Abstract
We present a case of a young male who presented with complaints of fever along with cough and sputum. He was diagnosed with having right pleural effusion. He was already taking anti-tubercular therapy for one month before presentation. He was started on intravenous antibiotics and continued on anti-tubercular therapy in our hospital, based on his high leukocyte count, pleural fluid analysis, and ultrasonographic report of multiple hypoechoic areas in the liver. His symptoms continued to worsen and he subsequently developed mediastinal widening and a left lung mass. Commuted tomography (CT)-guided biopsy of the lung mass revealed a desmoplastic small-round-cell tumor. Desmoplastic small-round-cell tumor is a rare and aggressive tumor, which presents rarely as a mediastinal and lung mass. This tumor has very poor prognosis.
KEY WORDS: Desmoplastic small-round-cell tumor, desmoplastic tumor of the lung, diagnosis, mediastinal
INTRODUCTION
Desmoplastic small-round-cell tumor is a very rare, but aggressive tumor, having an extremely poor prognosis. The average life span is less than two years.[1] This tumor predominantly affects young adolescent males. It most commonly originates in the abdomen, but desmoplastic small-round-cell tumors originating from other body parts are also known, although they are rare.[2] This tumor carries a characteristic t(11;22) (p13;q12) translocation, which leads to oncogenesis.[3] It shows immunohistochemical reactivity for epithelial, mesenchymal, and neural markers, thus suggesting that it has the capacity for multiphenotypic differentiation.[4] This property along with its characteristic translocation, differentiates this tumor from other round cell tumors. It is treated with a multimodality approach. Those undergoing surgery for tumor resection has better survival, but most of the times the tumor is unresectable.[5] We present a case that initially had pleural effusion and liver metastasis and later rapidly developed a lung and mediastinal mass, which turned out to be desmoplastic small-cell tumor.
CASE REPORT
Our patient, a 25-year-old male, presented with complaints of cough with minimal expectoration for the past four months followed by fever for the past four weeks, which was low-grade, continuous, and associated with a weight loss of around 10 kg and loss of appetite. He also had right hypochondrial pain, which was non-colicky, non-radiating, not associated with any bowel symptoms and not associated with jaundice. He was on treatment from another hospital where he was started presumptively on anti-tubercular therapy (isoniazid, rifampicin, pyrazinamide, ethambutol, and pyridoxine) in appropriate doses, in view of right pleural effusion. He was taking the medication regularly for four weeks before he presented to our hospital. There was no history of tuberculosis in the past or any case of active pulmonary tuberculosis in the family.
Examination revealed a thin young man, afebrile, with normal pulse, blood pressure, and respiratory rate. He had pallor, with decreased air entry in the right infrascapular and infra-axillary area, with tender hepatomegaly about 4-5 cm below the right costal margin. The rest of the general physical and systemic examination was essentially normal.
His initial investigations revealed a hemoglobin of 10.6 gm/ dL, leucocytosis (TLC = 24,000/cumm) with neutrophilia, with normal platelet count. His ESR was 60 mm in the first hour by the Wintrobe's method. Serum LDH was 798 U/L. The remaining biochemical parameters were essentially normal. The enzyme-linked immunosorbent assay (ELISA) for human immunodeficiency virus (HIV) was non-reactive. His chest radiograph revealed right pleural effusion. Analysis of pleural fluid showed 500 cells with a differential count of 80% lymphocytes and 20% polymorphs. The pleural fluid sugar level was 50 mg/dl and protein level was 3.2 gm/dl. Serum protein was 6.0 gm/dl. The Adenosine Deaminase (ADA) level in the pleural fluid was 48 U/L (Units/Liter) (Normal: <30 U/L, 30-60 was equivocal and >60 was considered highly suggestive of tuberculosis). Pleural fluid was negative for malignant cells and did not show any organisms either. The sputum was negative for acid fast bacilli. Ultrasonography of the abdomen showed an enlarged liver with multiple hypoechoic areas of variable sizes in both the lobes of the liver with minimal ascites. The rest of the abdominal ultrasound was essentially normal.
An initial impression of multiple liver abcesses with right-sided exudative pleural effusion (pleural fluid protein/ serum protein > 0.5) was made. Tubercular etiology was considered in view of long history and pleural fluid analysis and hence the antitubercular therapy was continued and he was started on intravenous antibiotics to cover possible pyogenic etiology of multiple liver abcesses.
An ultrasound-guided tap from the hypoechoic liver lesions was done and the aspirated fluid was sent for cytology, acid fast bacilli staining, Gram staining, and culture for bacterial pathogens.
Even as the above reports were expected, he began developing breathlessness along with abdominal distention, which on clinical examination was actually an enlarging liver. Repeat blood investigations revealed anemia (Hb = 10.2 g/dL) with leucocytosis (TLC = 22,800/cumm) with neutrophilia. The serum LDH increased to 1098 U/L. The rest of hematological and biochemical parameters were essentially normal. A repeat chest radiograph was done, which showed mediastinal widening with homogenous opacity in the left upper zone, along with the pre-existing right pleural effusion. A repeat pleural fluid analysis was negative for malignant cells. Bone marrow aspiration and biopsy were done. Contrast enhanced commuted tomography (CECT) of the chest and abdomen and a guided biopsy from the left lung mass were planned.
The cytology of the liver lesion aspirate yielded cells suspicious of being round cell tumor. The liver lesion aspirate showed no acid fast bacilli and on culture grew no pathological organisms. CECT of the chest and abdomen performed subsequently showed nodular lesions in the mediastinum and left upper lung, along with hepatomegaly with multiple hypodense lesions scattered within it [Figure 1]. A CECT-guided biopsy of the left lung mass was done, which revealed a cellular tumor comprising of nests and groups of polygonal-to-spindle-shaped pleomorphic cells, separated by fibrous septa, having a high nuclear to cytoplasmic ratio, high mitosis, and some areas of necrosis [Figure 2]. On immunohistochemistry, the tumour cells were negative for LCA (Leucocyte Common Antigen), strongly expressed Cytokeratin, Vimentin, Desmin, Neuron-specific enolase, and Epithelial Membrane Antigen (EMA), suggestive of DESMOPLASTIC SMALL-ROUND-CELL TUMOR [Figure 3]. Bone marrow aspirate and biopsy received subsequently were essentially normal.
Figure 1.
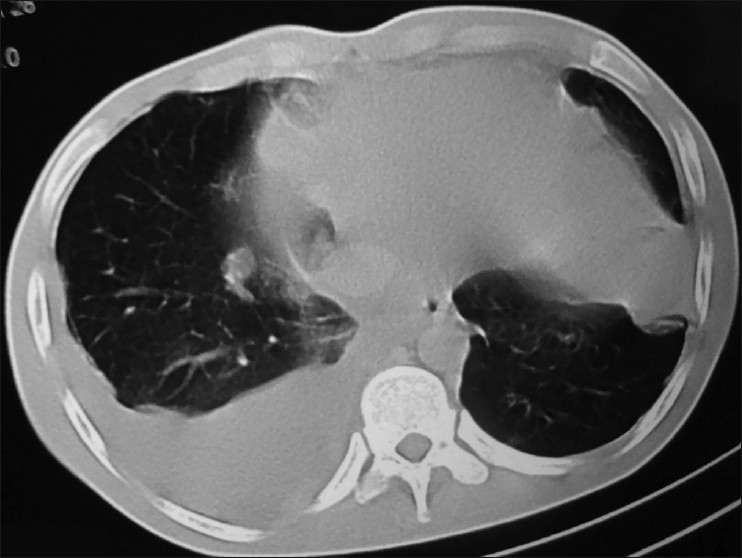
CECT chest showing mediastinal lymph nodes with lung mass
Figure 2.
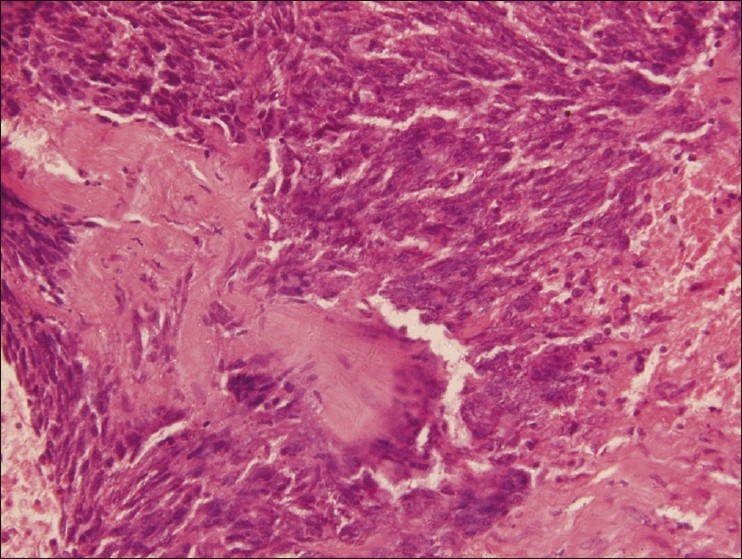
Tumor tissue, high power H and E stain
Figure 3.
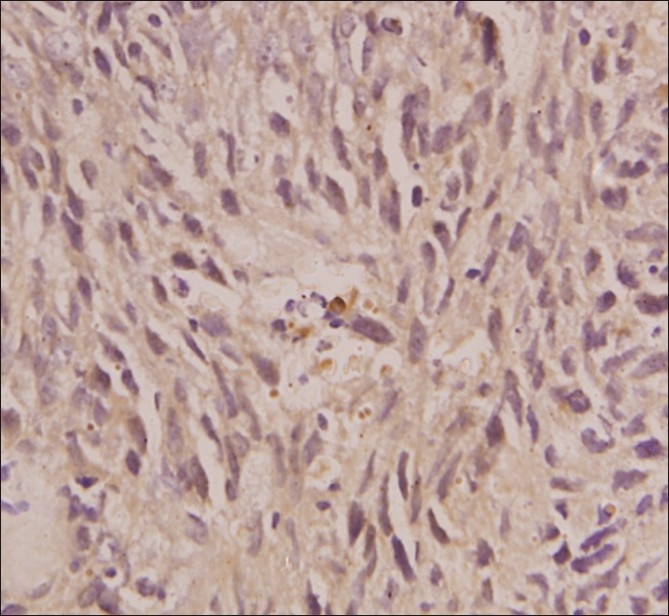
Cytokeratin stain
On being told about the illness and its prognosis, the patient and his family members decided against getting any chemotherapy or surgery and also declined to get polymerase chain reaction (PCR) done for detection of t(11;22) (p13;q12) translocation. He left against medical advice and expired after a few weeks in his native village.
DISCUSSION
Desmoplastic small-round-cell tumor is a rare, but aggressive tumor. It was first described by Gerald and Rosai in the year 1989, when they noticed a distinctive type of small cell tumor, which predominantly involved the abdomen and affected young males.[6] Gerald et al. in their study of 19 patients of desmoplastic small-round-cell tumor concluded that these tumors occurred predominantly in the abdomen, with the mean age of occurrence being 18.4 years, and had prelidiction for adolescent males. They also noticed that these tumors had inconstant distant organ involvement with immunohistochemical reactivity for epithelial, neural, and muscle markers and a highly aggressive behavior.[7] Ordonez in his observation of 39 patients with desmoplastic small-round-cell tumors noted that out of the 39 patients, one patient had tumor originating from the liver, one from the scrotum, and rest from the abdomen and/or pelvic peritoneum. Similar to Gerald et al., he also observed that this tumor predominantly involved young males.[8] Lee and Hsiao recently studied four patients with desmoplastic small-round-cell tumors and observed that the mean age of occurrence of this tumor was 24 years, and the tumor originated in the peritoneum of all the four patients.[9] This tumor occurred rarely in females and could even masquerade as ovarian cancer.[10]
In desmoplastic small-round-cell tumor, the characteristic chromosomal translocation t(11;22) (p13; q12) is seen, which brings the EWS gene on chromosome 22 to the WT1 gene on chromosome 11.[3] In a study by Lae et al., this translocation was present in 29 out of 32 tumors examined.[11] Fusion of the two genes leads to the production of two transcription factors EWS/WT1(–KTS) and EWS/ WT1(+KTS), which act on various genes like those of PDGF-A (platelet-derived growth factor A), IGFR1 (insulin-like growth-factor receptor 1), IL2RB (interleukin2 receptor beta), BAIAP3 (BAI1-associated protein 3), TALLA-1 (T-cell acute lymphoblastic leukemia associated antigen 1), and ENT4 (equilibrative nucleoside transporter 4), which leads to oncogenesis.[12]
Pathologically, the desmoplastic small-round-cell tumor consists of round, blue cells embedded in a desmoplastic stroma. To distinguish it from other small cell tumors, immunohistochemical markers are used. In the study by Ordonez, a variety of epithelial, mesenchymal, and neuronal markers have been detected in 39 tumors studied; cytokeratin in 37/39, epithelial membrane antigen in 24/25, desmin in 39/39, vimentin in 22/27, neuron-specific enolase in 18/25, synaptophysin in 3/19, chromogranin in 1/22, WT1 protein in 8/9, muscle-specific actin in 3/19, and alpha-smooth-muscle actin in 3/16.[8] Lee and Hsiao have observed that all the tumor cells are reactive to cytokeratin, desmin, vimentin, and WT-1. The EWS-WT1 fusion gene has been identified in three patients.[9] Thus, although it was initially thought to be of mesodermal origin, due to its site of origin, this tumor is now hypothesized to originate from a progenitor cell with multi-phenotypic differentiation.[4]
The desmoplastic small-round-cell tumor can be found at sites other than the abdomen. The paratesticular region, pleural serosa, posterior cranial fossa, bone, ovary, soft tissue, parotid gland, lung, kidney, pancreas, and sinonasal are the other sites from where desmoplastic small-cell tumors have been reported.[2,13–15] A case report of symptomatic hypoglycemia due to high serum IGF-II levels in a case of desmoplastic small-round-cell tumor has been recently published.[16] Abdominal pain, palpable abdominal mass, abdominal distention due to mass or ascites, hydronephrosis due to obstruction of the urinary tract by the tumor, hepatomegaly, bowel obstruction due to the pressure effect of the tumor, intra-abdominal lymphadenopathy, and liver metastasis are the common modes of presentation of this tumor.[2] Clinical manifestations also depend upon the site of the tumor. CECT and magnetic resonance imaging (MRI) of the involved site reveal desmoplastic small cell tumor to be a mass of heterogenous density/intensity. Flurodeoxyglucose-Positron Emission Technology/Commuted Tomography (FDG-PET/CT) can provide additional information on the stage of the tumor.[17]
Biswas G et al., recommended the multimodality approach comprising of chemotherapy, radiotherapy, and surgery, for the treatment of desmoplastic small-round-cell tumor. They found that surgical excision improved survival, but it was not possible in all the cases. They also observed that desmoplastic small-round-cell tumors were chemosensitive, but the overall results of the multimodality approach were unsatisfactory with the overall median survival in their study being only 6.5 months.[18] In another study by Lal et al., the multimodality approach resulted in a significant improvement in survival and it was also observed that surgical resection correlated with an improved outcome.[5] In recent times, Temsirolimus has been used for the treatment of metastatic desmoplastic small-round-cell tumor.[19]
Overall, the average survival of desmoplastic small-round-cell tumor is less than two years.[1]
There are two case reports of desmoplastic small-cell tumor involving the lung. The first case reported was of a young 22-year-old man who had wheezing for three months along with dry cough for two weeks, and his chest imaging demonstrated reticulonodular densities in the right lung and mediastinum, along with bilateral hilar and mediastinal lymphadenopathy. There was no fever, night sweats or weight loss. His transbronchial lung biopsy from the right lower lobe revealed a desmoplastic small-round-cell tumor. He was treated with four courses of doxorubicin, cisplatin, and ifosfamide and was doing well six months after the completion of chemotherapy.[20] The second case was a young 19-year-old boy, who had fever, cough, and right-sided chest pain for 20 days and on CECT chest had a mass in the right lower lung, the biopsy of which revealed desmoplastic small-round-cell tumor.[21] Our case is the third case of lung involvement by demoplastic small-round-cell tumor, which predominately involves the abdomen. Our patient had fever with cough and pleural effusion and was started on antitubercular therapy initially, on a presumptive basis. However, when the symptoms persisted, he was brought to our center, where further investigations established the diagnosis of desmoplastic small-cell tumor. He developed a mediastinal mass and left upper lung mass within a few days of presentation. This was similar to the rapid presentation seen in the case reported from China. This probably suggests that desmoplastic small-round-cell tumors of the lungs are very aggressive. Both the cases responded to treatment initially, but unfortunately, our patient declined treatment. Differential diagnoses of desmoplastic small-cell-tumor of the lung include:[21]
Small cell mesothelioma — This tumor is extremely rare in children and young adults. Negative staining for cytokeratin 5/6 and calretinin and dot-like staining for desmin can distinguish desmoplastic small-round-cell tumor from mesothelioma.
Primitive neuroectodermal tumor (PNET)/Ewing's sarcoma family of tumors — The age of presentation is similar. Histologically, they are composed of blue undifferentiated cells, with scant cytoplasm. Immunohistologically, PNET/Ewing's sarcomas usually differentiate toward neural elements. The abnormal translocation in the PNET/Ewing's sarcoma is t(11;22)(q24;q12).
Malignant non-Hodgkins lymphoma — It is excluded by the negative immunostaining of the LCA of the tumor cells.
Small cell carcinoma — There is lack of desmoplastic stroma, and the tumor cells stain negative for desmin and S100.
Diagnosis of desmoplastic small-round-cell tumor is clinched by a specific pathological and tumor-specific immunohistochemical staining pattern, not seen in other tumors.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kempson RL, Fletcher CDM, Evans HL, Henrickson MR, Sibley RS. Atlas of Tumor Pathology. 3rd Series, Fascicle 30. Washington, DC: Armed Forces Institute of Pathology; 2001. Tumors of the Soft tissue; pp. 452–8. [Google Scholar]
- 2.Chang F. Desmoplastic small round cell tumors: Cytologic, histologic, and immunohistochemical features. Arch Pathol Lab Med. 2006;130:728–32. doi: 10.5858/2006-130-728-DSRCTC. [DOI] [PubMed] [Google Scholar]
- 3.Sawyer JR, Tryka AF, Lewis JM. A novel reciprocal chromosome translocation t(11;22)(p13;q12) in an intraabdominal desmoplastic small round-cell tumor. Am J Surg Pathol. 1992;16:411–6. doi: 10.1097/00000478-199204000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Granja NM, Begnami MD, Bortolan J, Filho AL, Schmitt FC. Desmoplastic small round cell tumour: Cytological and immunocytochemical features. Cytojournal. 2005;2:6. doi: 10.1186/1742-6413-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lal DR, Su WT, Wolden SL, Loh KC, Modak S, La Quaglia MP. Results of multimodal treatment for desmoplastic round cell tumors. J Pediatr Surg. 2005;40:251–5. doi: 10.1016/j.jpedsurg.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 6.Gerald WL, Rosai J. Case 2. Desmoplastic small round cell tumor with divergent differentiation. Pediatr Pathol. 1989;9:177–83. doi: 10.3109/15513818909022347. [DOI] [PubMed] [Google Scholar]
- 7.Gerald WL, Miller HK, Battifora H, Miettinen M, Silva EG, Rosai J. Intraabdominal desmoplastic small round-cell tumor. Report of 19 cases of a distinctive type of high-grade polyphenotypic malignancy affecting young individuals. Am J Surg Pathol. 1991;15:499–513. [PubMed] [Google Scholar]
- 8.Ordonez NG. Desmoplastic small round cell tumor. I. A histopathologic study of cases with emphasis on unusual histologic patterns. Am J Surg Pathol. 1998;22:1303–13. doi: 10.1097/00000478-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Lee YS, Hsiao CH. Desmoplastic small round cell tumor: A clinicopathologic, immunohistochemical and molecular study of four patients. J Formos Med Assoc. 2007;106:854–60. doi: 10.1016/S0929-6646(08)60051-0. [DOI] [PubMed] [Google Scholar]
- 10.Bland AE, Shah AA, Piscitelli JT, Bentley RC, Secord AA. Desmoplastic small round cell tumor masquerading as advanced ovarian cancer. Int J Gynecol Cancer. 2008;18:847–50. doi: 10.1111/j.1525-1438.2007.01110.x. [DOI] [PubMed] [Google Scholar]
- 11.Lae ME, Roche PC, Jin L, Lloyd RV, Nascimento AG. Desmoplastic small round cell tumor: A clinicopathologic, immunohistochemical, and molecular study of 32 tumors. Am J Surg Pathol. 2002;26:823–35. doi: 10.1097/00000478-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Smolen GA, Beers LF, Xia L, Gerald W, Wang J, et al. Adenosine transporter ENT4 is a direct target of EWS/WT1 translocation product and is highly expressed in desmoplastic small round cell tumor. PLoS One. 2008;3:e2353. doi: 10.1371/journal.pone.0002353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su MC, Jeng YM, Chu YC. Desmoplastic Small Round Cell Tumor of the Kidney. Am J Surg Pathol. 2004;28:1379–83. doi: 10.1097/01.pas.0000128676.64288.0f. [DOI] [PubMed] [Google Scholar]
- 14.Bismar TA, Basturk O, Gerald WL, Schwarz K, Adsay NV. Desmoplastic small cell tumor in the pancreas. Am J Surg Pathol. 2004;28:808–12. doi: 10.1097/01.pas.0000126782.94781.dc. [DOI] [PubMed] [Google Scholar]
- 15.Finke NM, Lae ME, Lloyd RV, Gehani SK, Nascimento AG. Sinonasal desmoplastic small round cell tumor: A case report. Am J Surg Pathol. 2002;26:799–803. doi: 10.1097/00000478-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Barra WF, Castro G, Hoff AO, Siqueira SA, Hoff PM. Symptomatic hypoglycemia related to in appropriately high igf-ii serum levels in a patient with desmoplastic small round cell tumor. Case Report Med. 2010;2010:684045. doi: 10.1155/2010/684045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang WD, Li CX, Liu QY, Hu YY, Cao Y, Huang JH. CT, MRI, FDG- PET/ CT imaging findings of abdominopelvic desmoplastic small round cell tumors: Correlation with histopathological findings. Eur J Radiol. 2010 Jul; doi: 10.1016/j.ejrad.2010.06.046. doi:101016/j.ejrad.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 18.Biswas G, Laskar S, Banavali SD, Gujral S, Kurkure PA, Muckaden M, et al. Desmoplastic small round cell tumor: Extra abdominal and abdominal presentations and the results of treatment. Indian J Cancer. 2005;42:78–84. doi: 10.4103/0019-509x.16696. [DOI] [PubMed] [Google Scholar]
- 19.Thijs AM, van der Graaf WT, van Herpen CM. Temsirolimus for metastatic desmoplastic small round cell tumor. Pediatr Blood Cancer. 2010;55:1431–2. doi: 10.1002/pbc.22755. [DOI] [PubMed] [Google Scholar]
- 20.Syed S, Haque AK, Hawkins HK, Sorensen PH, Cowan DF. Desmoplastic small round cell tumor of the lung. Arch Pathol Lab Med. 2002;126:1226–8. doi: 10.5858/2002-126-1226-DSRCTO. [DOI] [PubMed] [Google Scholar]
- 21.Wang ZM, Xiao WB, Zheng SS. Desmoplastic small round cell tumor of the lung: Case report. Chin Med J(Engl) 2007;120:2327–8. [PubMed] [Google Scholar]


