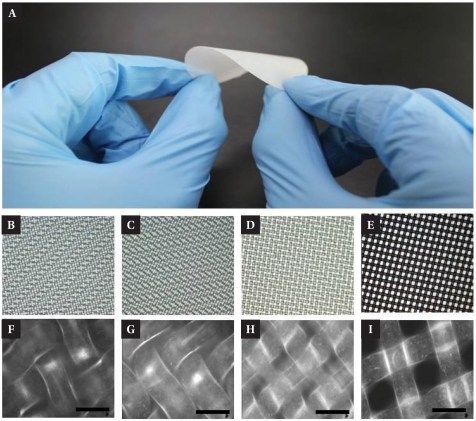
Fig. 1A shows the compliance of the NMM to the mechanical bending. It was also easy to handle by hand for forceps. Fig. 1 also shows the ordered structures of NMM in micrometer scales. The thickness of the individual nylon fiber was 30 or 35 µm and the thickness of NMM was 75 µm, 45 µm, 55 µm, 60 µm, respectively (Fig. 1B~E). NMMs have many regular pores with the pore diameters of 8 µm, 10 µm, 20 µm, and 40 µm (Fig. 1F~I). These properties made NMMs suitable for 3D cell culture scaffolds.
Fig. 1.
Nylon micro-mesh. (A) Actual image of NMM. Flexibility and hardness of the NMM show that it is simple to handle and not weak as a 3D culture scaffold. (B~E) Optical microscope image of the NMMs, diameter of pore is 8 µm, 10 µm, 20 µm, 40 µm, respectively and (F~I) the magnified image of those NMMs. Scale bar: 50 µm.
Cell Viability and biocompatibility
Biocompatibility of materials is a crucial parameter, since it can be decisive criteria as a culture scaffold. Most of the reported scaffolds were composed of ECM molecules (Badylak, 2002), or had bioactive molecules to modulate cellular activities (Zhu, 2010), or they had controllability in chemicals (Yildirim et al., 2010).
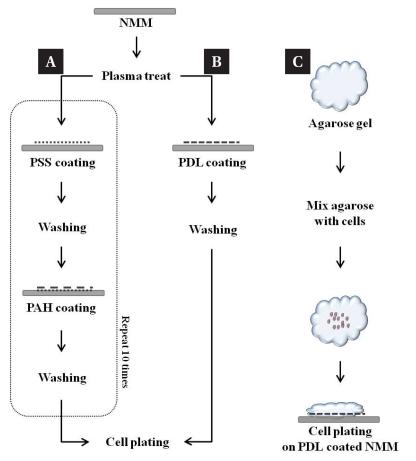
Fig. 2 shows the surface modification of NMMs using positively charged polymers. We coated NMMs with either poly-D-lysine (PDL) or polyelectrolyte multilayer (PEL). PDL is one of the most common polymer used as a cell adhesive coating material. PEL was used to modify the NMM surface with much denser positive charge layers through layer-by-layer coating technique, which turned out to be more efficient in terms of reproducibility and reliability.
Fig. 2.
Schematic illustration of the chemical coating and 3D entrapment procedures for cell culture. (A) PEL coating method. PSS and PAH transform the surface charge from positive to negative and vice versa, respectively. (B) PDL coating method. PDL, highly positive charged amino acid chain, promote cell adhesion in culture (C) low temperature agarose gel was used for 3D cell entrapment.
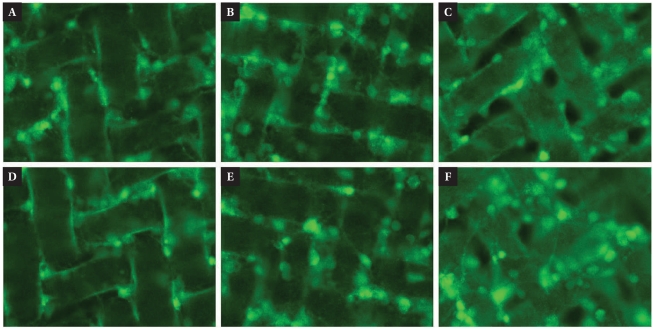
In order to investigate the effect of chemical controllability and viability of cells on the NMM, we prepared different pore size of NMMs coated by PDL and PEL and cell plate. Fig. 3 shows excellent cell viability at 9 DIV. Neurons tend to grow on and in-between the micro-fibers and pores were be filled with neuronal growth.
Fig. 3.
Neuron growth on the NMMs at 9DIV. PDL and PEL coated NMM was covered by hippocampal neurons and formed the neural tissue. Since chemical controllability and neuron viability on the NMM, it has sufficient qualifications for 3D cell culture scaffold. (A~C) Neuron growth on the PDL coated NMM. (D~F) PEL coated NMM. Diameters of pore are 8 µm (A, D), 10 µm (D, E) and 20 µm (C, F). All of viable neurons (green) were labeled with Calcein AM.
Entrapment of cells in three dimensional space
Entrapment of cells in three dimensional space has a special meaning that it provide distinct differences in cell morphogenesis, behavior, gene expression, differentiation from 2D environment (Holmes et al., 2000; Zaman et al., 2006). In other word, the recovered 3D environment cause different viability and functionality of cell.
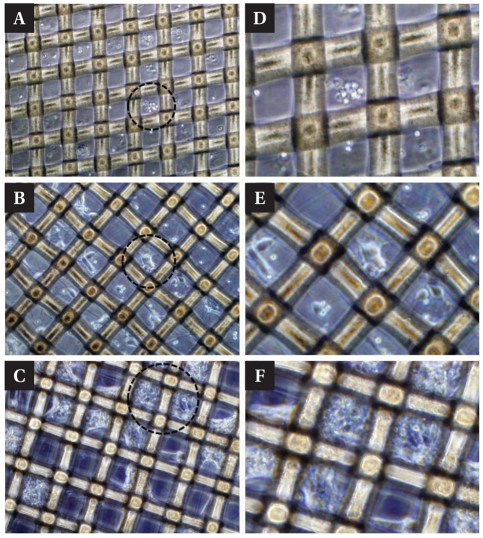
Many of the studies used natural hydrogels or synthetic gel such as alginate (Pangas et al., 2003), collagen (Batorsky et al., 2005) and poly ethylene glycol (PEG) (Elisseeff et al., 1999) to entrap cells. We used agarose hydrogel which is a common material in biological laboratories for the cell entrapment (Fig. 2). Fig. 4 shows the neuronal growth in agarose hydrogels. Neurons were well dispersed in the gel, and neurites were formed and extended (Fig. 4B, E). They tend to get clustered in the gel after forming thick fiber bundles. As the gel was not functionalized to be cell adhesive, the clustering process was likely to occur in the matured neuronal cultures. However, neurons were still viable and maintained in 3D NMM scaffolds.
Fig. 4.
3D entrapment of neuron on the NMM. After PDL coating the NMM, 0.7% agarose gel was used for 3D entrapment. (A) Agarose gel positioned the hippocampal neurons on the pore and fiber of NMM. And they generate the neurites at 2DIV, (B) growth and connected each other at 4DIV. (C) Finally, many of neurons established complex network on the NMM at 14DIV. Diameter of the pore, we used in 3D entrapment, is 40 µm (D~F). Magnified circle images in (A), (B), (C), respectively.
Three dimensional neurite guidance effect
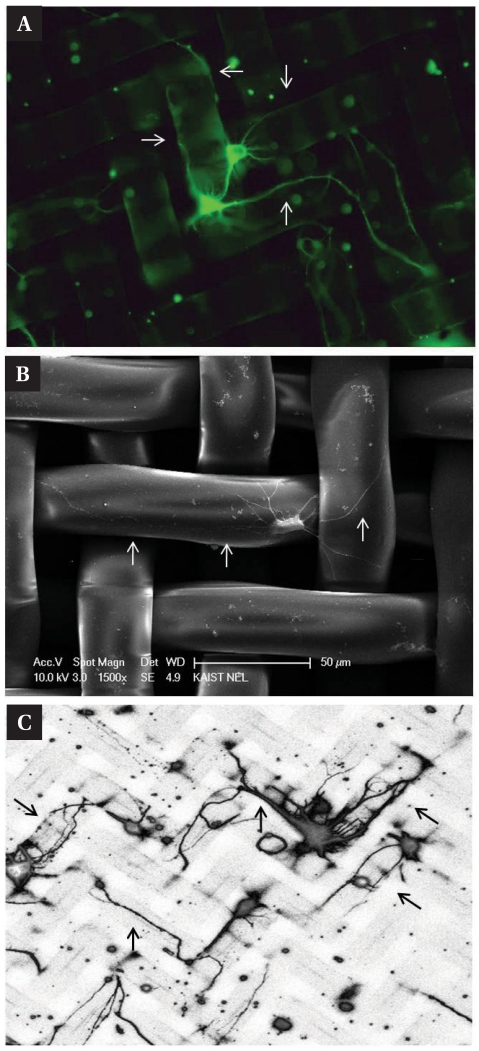
As NMMs had ordered structures in micrometer scales (Fig. 1), each nylon fiber can provide the topographical guidance cues to the growing neurites. Fig. 5A shows an example of the guided neurite outgrowth along the 3D mesh fibers. The fiber thickness was comparable to the size of soma and neurites followed the edge of the fibers. This is also shown in scanning electron micrograph in Fig. 5B. Fig. 5C shows more 'patterned' neurites on the mesh. Although the fiber itself was too thick to control thinner neurites, its edges clearly had an effect on neurite guidance.
Fig. 5.
3D neuron patterning on the NMM. Fibers of NMM compose 3D grid pattern which is a guide-way to the neurites and these 3D structural patterns of NMM appropriate to neuronal patterning. (A) Cacein AM labeled live neurons. Arrows indicate that neurites growth along with 3D grid pattern. (B) SEM image of patterned neuron. (C) Confocal laser scanning microscope image also show the patterned neurons on the NMM. All of neurons were extracted in hippocampus and stained at 6DIV. Diameter of pore is 20 µm.
DISCUSSION
In summary, we proposed NMMs as a simple but effective 3D cell culture scaffold. Unlike other 3D scaffold materials that requires expensive or special technicality, NMMs are an inexpensive material that is common biological laboratories. NMM had excellent mechanical and structural properties to support neuronal cell growth and gel based cell cultures. It was also chemically modifiable to convert the nylon surface into cell adhesive surfaces. Moreover, the micro-scale structural regularity could be potentially be applicable to 3D tissue engineering applications.
ACKNOWLEDGEMENTS
This work was supported by National Research Foundation of Korea Grant funded by the Korean Government (No. 2010-0000419, 2010-0028173) and the Brain Research Center of the 21st Century Frontier Research Program funded by MEST.
References
- 1.Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. 2002;13:377–383. doi: 10.1016/s1084952102000940. [DOI] [PubMed] [Google Scholar]
- 2.Batorsky A, Liao J, Lund AW, Plopper GE, Stegemann JP. Encapsulation of adult human mesenchymal stem cells within collagen-agarose microenvironments. Biotechnol Bioeng. 2005;92:492–500. doi: 10.1002/bit.20614. [DOI] [PubMed] [Google Scholar]
- 3.Bayliss SC, Buckberry LD, Fletcher I, Tobin MJ. The culture of neurons on silicon. Sens Actuators A Phys. 1999;74:139–142. [Google Scholar]
- 4.Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro--a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol. 2005;15:405–412. doi: 10.1016/j.semcancer.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Cheung HY, Lau KT, Lu TP, Hui D. A critical review on polymer-based bio-engineered materials for scaffold development. Composites Part B-Engineerig. 2007;38:291–300. [Google Scholar]
- 6.Curtis AS, Wilkinson CD. Reactions of cells to topography. J Biomater Sci Polym Ed. 1998;9:1313–1329. doi: 10.1163/156856298x00415. [DOI] [PubMed] [Google Scholar]
- 7.Dutta RC, Dutta AK. Cell-interactive 3D-scaffold; advances and applications. Biotechnology Advances. 2009;27:334–339. doi: 10.1016/j.biotechadv.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Elisseeff J, Anseth K, Sims D, Mcintosh W, Randolph M, Langer R. Transdermal photopolymerization for minimally invasive implantation. Proc Natl Acad Sci USA. 1999;96:3104–3107. doi: 10.1073/pnas.96.6.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes TC, De Lacalle S, Su X, Liu G, Rich A, Zhang S. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc Natl Acad Sci USA. 2000;97:6728–6733. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karageorgiou V, Kaplan D. Porosity of 3D biornaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev. 2008;14:61–86. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- 12.Lund AW, Yener B, Stegemann JP, Plopper GE. The natural and engineered 3D microenvironment as a regulatory cue during stem cell fate determination. Tissue Eng Part B Rev. 2009;15:371–380. doi: 10.1089/ten.teb.2009.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Connor SM, Stenger DA, Shaffer KM, Ma W. Survival and neurite outgrowth of rat cortical neurons in three-dimensional agarose and collagen gel matrices. Neurosci Lett. 2001;304:189–193. doi: 10.1016/s0304-3940(01)01769-4. [DOI] [PubMed] [Google Scholar]
- 14.Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng. 2003;9:1013–1021. doi: 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- 15.Shin H, Jo S, Mikos AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353–4364. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 16.Stegemann JP, Kaszuba SN, Rowe SL. Review: advances in vascular tissue engineering using protein-based biomaterials. Tissue Eng. 2007;13:2601–2613. doi: 10.1089/ten.2007.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teixeira AI, Ilkhanizadeh S, Wigenius JA, Duckworth JK, Inganas O, Hermanson O. The promotion of neuronal maturation on soft substrates. Biomaterials. 2009;30:4567–4572. doi: 10.1016/j.biomaterials.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Treloara LRG. Calculations of elastic moduli of polymer crystals: I. Polyethylene and nylon 66. Polymer. 1960;1:95–103. [Google Scholar]
- 19.Yamada KM, Cukierman E. Modeling tissue mor-phogenesis and cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Yildirim ED, Besunder R, Pappas D, Allen F, Guceri S, Sun W. Accelerated differentiation of osteoblast cells on polycaprolactone scaffolds driven by a combined effect of protein coating and plasma modification. Biofabrication. 2010;2:014109. doi: 10.1088/1758-5082/2/1/014109. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 22.Zahir N, Weaver VM. Death in the third dimension: apoptosis regulation and tissue architecture. Curr Opin Genet Dev. 2004;14:71–80. doi: 10.1016/j.gde.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Zaman MH, Trapani LM, Sieminski AL, Mackellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci USA. 2006;103:10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S. Beyond the Petri dish. Nat Biotechnol. 2004;22:151–152. doi: 10.1038/nbt0204-151. [DOI] [PubMed] [Google Scholar]
- 25.Zhu JM. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639–4656. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]