Abstract
Background:
Limited data is available on the clinical expression of chronic obstructive pulmonary disease (COPD) from India. The impact of gender on expression of COPD has received even less attention. Apart from tobacco smoke, indoor air pollution, especially from biomass fuel may play an important role in development of COPD in women.
Materials and Methods:
Seven hundred and two patients of COPD were studied regarding the etiological and risk factors leading to COPD, gender-related differences in clinical presentation, radiological expression of COPD and the co-morbidities in COPD.
Results:
Tobacco smoke in the form of beedi smoking was the predominant smoke exposure in males, whereas smoke from biofuel burning was the predominant exposure in females. As compared to males, females were younger, reported more dyspnea, more severe bronchial obstruction, more exacerbations, and exhibited higher prevalence of systemic features. Also, females smoked less and had lesser incidence of productive cough, lower body mass index, lesser co-morbidities and less number of hospital admissions as compared to males. Males were more likely than females to have an emphysema-predominant phenotype, while airway-predominant disease was more common among females.
Conclusion:
The current study shows that gender-related differences do exist in COPD patients. Understanding these differences in etiological agent and clinical picture will help early diagnosis of COPD in females.
KEY WORDS: Biomass fuel, chronic obstructive pulmonary disease, gender, indoor pollution, smoking
INTRODUCTION
By 2030, chronic obstructive pulmonary disease (COPD) will be the third most frequent cause of death in the world.[1] The influence of gender on the expression of COPD has received limited attention.
Silverman et al[2] indicated that women may be more susceptible to tobacco smoke than men and more likely to develop early-onset COPD. Also, the prevalence of women smokers in developing countries is predicted to rise to 20% by 2025.[3]
Indoor air pollution due to the use of unprocessed solid fuels such as coal and biomass (dung, crop residues, and wood) for cooking and space heating has been implicated as an important risk factor for the development of COPD.[4,5] About >71% of households in India use such fuels, and studies on characteristics of women with COPD exposed to biomass smoke and the degree to which they differ from COPD in men (mainly due to tobacco smoking) from Indian context are missing.
The prevailing notion that COPD primarily affects men may put women at particular risk of under-diagnosis.[6] In the general population, it has been reported that females report dyspnoea and cough more frequently and phlegm less often than males.[7]
Although airflow obstruction is the hallmark of COPD, this can develop on the basis of airways disease, emphysema, or both. Dransfield et al (2007)[8] had reported variability in obstruction/ emphysema in very severe cases of COPD and gender-related changes in lung parenchyma on high-resolution computed tomography (HRCT).
Women have been under-represented in many clinical trials, which may result in inadequate diagnosis and suboptimal management.
Also, the impact of gender on the development of comorbidities (such as cardiac disease, diabetes mellitus, hypertension, osteoporosis, etc) in relation to COPD is not known.
Thus, despite multiple investigations it is difficult to draw final conclusion on the impact of gender on COPD. Also, there is paucity of these data from Indian scenario.
AIMS AND OBJECTIVES
To study the etiological and risk factors leading to COPD, gender-related differences in clinical presentation, radiological expression of COPD, and the co-morbidities in COPD.
MATERIALS AND METHODS
The study was carried out during 2008-2009 period on 702 patients of COPD, diagnosed by global initiative against obstructive lung diseases (GOLD) guidelines.[9]
Patients with COPD symptoms, i.e., dyspnea, chronic cough or sputum production, and/or a history of exposure to risk factors for the disease and, forced expiratory volume in 1st second/forced vital capacity (FEV1/FVC) ≤ 70% on spirometry, FEV1 ≤80% predicted normal, an increase of less than 12% or 200 ml of FEV1 after bronchodilator reversibility testing on spirometry, were included.
Patients with history of asthma or FEV1 increased more than 12% and 200 ml after bronchodilatation, patients not willing to be evaluated, patients with active pulmonary tuberculosis (due to the effect of active tuberculosis on the symptoms and signs of the patients), and other significant extra pulmonary diseases that can influence the results of the study, were excluded.
Patients were interviewed regarding symptoms, patient's exposure to risk factors, occupation, past medical history, family history of COPD or other chronic respiratory disease, history of exacerbations or previous hospitalizations for respiratory disorder, and presence of complications and co-morbidities.
All the patients were subjected to spirometric evaluation and measurements were evaluated by comparison with reference values based on age, height, sex, and race. Bronchodilator reversibility testing was done using drug protocols given in GOLD (2007)[9] guidelines.
Chest X-ray posteroanterior view was done in all patients to see for radiological changes associated with COPD, i.e., signs of hyperinflation, hyperlucency of the lungs, and rapid tapering of the vascular markings.[9] Chest X-ray was also used to evaluate the severity and for detecting co-morbidities and complications present.
Based on clinical suspicion and chest radiograph findings, patients were subjected to computed tomography (CT) of the chest, if required, to evaluate the presence of complications and/or co-morbidities such as bronchiectasis, carcinoma lung, pneumonia, pneumothorax, and pulmonary embolism.
Arterial blood gas analysis was done in patients with severe disease or if clinical suspicion of respiratory failure was noted.
Appropriate standard tests were performed to look for or exclude the presence of co-morbidities/complications like diabetes mellitus, anemia, osteoporosis, rheumatoid arthritis, obstructive sleep apnea etc.
The data collected were analyzed by using GraphPad InStat computer software. Chi Square and Student t test and proportion tests were applied for statistical significance.
OBSERVATIONS
The study included 702 patients of COPD of which 493 (70.2%) were males and 209 (29.8%) females. Table 1 shows the general characteristics of the patients. The mean age of the patients was 60.61 ± 10.36 years. All the patients complained of dyspnea of varying grades. About 49.1% patients presented at grade 2 dyspnea. Other common symptoms reported by patients were cough (96%), fever (35.3%), edema feet (12.8%), chest pain (7.7%), anorexia (5.3%), abdominal pain (4.8%), body ache (4.1%), and hemoptysis (2.7%).
Table 1.
General characteristics and symptoms
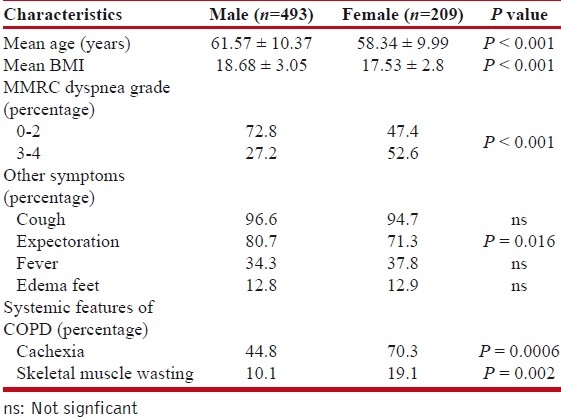
Average body mass index (BMI) of the patients was 18.36 ± 3.02. Females had lower BMI than males (17.53 ± 2.8 v/s 18.68 ± 3.05, P < 0.001). Also low BMI was found to be associated with more exacerbations, especially in females and statistically significant lower FEV1 values on spirometry (P< 0.001).
About 66.1% patients (313 males and 151 females) had exacerbations, which did not lead to admission. Out of the 313 males, 65.8% males had 0-1 such exacerbations. Out of 151 females, 53.6% had 0-1 such exacerbations. About 33.9% patients had history of hospitalization, with majority 53.8% had history of one such episode. Average duration of hospital stay was 6.6 ± 2.9 days. Among the males, heart failure was noted in 25%, pneumonia in 12.8%, pneumothorax in 5.6%, and pulmonary embolism in 1.1% at the time of hospitalization. In females, 17.2% had heart failure, 12.2% had pneumonia and 1.7% had pneumothorax at the time of hospitalization.
As shown in Table 2, about 84.8% males smoked beedi, 11.8% smoked cigarette, and 1.8% smoked tobacco in form of hookah and/or chillum. Also 89% of females had exposure to chula (biomass smoke). Most (68%) of these female patients used wood, rest 32% also used dung (kanda), grass, leaves, etc. Wood was used for cooking purposes and rest of the material for other purposes like heating water. Significant proportion, i.e., 42.1% females had exposure to both chula and tobacco smoke.
Table 2.
FEV1, type of smoke exposure and radiological findings
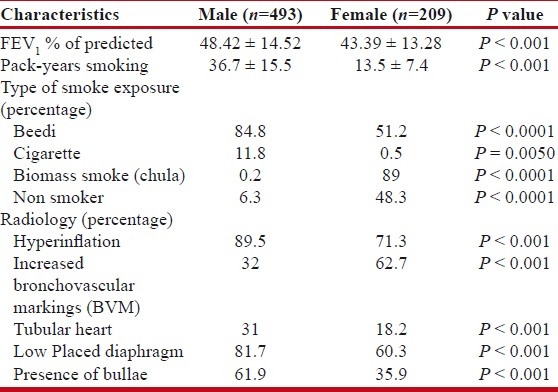
On chest X-ray, males had more prevalence of hyperinflation (89.5% v/s 71.3%, respectively, P<0.001). Typical tubular heart was seen in 31% males as compared to 18.2% females. Presence of bullae was noted more frequently in males (61.9%) than females (35.9%). Basal bullae were observed more frequently than apical bullae (56.2% v/s 28.2% in males and 29.2% v/s 11.5% in females).
Mean FEV1 of the patients was 46.93 ± 14.32 of the predicted. Females had considerably lower FEV1 values as compared to males (mean FEV1 of 43.39 ± 13.28 in females v/s 48.42 ± 14.52 in males). Among the non-smoking population, females showed more severe obstruction as compared to males (mean FEV1 of 45 ± 13.3 v/s 48.5 ± 17.1, respectively). Among the smokers, the mean FEV1 of females was even lower, i.e., 41.9 ± 13.2 as compared to males (48.4 ± 14.3).
Table 3 shows the prevalence of co-morbidities and complications. The most common co-morbid conditions observed were acid peptic disease/ gastro-esophageal reflux disease, carcinoma lung, and cardiovascular diseases like ischemic heart disease, systemic hypertension, pulmonary arterial hypertension, arrhythmias, etc. Males had higher prevalence of carcinoma lung (P = 0.003), cardiovascular diseases (P = 0.01), and acid peptic disease/gastro-esophageal reflux disease (P = 0.0002) as compared to females. Presence of diabetes was noted in 3.1% patients. About 4.4% patients had other associated diseases like silicosis, carcinoma esophagus, carcinoma breast, oral cancer, alcoholic liver disease, hyperthyroidism, obstructive sleep apnea, hernia, etc. Around 28.8% patients had history of pulmonary tuberculosis in past. These patients had pulmonary tuberculosis 29.1 ± 8.8 years earlier.
Table 3.
Co-morbidities and Complications
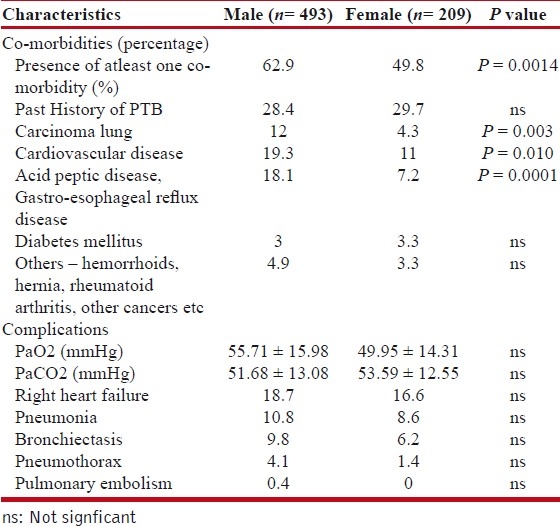
Right heart failure, pneumonia, bronchiectasis and pneumothorax were most common complications noted.
DISCUSSION
Females were found to be younger as compared to males (mean age 58.34 ± 9.99 years v/s 61.57 ± 10.37 years, P < 0.001). This could be because biomass smoke exposure begins early in females who start cooking using wood stoves at a very early age and also, sleep and eat in the same room where they cook.
Males smoked more than females (mean pack year smoking of 36.7 ± 15.5 v/s 13.5 ± 7.4, P < 0.001) and majority male smokers (93.7%), smoked beedi. Jindal et al (2006)[10] indicated a higher prevalence of COPD among beedi smokers than cigarette smokers (8.2% v/s 5.9%).
Some 186 (89%) females were exposed to smoke from biomass burning. This biomass smoke has been proposed to produce pathologic changes of COPD similar to those observed in cigarette smokers by Rivera et al (2008).[11] Also, several researchers have reported prevalence of COPD/chronic bronchitis in people exposed to biomass smoke especially from wood and cow dung.[12–16]
Patients exposed to both biomass smoke and tobacco smoke had the worst FEV1 scores of 42.39 ± 12.43. This combined exposure is suspected to have an additive effect,[15] probably leading to increased severity of the obstructive airway disease.
The average duration of exposure to biomass smoke in the female patients was around 29 ± 8 years. The average daily time spent in the kitchen was around 8-10 hours. Studies have shown that the risk of airflow obstruction increases with increasing hour-years exposure of biomass smoke.[17,18] The exact hour-year exposure was not calculated, but a similar trend was noted in the current study.
In comparison to males, females reported more dyspnea at presentation (grade 3 v/s grade 2, P < 0.001). This could be due to females displaying more bronchial obstruction than males (mean FEV1 of 43.39 ± 13.28 v/s 48.42 ± 14.52, respectively, P < 0.001). This could also be related to the increased susceptibility of Indian females to etiological agents (tobacco and biomass smoke) or delay in the diagnosis of COPD in females at primary health care level and/or late referral to a tertiary care hospital (which may be due to knowledge gap regarding COPD in females and/or less use of spirometry to diagnose or grade COPD severity).
The lower mean FEV1 values of the patients as compared to their western counterparts,[8,19] could be due to increased susceptibility of the Indian patients to tobacco smoke or increased toxic effects of beedi smoking. A recent study has demonstrated this fact that pulmonary functions are more affected in beedi smokers than in cigarette smokers.[20]
FEV1 values in female patients who smoked were considerably lower than those observed in nonsmoker females (mean FEV1 of 41.9 ± 13.2 and 45 ± 13.3, respectively). This difference in males was not significant. This may imply that smoking disproportionately affects females than males.[21]
Females had more exacerbations than males, but had relatively lesser number of hospital admissions in the preceding year. This may imply that even though females have more symptomatic exacerbations of the disease that requires medical attention; they receive fewer specialist referrals than males due to ignorance or gender bias that exists in availing the hospital facilities or simply these exacerbations were not severe enough to require hospital admission.
Males had more prevalence of emphysema seen on chest radiograph as compared to females. Females were more likely to have “increased markings” pattern (that resembles the “dirty chest” appearance seen in chronic bronchitis) than males (62% v/s 32% respectively, P < 0.001). These findings are similar to that reported by various researchers that male smokers with COPD have more extensive emphysema than women.[8,22,23] Also, about 90% of the females in the current study had exposure to biomass smoke, which has been described as the predisposing factor for development of chronic bronchitis.[15,16,24,25]
Contrary to the previous data, no association was found in the occurrence of pneumonia and type of inhaled corticosteroid used (budesonide v/s fluticasone). No significant difference was observed between males and females with regard to presence of complications such as pneumonia, right heart failure, pneumothorax, pulmonary embolism, etc.
Presence of atleast one co-morbid conditions was noted in 310 (62.9%) males and 104 (49.8%) females (P = 0.0014). More than 55% patients had 1-2 co-morbid conditions, and 3% had 3-4 co-morbid conditions. The lesser number of co-morbidities than reported by Van Manen and colleagues[26] could be due to the fact that we studied only a limited number of such conditions.
Anemia (associated with higher morbidity and mortality in COPD) was seen more commonly in females as compared to males (23.4% and 10.1%, respectively, P < 0.0001). Potential explanations could be malnutrition, particularly poor dietary intake of iron, increased blood loss during menstruation/child birth, or due use of oral steroids.
Higher prevalence of carcinoma lung and cardiovascular diseases in males could be attributed to the fact that smoking is a common risk factor for them and there were more number of male who smoked with heavier smoking history than females. Also, alcohol consumption and smoking are associated with increased incidence of acid peptic disease, both of which were more common in males.
Cachexia, skeletal muscle wasting and depression were noted more in females as compared to males, probably displaying a severe expression of COPD in females. This also stresses on the fact to look for these systemic features, more so in females so as to offer the patient a less morbid life.
Chronic airway obstruction occurs following tuberculosis[27–29] and is reported to be functionally comparable to COPD patients.[27] In the current study, past history of pulmonary tuberculosis (TB) was noted in 28.8% of the cases (similar to a recent study from India,[30] which reported the prevalence to be 28.4% in the COPD patients). These patients with COPD post-TB were younger as compared to COPD patients with no past history of TB (mean age of 59 ± 9 years and 61 ± 10 years, respectively, P = 0.01). On spirometry, patients with COPD post-TB had FEV1 values better than the other group (mean FEV1 of 48 ± 14 and 46 ± 14, respectively, P = 0.02).
Since women tend to have untreated or delayed treatment of TB due to prevailing socio-cultural systems,[5] cases due to COPD post-tuberculosis may see a rise in future specially in females. Larger field based studies are needed to further define this fact.
Thus, in comparison to males, females were younger, reported more dyspnea, more severe bronchial obstruction, more exacerbations and higher prevalence of systemic features. Also, females smoked less and had lesser incidence of productive cough, lower BMI, lesser co-morbidities and less number of hospital admissions as compared to males. Tobacco smoke in the form of beedi smoking was the predominant smoke exposure in males, whereas smoke from biofuel burning was the predominant exposure in females. Males were more likely than females to have an emphysema-predominant phenotype, while airway-predominant disease was more common among females.
To conclude, there are several gaps in the current knowledge regarding COPD in women. There is an urgent need for larger cohort or population studies with a significant number of women exclusively designed to evaluate the effect of gender on the clinical manifestations of COPD and the response to treatment.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverman EK, Weiss ST, Drazen JM, Chapman HA, Carey V, Campbell EJ, et al. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:2152–8. doi: 10.1164/ajrccm.162.6.2003112. [DOI] [PubMed] [Google Scholar]
- 3.Mackay J, Amos A. Women and tobacco. Respirology. 2003;8:123–30. doi: 10.1046/j.1440-1843.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- 4.Albalak R, Frisancho AR, Keeler GJ. Domestic biomass fuel combustion and chronic bronchitis in two rural Bolivian villages. Thorax. 1999;54:1004–8. doi: 10.1136/thx.54.11.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varkey AB. Chronic obstructive pulmonary disease in women: Exploring gender differences. Curr Opin Pulm Med. 2004;10:98–103. doi: 10.1097/00063198-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Chapman KR, Tashkin DP, Pye DJ. Gender Bias in the Diagnosis of COPD. Chest. 2001;119:1691–5. doi: 10.1378/chest.119.6.1691. [DOI] [PubMed] [Google Scholar]
- 7.Becklake MR, Kauffmann F. Gender differences in airway behavior over the human life span. Thorax. 1999;54:1119–38. doi: 10.1136/thx.54.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dransfield MT, Washko GR, Foreman MG, Estepar RS, Reilly J, Bailey WC. Gender differences in the severity of CT emphysema in COPD. Chest. 2007;132:464–70. doi: 10.1378/chest.07-0863. [DOI] [PubMed] [Google Scholar]
- 9.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 10.Jindal SK, Aggarwal AN, Chaudhry K, Chhabra SK, D’Souza GA, Gupta D, et al. Asthma Epidemiology Study Group. A Multicentric Study on Epidemiology of Chronic Obstructive Pulmonary Disease and its Relationship with Tobacco Smoking and Environmental Tobacco Smoke Exposure. Indian J Chest Dis Allied Sci. 2006;48:23–9. [PubMed] [Google Scholar]
- 11.Rivera RM, Cosio MG, Ghezzo H, Salazar M, Pérez-Padilla R. Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. Int J Tuberc Lung Dis. 2008;12:972–7. [PubMed] [Google Scholar]
- 12.Kiraz K, Kart L, Demir R, Oymak S, Gulmez I, Unalacak M, et al. Chronic pulmonary disease in rural women exposed to biomass fumes. Clin Invest Med. 2003;26:243–8. [PubMed] [Google Scholar]
- 13.Ekici A, Ekici M, Kurtipek E, Akin A, Arslan M, Kara T, et al. Obstructive airway diseases in women exposed to biomass smoke. Environ Res. 2005;99:93–8. doi: 10.1016/j.envres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Saha A, Rao NM, Kulkarni PK, Majumdar PK, Saiyed HN. Pulmonary function and fuel use: A population survey. Respir Res. 2005;6:127. doi: 10.1186/1465-9921-6-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu G, Zhou Y, Tian J, Yao W, Li J, Li B, et al. Risk of COPD from exposure to biomass smoke: a metaanalysis. Chest. 2010;138:20–31. doi: 10.1378/chest.08-2114. [DOI] [PubMed] [Google Scholar]
- 16.Orozco-Levi M, Garcia-Aymerich J, Villar J, Ramírez-Sarmiento A, Antó JM, Gea J. Wood smoke exposure and risk of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:542–6. doi: 10.1183/09031936.06.00052705. [DOI] [PubMed] [Google Scholar]
- 17.Pérez-Padilla R, Regalado J, Vedal S, Paré P, Chapela R, Sansores R, et al. Exposure to biomass smoke and chronic airway disease in Mexican woman: A case-control study. Am J Respir Crit Care Med. 1996;154:701–6. doi: 10.1164/ajrccm.154.3.8810608. [DOI] [PubMed] [Google Scholar]
- 18.Ramírez-Venegas A, Sansores RH, Pérez-Padilla R, Regalado J, Velázquez A, Sánchez C, et al. Survival of Patients with Chronic Obstructive Pulmonary Disease Due to Biomass Smoke and Tobacco. Am J Respir Crit Care Med. 2006;173:393–7. doi: 10.1164/rccm.200504-568OC. [DOI] [PubMed] [Google Scholar]
- 19.Laitinen T, Hodgson U, Kupiainen H, Tammilehto L, Haahtela T, Kilpelainen M, et al. Real-world clinical data identifies gender-related profiles in chronic obstructive pulmonary disease. COPD: J Chron Obstruct Pulmon Dis. 2009;6:256–62. doi: 10.1080/15412550903051799. Available from: http://informahealthcare.com/doi/abs/10.1080/15412550903051799 . [DOI] [PubMed] [Google Scholar]
- 20.Padmavathy KM. Comparative study of pulmonary function variables in relation to type of smoking. Indian J Physiol Pharmacol. 2008;52:193–6. [PubMed] [Google Scholar]
- 21.Prescott E, Bjerg AM, Andersen PK, Lange P, Vestbo J. Gender difference in smoking effects on lung function and risk of hospitalization for COPD: Results from a Danish longitudinal population study. Eur Respir J. 1997;10:822–7. [PubMed] [Google Scholar]
- 22.Martinez FJ, Curtis JL, Sciurba F, Mumford J, Giardino ND, Weinmann G, et al. Sex Differences in Severe Pulmonary Emphysema. Am J Respir Crit Care Med. 2007;176:243–52. doi: 10.1164/rccm.200606-828OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tatsumi K, Kasahara Y, Kurosu K, Tanabe N, Takiguchi Y, Kuriyama T. Respiratory Failure Research Group in Japan: Clinical phenotypes of COPD: Results of a Japanese epidemiological survey. Respirology. 2004;9:331–6. doi: 10.1111/j.1440-1843.2004.00611.x. [DOI] [PubMed] [Google Scholar]
- 24.Sin DD, Cohen SB, Day A, Coxson H, Paré PD. Understanding the Biological Differences in Susceptibility to Chronic Obstructive Pulmonary Disease between Men and Women. Proc Am Thorac Soc. 2007;4:671–4. doi: 10.1513/pats.200706-082SD. [DOI] [PubMed] [Google Scholar]
- 25.Kara M, Bulut S, Tas F, Akkurt I, Seyfikli Z. Evaluation of pulmonary changes due to biomass fuels using high-resolution computed tomography. Eur Radiol. 2003;13:2372–7. doi: 10.1007/s00330-003-1925-5. [DOI] [PubMed] [Google Scholar]
- 26.Van Manen JG, Bindels PJ, IJzermans CJ, van der Zee JS, Bottema BJ, Schadé E. Prevalence of comorbidity in patients with a chronic airway obstruction and controls over the age of 40. J Clin Epidemiol. 2001;54:287–93. doi: 10.1016/s0895-4356(01)00346-8. [DOI] [PubMed] [Google Scholar]
- 27.Jiménez P, Torres V, Lehman P, Hernández E, Alvarez M, Meneses M, et al. Chronic airflow limitation in patients with pulmonary tuberculosis sequelae. A comparison with COPD. [Article in spanish] Rev Chil Enf Respir. 2006;22:98–104. Available from: http://www.scielo.cl/scielo.php?script=sci_arttextandpid=S0717-73482006000200004 . [Google Scholar]
- 28.Menezes AM, Hallal PC, Perez-Padilla R, Jardim JR, Muiño A, Lopez MV, et al. Tuberculosis and airflow obstruction: Evidence from the PLATINO study in Latin America. Eur Respir J. 2007;30:1180–5. doi: 10.1183/09031936.00083507. [DOI] [PubMed] [Google Scholar]
- 29.Lam KB, Jiang CQ, Jordan RE, Miller MR, Zhang WS, Cheng KK, et al. Prior TB, smoking, and airflow obstruction: A cross-sectional analysis of the Guangzhou Biobank Cohort Study. Chest. 2010;137:593–600. doi: 10.1378/chest.09-1435. [DOI] [PubMed] [Google Scholar]
- 30.Mohan A, Premanand R, Reddy LN, Rao MH, Sharma SK, Kamity R, et al. Clinical presentation and predictors of outcome in patients with severe acute exacerbation of chronic obstructive pulmonary disease requiring admission to intensive care unit. BMC Pulm Med. 2006;6:27. doi: 10.1186/1471-2466-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]


