Abstract
One mechanism of immune evasion utilized by human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) envelope glycoproteins is the presence of a dense carbohydrate shield. Accumulating evidence from in vitro and in vivo experiments suggests that alterations in N-linked glycosylation of SIV gp120 can enhance host humoral immune responses that may be involved in immune control. The present study was designed to determine the ability of glycosylation mutant viruses to redirect antibody responses to shielded envelope epitopes. The influence of glycosylation on the maturation and specificity of antibody responses elicited by glycosylation mutant viruses containing mutations of specific N-linked sites in and near the V1 and V2 regions of SIVmac239 gp120 was determined. Results from these studies demonstrated a remarkably similar maturation of antibody responses to native, fully glycosylated envelope proteins. However, analyses of antibodies to defined envelope domains revealed that mutation of glycosylation sites in V1 resulted in increased antibody recognition to epitopes in V1. In addition, we demonstrated for the first time that mutation of glycosylation sites in V1 resulted in a redirection of antibody responses to the V3 loop. Taken together, these results demonstrate that N-linked glycosylation is a determinant of SIV envelope B-cell immunogenicity in addition to in vitro antigenicity. In addition, our results demonstrate that the absence of N-linked carbohydrates at specific sites can influence the exposure of epitopes quite distant in the linear sequence.
Immune responses to human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) appear early in infection, limiting virus replication and controlling the initial primary viremic episode. While host immune responses are capable of controlling virus replication for several months to years following HIV and SIV infection, these viruses eventually escape the apparent immune control and result in the ultimate destruction of the host immune system. Understanding the early virus-host interactions that result in immune control and the mechanisms responsible for immune evasion by these viruses is critical to the development of effective vaccine strategies. Detailed characterizations of antibody responses directed to SIV and HIV type 1 (HIV-1) envelope proteins revealed a complex maturation process characterized by gradual, ongoing changes in both quantitative and qualitative antibody properties during the first 6 to 10 months following infection (9, 11, 13, 14, 33). This antibody maturation process has been associated with the development of protective immunity (9, 13, 20, 33, 48). However, further studies are needed to understand the prolonged time needed to achieve this maturation process and to define the mechanisms used by lentivirus envelope proteins for the early evasion of immune recognition and control.
The envelope proteins of HIV and SIV are heavily glycosylated, containing approximately 24 N-linked (Asn-X-Ser/Thr) glycosylation sites (35). These carbohydrates comprise about 50% of the total glycoprotein mass and are required to generate properly folded and processed proteins (29). However, once fully glycosylated proteins have been produced, these carbohydrate moieties do not appear to be required to maintain native protein structure, since enzymatically deglycosylated core envelope proteins retain their ability to bind CD4 and many conformationally dependent antibodies (28-30). In addition, despite the general requirement for carbohydrates in the production of envelope proteins, it is possible to remove some individual sites without impairing the ability of these glycosylation mutant envelope proteins to bind CD4 or yield replication-competent viruses (3, 4, 26). For years, it has been suggested that these carbohydrates serve as a barrier to shield the virus from effective immune recognition and control. The first evidence of this comes from studies with caprine arthritis encephalitis virus (CAEV). While treatment of CAEV with neuraminidase did not reduce infectivity of the virus particles, it enhanced the kinetics of neutralization of the virus by goat antibodies (19). These results strongly suggest that carbohydrates on the surface of CAEV are important in protection of the virus from rapid neutralization by antibodies. A second line of evidence comes from studies with both human and animal lentiviruses, where variation in the envelope glycoproteins frequently results in the deletion, addition, or relocation of potential N-linked glycosylation sites, suggesting a role for immune selection in the evolution of viral variants. These variation studies are further supported by the observation that the binding and neutralizing properties of some HIV-1 monoclonal antibodies (MAbs) are affected by changes in N-linked glycosylation (2, 4, 5, 16, 45). Recent studies also suggest that glycosylation in the V1, V2, and V3 regions may play critical roles in determining HIV-1 gp120 interactions with receptors (22, 37, 38) and in preventing access of neutralizing antibodies to the receptor binding domains (31).
While these studies demonstrate the effects of glycosylation on in vitro antigenicity, little has been reported on the role of glycosylation on immunogenicity in vivo. Nara et al. first demonstrated the emergence of V3-specific neutralization-resistant viruses in plasma samples from chimpanzees infected with HIV-1 (36). These results were suggested to be the result of immune pressure, resulting in the emergence of viral variants exhibiting conformational changes in gp120 that prevented antibody recognition of the V3 loop. While the relevance of these studies was originally questioned due to concerns over the use of the highly adapted HIV-1/IIIB laboratory strain, more recent studies have demonstrated that the V3 region may be an early neutralization target in primary HIV-1 isolates as well (23). Chackerian et al. reported that the evolution of SIVmne variants containing an additional N-linked glycosylation site in V1 in vivo were neutralized less efficiently by monkey sera than was the parental virus strain in vitro (7). Studies by Reitter et al. demonstrated that mutation of specific N-linked glycosylation sites in the V1 and V2 regions of SIVmac239 gp120 resulted in attenuated but replication-competent viruses (41) capable of eliciting increased levels of neutralizing antibody to the wild-type (WT) virus in vivo (42). More recently, Johnson et al. reported that mutations to remove glycosylation sites in the SIVmac239 gp41 ectodomain resulted in attenuated, replication-competent viruses that were more sensitive to in vitro antibody-mediated neutralization with WT SIVmac239-infected monkey serum (21). In addition, mapping of T-helper epitopes elicited by attenuated SIV infection demonstrated a suppressed recognition of T-helper epitopes in glycosylated regions of gp120 (44). Finally, recent studies in the HIV-1 and EIAV systems have demonstrated the emergence of neutralization-resistant viruses early after infection that contained mutations to introduce or relocate potential N-linked glycosylation sites in gp120 (17, 32, 49). Taken together, these results suggest that alterations in N-linked glycosylation of SIV gp120 can substantially alter host humoral immune responses that may be involved in immune control.
The present study was designed to examine the influence of glycosylation on the development of antibody responses elicited by glycosylation mutant viruses in which specific N-linked sites in and near the V1 and V2 regions of SIVmac239 gp120 were removed. Results from these studies demonstrated for the first time that mutation of glycosylation sites in V1 resulted in the redirection of antibody responses to V3 and that N-linked glycosylation is a determinant of SIV envelope B-cell immunogenicity in addition to in vitro antigenicity.
MATERIALS AND METHODS
Sources of antibody.
Longitudinal serum samples were collected from two individual monkeys per group inoculated with either WT SIVmac239 or SIVmac239 containing genetic mutations of N-linked glycosylation sites 4 and 5 (g45), 4 and 6 (g46), 5 and 6 (g56), or 5, 6, 8, 12, and 13 (M5) in V1 and V2 regions, previously described (41). All serum samples used in these studies were taken at time points after infection when no reversion of the original mutation(s) was observed (42). Reference immature (1 to 3 months) and mature (>12 months) immune sera obtained from monkeys inoculated with SIV have been described previously (9, 13, 14) and were used as controls in all experiments. Reference HIV-1 immune serum 25814-52 was obtained from the AIDS Research and Reference Reagent Program, National Institutes of Health (NIH), Rockville, Md.
Rhesus MAbs were generated from four individual monkeys infected with SIV/17E-CL for at least 12 months (9). All rhesus MAbs were produced, purified by immunoaffinity chromatography, and characterized as described previously (10, 43).
MAbs 50-69, Md-1, 98.6, 126-6, and F240 that recognize epitopes in the ectodomain of HIV-1 gp41 were obtained from the NIH AIDS Research and Reference Reagent Program of NIH.
SIV envelope glycoprotein antigens.
Native envelope glycoproteins (gp120 and gp41) were derived from detergent-disrupted, gradient-purified SIVsmB7 (24) as described previously (13). SIVsmB7 was chosen as our reference source of viral envelope following comparison of several SIV strains (including SIVsmB7, SIVmac251, and SIVmac239) in our panel of serological assays; results from these analyses revealed similar antibody maturation profiles.
Synthetic 20-mer peptides (overlapping by 10 amino acids) representing the 5′ half of the primary sequence of SIVmac239 gp120 (obtained from the NIH AIDS Research and Reference Reagent Program) were used to measure antibody responses directed to linear envelope determinants.
Baculovirus recombinant envelope proteins containing small segments of the SIV/17E-CL gp120 in the HIV-1/HXB2 envelope (gp120 and the ectodomain of gp41) backbone were constructed essentially by the HIV-2/HIV-1 chimeric envelope antigen construction method of Morikawa et al. (34). For production of these chimeras, the HIV-1/HXB2 envelope (gp120 and the ectodomain of gp41) was subcloned into a baculovirus expression system (Gibco/BRL) to produce multimeric envelope proteins. Using an overlapping PCR strategy, recombinant envelope DNA that contained the SIV/17E-CL region of interest flanked by conserved HIV-1/HXB2 backbone sequence and that contained restriction sites unique to the HIV-1 sequence was generated. The chimeric DNA was then subcloned into the HIV-1/HXB2 envelope backbone, and recombinant baculoviruses were generated. Recombinant, chimeric envelope proteins were produced by the infection of Sf21 insect cells, and secreted envelope proteins were purified by immunoaffinity chromatography. Using the same baculovirus expression system, native SIV/17E-CL and HIV-1/HXB2 envelope proteins were also produced. Due to a lack of cross-reactivity between SIV serum antibodies and the HIV-1/HXB2 envelope protein, these chimeric antigens allowed for the measurement of antibody responses directed against defined SIV epitopes expressed in the context of a native envelope protein. For analyses described in this study, chimeric envelope proteins containing the V1 (HSgpV1) or the V2 (HSgpV2) regions of SIV were used (see Fig. 6).
FIG. 6.
Schematic of HIV-1/SIV chimeric envelope proteins. (A) Two-dimensional schematic of recombinant HIV-1(SIV) chimeric envelope proteins, highlighting the SIV variable region (white squares) inserted into the corresponding HIV-1 variable region (black circles). The remaining HIV-1 gp120 backbone is shown by white circles. aa, amino acids. (B) Alignment of SIV and HIV-1 amino acid sequences. SIV/17E-CL inserted sequences are aligned with HIV-1/HXB2 deleted sequences. The variable regions are shown by bold letters, and the residues comprising the referenced N-linked glycosylation sites are shown boxed. Alignment spacers are indicated by dashes.
Measurement of SIV envelope-specific antibody responses.
Antibody responses directed against the native viral SIV envelope (gp120 and gp41) proteins were measured in a concanavalin A (ConA) enzyme-linked immunosorbent assay (ELISA) as previously described (13). Briefly, envelope glycoproteins from detergent-disrupted SIVsmB7 virus were captured on ConA-coated plates, and serum antibodies or MAbs were allowed to incubate with the ConA-anchored envelope proteins for 1 h at room temperature. After the appropriate wash step (phosphate-buffered saline [PBS] alone for endpoint titer and conformational dependence; 8 M urea versus PBS for antibody avidity), all wells were incubated with peroxidase-labeled goat anti-monkey immunoglobulin G (IgG) or goat anti-mouse IgG (Nordic Immunology) for 1 h at room temperature. All wells were incubated with TM blue (Serologicals Corp., Gaithersburg, Md.) substrate for 20 min at room temperature, color was developed by the addition of 1 N sulfuric acid, and the optical density at 450 nm (OD450) was read using an automated ELISA plate reader (Dynex Technologies). Endpoint titers were determined to be the last twofold dilution with an OD450 twice that of healthy monkey serum. Conformational dependence was determined by measuring the reactivity to native versus denatured envelope proteins, where a ratio of >1 reflects predominant reactivity with native envelope proteins and a ratio of <1 reflects predominant reactivity with denatured envelope proteins. Antibody avidity was determined by measuring the stability of the antigen-antibody complexes to 8 M urea and is expressed as follows: percent antibody avidity = (OD of wells washed with 8 M urea/OD of wells washed with PBS) × 100. The results are averages of at least two independent experiments, with variation in individual antibody avidity and conformational dependence values of less than 10%.
Antibody responses directed to synthetic 20-mer peptides, overlapping by 10 amino acids and representing the first 350 amino acids of the SIVmac239 gp120 sequence (kindly provided by the NIH AIDS Research and Reference Reagent Program), were measured in a poly-l-lysine (PLL) peptide ELISA as described previously (12). Briefly, synthetic peptides were fixed in the PLL-coated wells of microtiter plates in an overnight incubation at room temperature. The remaining ELISA procedure was performed as described above for the ConA ELISA. Antibody reactivity to the peptides was determined by using a 1:200 dilution of serum. The results are reported as the OD450 and represent the average of two independent experiments.
Antibody responses directed to conformational epitopes as expressed in recombinant chimeric envelope proteins (HSgpV1 and HSgpV2) were measured in a ConA ELISA using the method described above for endpoint titers to native SIVsmB7 envelope proteins. The full-length native SIV (Sgp) and HIV-1 (Hgp) envelope proteins were included as controls, and antibody reactivity to both native and chimeric recombinant envelope proteins was determined by using a 1:200 dilution of serum. The results are reported as the OD450 and represent the average of two independent experiments.
SIV and HIV-1 MAb reactivity with native and chimeric envelope glycoproteins were determined in a ConA ELISA using the method described above for endpoint titers to native SIVsmB7 envelope proteins.
CD4 binding ELISA.
Reactivity of native and chimeric envelope antigens to recombinant soluble CD4 (rsCD4) was determined in a ConA ELISA. Native or chimeric envelope proteins were captured in ConA-coated wells as described above. All wells were blocked with 10% powdered milk in PBS before being incubated with 10 ng of rsCD4 (kindly provided by the NIH AIDS Research and Reference Reagent Program) per well for 1 h at room temperature. All wells were incubated with a 1:1,000 dilution of rabbit anti-CD4 antibody (kindly provided by the NIH AIDS Research and Reference Reagent Program) for 1 h at room temperature, followed by incubation with peroxidase-labeled goat anti-mouse IgG (Nordic Immunology) for 1 h at room temperature. TM blue substrate (Serologicals Corp.) was added to each well for 20 min at room temperature, color was developed by the addition of 1 N sulfuric acid, and the OD450 was read using an automated ELISA plate reader (Dynex Technologies).
RESULTS
Antibody maturation to native, fully glycosylated SIV envelope proteins in monkeys inoculated with glycosylation mutant SIVmac239 viruses.
To determine the effects of mutations of N-linked glycosylation sites on antibody maturation to native, fully glycosylated envelope proteins, plasma samples from monkeys inoculated with either WT SIVmac239 or viruses containing mutations to remove specific N-linked glycosylation sites in and around the V1 and V2 regions of gp120 were analyzed (42). Heat-inactivated plasma samples taken monthly for the first year following infection with wild-type SIVmac239 or viruses containing mutations of glycosylation sites 4 and 5 (g45), sites 4 and 6 (g46), sites 5 and 6 (g56), or sites 5, 6, 8, 10, and 11 (M5) were analyzed for reactivity with native, fully glycosylated envelope proteins in a ConA ELISA. A schematic of the putative N-linked glycosylation sites for SIVmac239 and the locations of the mutated sites in and near the V1 region are shown in Fig. 1. Previously, rhesus macaques infected with glycosylation mutant viruses were monitored for levels of virus replication, disease progression, and envelope sequence changes that effectively restored the original glycosylation site or added a new glycosylation site in close proximity to the original mutation site (42). While removal of glycosylation sites in the V1 and V2 regions of SIVmac239 gp120 resulted in attenuation of the virus, the four mutant viruses replicated to levels similar to that of the WT virus, as determined by similar peak viral loads in vivo. In addition, since sequence changes that resulted in the restoration of specific N-linked glycosylation sites could influence the specificity of immune responses to SIV envelope proteins, we selected samples from time points at which no variation in the respective infecting viral envelope were detected. In this manner, the maturation of antibody responses to fully glycosylated SIV envelope proteins could be directly compared to those responses elicited by infection of viruses lacking glycosylation at specific sites in the V1 region.
FIG. 1.
Schematic of SIVmac239 gp120. Amino acids representing the entire primary sequence of SIVmac239 were drawn schematically based on the known disulfide bonds for HIV-1 (27) and the hypothetical disulfide bonds for SIV and HIV-2 (18). The variable regions were based on the designations defined by Choi et al. (8), and the V3 region corresponds to the analogous V3 loop of HIV-1. All 23 potential N-linked (N-X-S/T) glycosylation sites for SIVmac239 are indicated by the addition of a CHO symbol at the asparagine (N) residue. In addition, nine amino acid changes between the parental SIVmac239 and the recombinant SIV/17E-CL used to produce chimeric envelope proteins are designated at the appropriate residue with a square denoting the amino acid change (1).
As shown in Fig. 2, monkeys infected with either WT virus or glycosylation mutant viruses demonstrated antibody titers that emerged quickly, reaching maximum, steady-state, log reciprocal endpoint titers ranging from 12,800 to 102,400 by approximately 10 weeks after infection. The g45, g46, and g56 mutant viruses elicited quantitative antibody titers that were similar to those achieved by WT SIVmac239 infection, while infection of monkeys with the M5 mutant resulted in maximum endpoint titers that were 1 to 2 log units lower than those observed in SIVmac239-infected monkeys (Fig. 2). These differences in the quantitative levels of antibody elicited by the M5 mutant are likely due to the level of attenuation created by generating the specific N-linked glycosylation site mutations (42).
FIG. 2.
Maturation of envelope-specific antibody titers in monkeys infected with glycosylation mutant viruses. Serum antibody responses from monkeys infected with SIVmac239 (WT) or viruses with envelope proteins containing mutations of N-linked sites 4 and 5 (g45), sites 4 and 6 (g46), sites 5 and 6 (g56), or sites 5, 6, 8, 12 and 13 (M5) were analyzed for reactivity to SIVsmB7 envelope proteins in the ConA ELISA. Serum antibody responses from monkeys infected with each glycosylation mutant virus are shown as open symbols and compared to the average response from monkeys infected with WT virus (black squares). Endpoint titers were determined to be the last twofold dilution with an OD450 twice that of normal monkey serum and are reported as the log10 of the reciprocal endpoint titer.
Once quantitative titers were determined, the qualitative properties of antibody avidity and conformational dependence were analyzed. Antibody avidity, or the resistance of the plasma antibody-envelope glycoprotein complexes to treatment with 8 M urea, was determined for each plasma time point listed above (Fig. 3). In general, the maximum antibody avidity values achieved were similar in all monkeys infected with either WT virus or glycosylation mutant viruses, where maximum, steady-state antibody avidity values ranging from 46 to 52% were achieved. Despite similar maximum avidity values in all five groups of monkeys, the time to achieve maturation of antibody avidity differed slightly. For example, maturation of antibody avidity in monkeys infected with g45, g56, and M5 mutant viruses was slightly delayed, requiring approximately 40 weeks compared to the 30 weeks observed in monkeys infected with the wild-type virus (Fig. 3). Alternately, antibody avidity to the g46 mutant virus demonstrated a slightly faster maturation, achieving maximum avidity values by 20 weeks in one of the two monkeys (Fig. 3).
FIG. 3.
Maturation of envelope-specific antibody avidity in monkeys infected with glycosylation mutant viruses. Serum antibody responses from monkeys infected with SIVmac239 (WT) or glycosylation mutant viruses g45, g46, g56, or M5 were analyzed for reactivity to SIVsmB7 envelope proteins in the ConA ELISA. Serum antibody responses from monkeys infected with each glycosylation mutant virus are shown as open symbols and compared to the average response from monkeys infected with WT virus (black squares). Antibody avidity was determined by measuring the stability of the antigen-antibody complexes to 8 M urea and is expressed as the (OD of wells washed with 8 M urea/OD of wells washed with PBS) × 100.
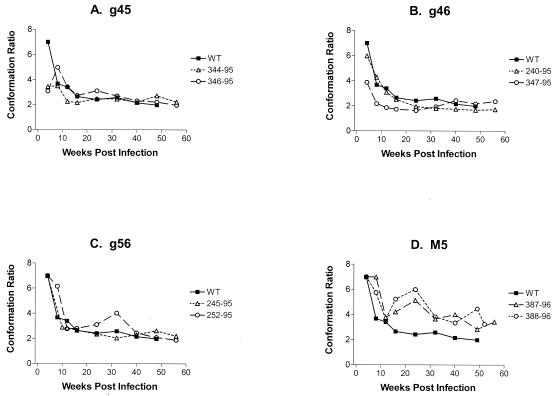
To complement the studies of antibody avidity, we also assayed for conformational dependence at these same time points. As shown in Fig. 4, again only minor differences in the time required for maturation to steady-state conformation ratios were observed in monkeys infected with the double mutant viruses. Both monkeys infected with the g45 or g46 mutant virus and one of the two monkeys infected with the g56 virus demonstrated antibody responses with conformational dependence similar to that of SIVmac239 (Fig. 4). The other monkey infected with the g56 mutant required 40 weeks to achieve a level of maturation similar to that observed for the monkeys infected with the WT virus (Fig. 4). In contrast, the monkeys infected with the M5 mutant displayed high conformational dependence at all time points tested, never reaching a mature state by 52 weeks after infection (Fig. 4).
FIG. 4.
Maturation of envelope-specific antibody conformational dependence in monkeys infected with glycosylation mutant viruses. Serum antibody responses from monkeys infected with SIVmac239 (WT) or glycosylation mutant viruses g45, g46, g56, or M5 were analyzed for reactivity to SIVsmB7 envelope proteins in the ConA ELISA. Serum antibody responses from monkeys infected with each glycosylation mutant virus are shown as open symbols and compared to the average response from the monkeys infected with WT virus (black squares). Conformational dependence was determined by measuring the reactivity to native versus denatured envelope proteins; ratios of >1 reflect predominant reactivity with native envelope proteins, while ratios of <1 reflect predominant reactivity with denatured envelope proteins.
Taken together, these analyses of both the quantitative and qualitative antibody properties elicited by these glycosylation mutant viruses demonstrated only minor differences in the maturation of antibody responses to native, fully glycosylated envelope glycoproteins that were not considered significant. These studies demonstrated an averaging effect when using whole, native envelope proteins and indicated the need for a higher-resolution analysis of antibody responses to specific envelope domains.
Removal of glycosylation sites in V1 increased antibody reactivity to V1 and redirected reactivity to linear envelope determinants in V3.
To address the need for higher-resolution analyses of antibody responses to envelope glycoproteins, we have developed additional serological assays to monitor antibody responses to defined envelope domains (12). Plasma antibody responses to linear envelope determinants were measured in plasma samples taken 8, 24, and 40 weeks after infection with WT SIVmac239 or glycosylation mutant viruses. For these assays, antibody reactivity to a panel of overlapping 20-mer synthetic peptides representing the 5′ half of the SIVmac239 amino acid sequence was used. Plasma samples were diluted 1:200 and assayed for reactivity to individual peptides in a PLL peptide ELISA (12). Results from these studies are summarized in Fig. 5. PLL peptide ELISAs revealed a limited reactivity of these plasma samples for linear envelope determinants, demonstrating reactivity to only 7 of the 35 peptides screened for any of the monkeys tested. This result was not surprising, since we have previously shown that the predominant antibody response to SIV envelope proteins is directed against conformational epitopes (9, 11-14, 33). A summary of the specificity of these reactive peptides is shown in Table 1.
FIG. 5.
Serum antibody reactivity to linear envelope determinants. Sera obtained from monkeys infected with g45 (A), g46 (B), g56 (C), or M5 (D) glycosylation mutant viruses at the indicated time points after infection were analyzed for antibody reactivity to SIVmac239 synthetic peptides spanning the first 350 amino acids of the gp120 in a PLL peptide ELISA. Reactivity of sera (1:200 dilution) to 6 of 35 peptides is reported as the OD450. The antibody reactivities of sera from monkeys infected with the WT or mutant viruses are indicated by four bars for each peptide; the bars in the graphs from left to right correspond to the bars in the symbol keys from top to bottom.
TABLE 1.
SIVmac239 reactive peptides
| Peptide | Amino acids | Envelope region | Amino acid sequence | N-linked site(s) |
|---|---|---|---|---|
| 12 | 111-130 | V1 | MRCNKSETDRWGLTKSITTT | g4 |
| 15 | 141-160 | V1 | KVDMVNETSSCIAQDNCTGL | g5, g6 |
| 16 | 151-170 | CIAQDNCTGLEQEQMISCKF | g6 | |
| 17 | 161-180 | EQEQMISCKFNMTGLKRDKK | g7 | |
| 32 | 311-330 | V3 | CRRPGNKTVLPVTIMSGLVF | g18 |
| 33 | 321-340 | V3 | PVTIMSGLVFHSQPINDRPK | |
| 34 | 331-350 | V3 | HSQPINDRPKQAWCWFGGKW |
While plasma samples from all monkeys reacted with these same seven peptides, the quantitative levels of reactivity were not equal. Monkeys infected with the WT SIVmac239 virus demonstrated only low antibody reactivity to these seven peptides, while monkeys receiving the g45 and g46 mutant viruses demonstrated marked increases in antibody reactivity to peptides in both the V1 and V3 loop regions of gp120. As shown in Fig. 5A, one of the two monkeys infected with the g45 mutant (monkey 346-95) demonstrated an eightfold increase in reactivity to peptide 12 at 8 weeks postinfection compared to monkey 344-95 (g45) or the two monkeys infected with WT virus. In addition, reactivity to the three peptides representing the V3 loop at this same 8 week time point was similar for both the WT and g45 viruses. In contrast, by 24 weeks postinfection, both monkeys infected with the g45 mutant virus demonstrated at least a 10-fold increase in reactivity to V1 peptide 12 and to the V3 loop peptide 32. These marked increases in reactivity to linear determinants in the V1 and cysteine loop regions were maintained at 40 weeks postinfection.
Monkeys infected with the g46 mutant virus also demonstrated increased antibody reactivity to the V1 and V3 loop regions of SIVmac239 gp120 (Fig. 5B). However, the overall levels of increased reactivity in the monkeys infected with the g46 mutant were five- to sevenfold lower than the increases observed in the g45-infected monkeys. Plasma antibody samples from monkeys infected with the g46 mutant also demonstrated an increased breadth of reactivity compared to the g45-infected monkeys. Monkeys infected with the g46 mutant not only demonstrated increased reactivity to peptides 12 (V1) and 32 (V3) previously observed with the g45 mutant but expanded this increased reactivity to include peptides 15 and 33, also in the V1 and V3 loop regions, respectively. This enhanced antibody reactivity to linear envelope determinants in both V1 and V3 regions increased from 8 to 24 weeks and was maintained at 40 weeks postinfection.
To determine whether the observed increases in antibody reactivity to V1 (peptide 12) and V3 (peptide 32) in monkeys infected with the g45 and g46 mutant viruses were significant, a t test was performed. Results from these analyses demonstrated statistically significant increases in reactivity to both peptides 12 (V1) and 32 (V3) at 24 weeks postinfection in the g45-infected monkeys (P = 0.001 and P = 0.016, respectively) compared to antibody reactivity observed in monkeys infected with WT SIVmac239. Monkeys infected with the g46 mutant virus demonstrated significant increases to peptide 12 (P = 0.042) but not to peptide 32 (0.282) compared to monkeys infected with WT virus. No significant increases in antibody reactivity to any of the peptides were observed in either the g56- or M5-infected monkeys. Taken together, these results demonstrated that mutation of the g4 N-linked glycosylation site in V1 resulted in increased antibody reactivity to linear envelope determinants in close proximity to the glycosylation site and in redirection of antibody reactivity to sites located more distal in the linear amino acid sequence from the V1 region of gp120 (i.e., the V3 loop).
Removal of glycosylation sites in V1 increased antibody reactivity to chimeric HIV-1 envelope glycoproteins containing the SIV V1 region.
Since the predominant antibody response to SIV envelope proteins is directed against conformational epitopes (9, 11-14, 33), it was necessary to further compare the antibody responses of SIVmac239-infected monkeys with those of monkeys infected with glycosylation mutant viruses to distinct envelope epitopes, as they are expressed in the context of a full-length, recombinant envelope glycoprotein. For these studies, chimeric envelope proteins in which small segments of SIV gp120 containing defined variable regions were placed in the HIV-1 envelope backbone (gp120 and the ectodomain of gp41) were constructed and expressed using a baculovirus expression system. A schematic of the two chimeric envelope proteins (HSgpV1 and HSgpV2 containing either the V1 or V2 region of SIV/17E, respectively, in the HIV-1/HXB2 backbone) used in these studies is shown in Fig. 6. SIV/17E-CL is a recombinant virus in which gp120 cloned from a brain isolate derived from SIVmac239 was cloned into the SIVmac239 backbone (1); it differs from SIVmac239 by 10 amino acids in gp120. These amino acid changes are highlighted in Fig. 1.
Once produced, immunoaffinity-purified chimeric envelope glycoproteins were extensively characterized in an attempt to determine the antigenic integrity and specificity of SIV-infected monkey serum reactivity directed to the SIV-specific amino acid fragments contained in the HSgpV1 and HSgpV2 chimeric antigens. Results from these characterizations are shown in Fig. 7. To quantitatively characterize these envelope proteins, antibody reactivity to Sgp, Hgp, HSgpV1, and HSgpV2 was determined in the ConA ELISA against high-titer reference immune serum from an SIV-infected monkey or an HIV-1-infected patient. As shown in Fig. 7A, serum reactivity with the native Sgp and Hgp envelope proteins was specific, i.e., reactivity of Sgp was observed only with SIV-infected monkey serum antibody and reactivity of Hgp was observed only with HIV-1-infected patient serum antibody. As expected, the levels of reactivity to HSgpV1 and HSgpV2 with SIV-specific serum were lower than that of the native Sgp envelope protein. These quantitative differences were not surprising, given that less than 10% of the total SIV envelope was expressed in each chimera. In contrast, similar levels of reactivity with HIV-specific serum were observed with Hgp, HSgpV1, and HSgpV2. These results confirmed the specificity of antibody reactivity with the native envelope proteins and demonstrated a lack of cross-reactivity with SIV and HIV-1 envelope proteins using polyclonal immune serum.
FIG. 7.
Characterization of HIV-1(SIV) chimeric envelope proteins. Antibody reactivity to native and chimeric envelope proteins was determined in the ConA ELISA against high-titer reference immune serum from an SIV-infected monkey or an HIV-1-infected patient (A), SIV V1-specific MAb 3.10A (B), or rsCD4 (C).
To demonstrate that the SIV epitope expressed in the HIV-1 envelope backbone has a conformation similar to its conformation in the native SIV envelope, the chimeras were used as antigens in the ConA ELISA to demonstrate binding to SIV-specific MAbs. Rhesus MAb 3.10A, previously shown to bind to the SIV V1 region (10), demonstrated similar levels of binding to the native SIV envelope (Sgp) and the V1 chimera (HSgpV1), while no reactivity with the native HIV-1 envelope backbone (Hgp) or the V2 chimera (HSgpV2) was observed (Fig. 7B). In addition, the native and chimeric envelope antigens also showed similar levels of binding to a panel of conformationally dependent HIV-1 MAbs (data not shown) and rsCD4 (Fig. 7C). Finally, the premise for using the chimeric envelope antigens is that the presentation of a small peptide segment in the context of a complete protein will assume a more specific conformation, and thus antibody reactivity, compared to a linear synthetic peptide. To test this claim directly, we compared the antigenicity of our HSgpV1 chimera with a purified V1 synthetic peptide containing the same 47 amino acids. Results from these studies revealed that a reference serum sample with the V1 peptide required 109 more copies to achieve a level of reactivity similar to that observed with HSgpV1 (i.e., for every nanomole of V1 chimera, we needed at least 1 mol of V1 peptide to obtain similar quantitative levels of reactivity with a reference serum sample), suggesting that the HSgpV1 chimera is more than one million times more reactive per antigen copy that the V1 peptide (data not shown). Taken together, these data suggest that presentation of a small SIV epitope in the context of an HIV-1 envelope backbone results in a conformational presentation that is similar to the presentation of the native SIV envelope protein.
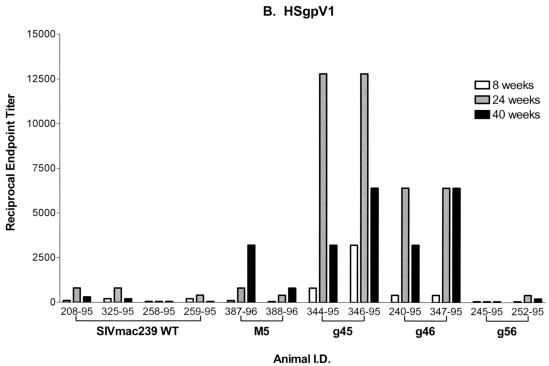
Once produced and characterized, the immunoaffinity-purified chimeric envelope proteins containing either the V1 (HSgpV1) or V2 (HSgpV2) region of SIV gp120 were used as antigens in the ConA ELISA. As shown in Fig. 8A, plasma antibodies from monkeys infected with WT or g45 mutant virus and one of two monkeys infected with g46 demonstrated similar reactivities to fully glycosylated, SIVsmB7 viral envelope proteins, with reciprocal endpoint titers ranging from 51,200 to 102,400. In contrast, two- to fivefold-lower levels of reactivity were observed for the g56 and M5 mutants, with maximum endpoint titers of 37,500 and 12,800, respectively. This variation in the levels of reactivity to native envelope proteins is likely to be related to either the level of attenuation of the mutant viruses or the masking of specific antibody epitopes available in the fully glycosylated protein. Reactivity to the HSgpV1 (Fig. 8B) and HSgpV2 (Fig. 8C) chimeric envelope proteins in all plasma antibody samples tested was 10- to 100-fold lower than that observed for SIVsmB7 envelope proteins. These results were expected, since reactivity to these chimeras reflects the measurement of polyclonal antibody reactivity to a single SIV variable region that comprises less than 10% of total gp120 protein.
FIG. 8.
Serum antibody reactivity to HSgpV1 and HSgpV2 chimeric envelope proteins. Sera obtained at the indicated time points after infection were analyzed for antibody reactivity to SIVsmB7 native envelope proteins (A), HSgpV1 (B), and HSgpV2 (C) in a ConA ELISA. HSgpV1 is a chimeric envelope protein containing the V1 region of SIV/17E-CL in the HIV-1/HXB2 envelope backbone, and HSgpV2 is a chimeric envelope antigen containing the V2 region of SIV/17E-CL in the HIV-1/HXB2 envelope backbone. Endpoint titers were determined to be the last twofold dilution with an OD450 twice that of normal monkey sera. Animal I.D., animal (monkey) identification number.
Despite lower overall levels of plasma antibody reactivity to the chimeric envelope proteins, significant differences were observed in a comparison of the WT and glycosylation mutant viruses. Monkeys infected with either the g45 or g46 mutant virus demonstrated significantly higher levels of reactivity (up to 10-fold) to HSgpV1 than monkeys infected with SIVmac239. Maximum reciprocal endpoint titers of 6,400 to 12,800 were observed in monkeys infected with the g45 and g46 mutant viruses compared to titers of 800 to 1,200 for monkeys infected with the WT virus. Using a t test, increases in antibody reactivity to HSgpV1 in monkeys infected with the g45 and g46 mutant viruses were considered statistically significant (P < 0.0001). While one of the two monkeys infected with the M5 mutant virus demonstrated a threefold increase in reactivity to HSgpV1, the second M5-infected monkey and the two g5-infected monkeys demonstrated levels of reactivity to HSgpV1 that were comparable to those observed for monkeys infected with WT virus. These differences were not considered statistically significant. Finally, seven of the eight monkeys infected with glycosylation mutants demonstrated no significant differences in plasma reactivity to HSgpV2 compared to monkeys infected with WT virus. While one of the two monkeys infected with M5 demonstrated a two- to threefold increase in reactivity to HSgpV2 compared to that of monkeys infected with WT virus, it appears that removal of glycosylation sites in the V1 and V2 regions did not enhance reactivity to epitopes in V2. Taken together, these studies using synthetic peptide and chimeric envelope antigens demonstrated the need for a higher-resolution analysis of serum antibodies in monkeys infected with glycosylation mutant viruses and stress the need to focus studies on defined envelope epitopes to identify differences in immune responses between WT and mutant virus infections.
DISCUSSION
The addition of complex carbohydrates to the surface envelope proteins of SIV and HIV-1 has long been proposed to serve as a shield to protect the virus from immune control. Previous studies have clearly demonstrated that mutations of defined N-linked glycosylation sites at the molecular level result in replication-competent viruses that are capable of eliciting stronger antibody responses against both mutant and WT viruses (21, 41, 42). In the present study, we analyzed the ability of SIVmac239 viruses containing mutations of specific N-linked glycosylation sites in the V1 and V2 regions to redirect B-cell responses in vivo. While no significant differences in maturation of antibody responses to native envelope proteins were observed, domain-specific serological analyses revealed striking differences in a comparison of antibody responses elicited by glycosylation mutant viruses to those elicited by WT SIVmac239. Consistent with previous findings by Reitter et al. (42), removal of N-linked glycosylation sites in the V1 region served to increase antibody reactivity to V1 determinants (represented by linear peptides and in the context of a conformationally intact chimeric envelope protein). More striking was the observation that mutation of glycosylation site 4 in V1 also resulted in enhanced antibody recognition of epitopes in the region of SIVgp120 analogous to the HIV-1 V3 loop. This is the first report to our knowledge demonstrating that removal of N-linked glycosylation sites in SIV gp120 resulted in the redirection of B-cell responses to regions that are distal in the linear amino acid sequence.
Two possible explanations for this observation exist. First, while located distally in the linear sequence, the V1 and V3 regions of SIVmac239 may lie in close proximity when the envelope glycoproteins are folded in their native, oligomeric states. Thus, glycosylation present in the V1 loop (i.e., the g4 site) may simply serve as steric hindrance for antibody binding sites present in the V3 loop. Alternately, these data may suggest that glycosylation at the g4 site plays a role in maintaining structural conformation that serves to shield critical epitopes in V3 from immune recognition. On the basis of the crystallized HIV-1 gp120 molecule (51), the V1, V2, and V3 loop stems flank proposed coreceptor binding sites including CCR5, the major coreceptor used by SIV strains. If we believe that the SIV gp120 envelope protein is similar to that of HIV-1, it could be predicted that removal of carbohydrates that lie in or near this coreceptor binding region may serve to remove the shield that limits immune recognition. By exposing the CCR5 binding site, coreceptor binding and in turn virus-cell and cell-cell fusion would be facilitated in the absence of CD4. This would allow for more efficient virus entry during the earlier stages of infection when the immune response has not fully matured and also may result in more efficient cell-to-cell spread of virus. This hypothesis is supported by the recent findings of Puffer et al. (39) that envelope proteins containing pairs of mutant N-linked glycosylation sites (g45, g46, and g56) are capable of facilitating cell-cell fusion in a CD4-independent manner. In addition, recent site-directed mutagenesis studies suggest that the presence of N-linked glycosylation sites in the V1, V2, and V3 regions may strongly influence HIV-1 gp120 interactions with coreceptors and may serve to limit recognition of these receptor regions from effective immune recognition (22, 31, 37, 38).
The ability of N-linked glycosylation in the SIVmac239 V1 region to inhibit antibody recognition has clearly been demonstrated (42), but the relevance of shielding these regions from immune recognition is not totally understood. SIVmac239 viruses containing deletions of 100 amino acids, including the V1 and V2 loops, remain replication competent (21). The fact that the V1 and V2 regions of SIVmac239 are dispensable for virus replication and infection may suggest that these regions are not important targets for effective immune control in vivo. In contrast, removal of the V1 and V2 loops of HIV-1 renders the virus replication deficient (6). While the two results appear to contradict one another at first glance, the most likely explanation for this difference comes from the inherent differences in the need for CD4 and/or CCR5 binding. HIV-1 requires CD4 binding to induce the conformational changes necessary for efficient coreceptor binding and infection of target cells (25, 46, 47, 50); in contrast, most macrophage-tropic SIV strains are capable of infecting target cells in a CD4-independent manner (15, 39). SIVmac239 gp120 is CD4 dependent, while the g45, g46 and g56 gp120 proteins are capable of inducing cell-cell fusion in a CD4-independent manner (39). Thus, the data reported in the present study strongly suggest that removal of glycosylation in the V1 region of SIVmac239 resulted in envelope proteins that contain a more exposed coreceptor binding site.
The ability of glycosylation in the V1 region of SIVmac239 to shield epitopes in the V3 loop also has important implications for virus neutralization. Previous findings by Reitter et al. demonstrated that viruses containing mutations of glycosylation sites in the V1 and V2 regions elicited better neutralizing antibody responses in vivo than did WT virus (42). This is consistent with findings in the present study suggesting that glycosylation at site 4 in the V1 region hinders antibody recognition of the V3 region, including the coreceptor binding site. Recent studies of HIV-1-infected patients receiving antiretroviral therapy (32, 49) and EIAV-infected horses (17) also demonstrate the emergence of neutralization-resistant viruses early after infection that were characterized by the evolution of sequence changes in the surface envelope glycoprotein to create or relocate potential N-linked glycosylation sites. In addition, a recent report by Quinones-Kochs et al. (40) suggests that the role of N-linked glycans in the HIV-1 and SIV envelope proteins may be different; while HIV-1 V1/V2 glycosylation mutant gp120 proteins were not significantly better at inducing neutralizing antibodies compared to wild-type gp120, the mutant envelope proteins were more sensitive to neutralization by antibodies raised against wild-type HIV-1 gp120 (40). Thus, while it is possible that the increased levels of virus neutralization demonstrated in SIV-infected macaques are the result of exposing V1 epitopes for antibody recognition, the more plausible explanation is that removal of V1 glycosylation removes the shield necessary to prevent effective immune recognition of other critical envelope epitopes, such as the coreceptor binding site.
Taken together, these studies support the hypothesis that the addition of complex carbohydrates to SIV (and HIV-1) gp120 and gp41 envelope proteins are most likely one mechanism by which the virus escapes effective immune recognition. These studies with glycosylation mutant viruses have clearly demonstrated the ability of such a strategy to augment B-cell responses in vivo and have important implications on the rationale for future therapeutic and vaccine studies. Designing immunogens that expose critical envelope epitopes for immune recognition using strategies such as the creation of glycosylation mutant envelope proteins and viruses may serve to prime and enhance effective immune control upon wild-type viral challenge.
Acknowledgments
We thank the NIH AIDS Research and Reference Reagent Program for providing the SIVmac239 gp120 overlapping peptide panel, Jodi Craigo Steckbeck for the two-dimensional schematic of SIVmac239 gp120, and Florin Vaida for help with statistical analyses.
This work was supported in part by NIH grants AI-28243 and AI-47758 and amfAR grant 02803-30-RGV (K.S.C.).
REFERENCES
- 1.Anderson, M. G., D. P. Hauer, S. V. Joag, O. Narayan, C. M. Zink, and J. E. Clements. 1993. Analysis of envelope changes acquired by SIVmac239 during neuroadaptation in rhesus macaques. Virology 195:616-626. [DOI] [PubMed] [Google Scholar]
- 2.Back, N. K. T., L. Smit, J.-J. De Jong, W. Keulen, M. Schutten, J. Goudsmit, and M. Tersmette. 1994. An N-glycan within the human immunodeficiency virus type 1 gp120 V3 loop affects virus neutralization. Virology 199:431-438. [DOI] [PubMed] [Google Scholar]
- 3.Bolmstedt, A., A. Hemming, P. Flodby, P. Berntsson, B. Travis, J. P. C. Lin, J. Ledbutter, T. Tsu, H. Wigzell, S. L. Hu, and S. Olofsson. 1991. Effects of mutations in glycosylation sites and disulphide bonds on processing, CD4-binding and fusion activity of human immunodeficiency virus envelope glycoproteins. J. Gen. Virol. 72:1269-1277. [DOI] [PubMed] [Google Scholar]
- 4.Bolmstedt, A., S. Olofsson, E. Sjogren-Jansson, S. Jeansson, I. Sjoblom, L. Akerblom, J. E. Hansen, and S. L. Hu. 1992. Carbohydrate determinant NeuAc-Galβ(1-4) of N-linked glycans modulates the antigenic activity of human immunodeficiency virus type 1 glycoprotein gp120. J. Gen. Virol. 73:3099-3105. [DOI] [PubMed] [Google Scholar]
- 5.Bolmstedt, A., S. Sjolander, J. E. Hansen, L. Akerblom, A. Hemming, S. L. Hu, B. Morein, and S. Olofsson. 1996. Influence of N-linked glycans in V4-V5 region of human immunodeficiency virus type 1 envelope glycoprotein. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 12:213-220. [DOI] [PubMed] [Google Scholar]
- 6.Cao, J., N. Sullivan, E. Desjardin, C. Parolin, J. Robinson, R. Wyatt, and J. Sodroski. 1997. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1 and V2 variable loops of the gp120 envelope glycoprotein. J. Virol. 71:9808-9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chackerian, B., L. M. Rudensey, and J. Overbaugh. 1997. Specific N-linked and O-linked glycosylation modifications in the envelope V1 domain of simian immunodeficiency virus that evolve in the host alter recognition by neutralizing antibodies. J. Virol. 71:7719-7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi, W. S., C. Xollignon, C. Thriart, D. P. W. Burns, E. J. Stott, K. A. Kent, and R. C. Desrosiers. 1994. Effects of natural sequence variation of recognition by monoclonal antibodies that neutralize simian immunodeficiency virus infectivity. J. Virol. 68:5395-5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clements, J. E., R. C. Montelaro, C. Zink, A. M. Amadee, S. Miller, A. M. Trichel, B. Jagerski, D. Hauer, L. N. Martin, R. P. Bohm, and M. Murphey-Corb. 1995. Cross-protective immune responses induced in rhesus macaques by immunization with an attenuated macrophage-tropic simian immunodeficiency virus. J. Virol. 69:2737-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole, K. S., M. Alvarez, D. H. Elliott, H. Lam, E. Martin, T. Chau, K. Micken, J. L. Rowles, J. E. Clements, M. Murphey-Corb, R. C. Montelaro, and J. E. Robinson. 2001. Characterization of neutralization epitopes of simian immunodeficiency virus recognized by rhesus monoclonal antibodies derived from monkeys infected with an attenuated SIV strain. Virology 290:59-73. [DOI] [PubMed] [Google Scholar]
- 11.Cole, K. S., M. Murphey-Corb, O. Narayan, S. V. Joag, G. M. Shaw, and R. C. Montelaro. 1998. Common themes of antibody maturation to simian immunodeficiency virus, simian-human immunodeficiency virus, and human immunodeficiency virus type 1 infections. J. Virol. 72:7852-7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole, K. S., M. J. Paliotti, M. Murphey-Corb, and R. C. Montelaro. 2000. Maturation of envelope-specific antibody responses to linear determinants in monkeys inoculated with attenuated SIV. J. Med. Primatol. 29:220-239. [DOI] [PubMed] [Google Scholar]
- 13.Cole, K. S., J. L. Rowles, M. Murphey-Corb, J. E. Clements, T. Unangst, J. Robinson, R. C. Desrosiers, M. S. Wyand, and R. C. Montelaro. 1997. Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. J. Virol. 71:5069-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole, K. S., J. L. Rowles, M. Murphey-Corb, J. E. Clements, J. Robinson, and R. C. Montelaro. 1997. A model for the maturation of protective antibody responses to SIV envelope proteins in experimentally immunized monkeys. J. Med. Primatol. 26:51-62. [DOI] [PubMed] [Google Scholar]
- 15.Edinger, A. L., M. Ahuja, T. Sung, K. C. Baxter, B. Haggarty, R. W. Doms, and J. A. Hoxie. 2000. Characterization and epitope mapping of neutralizing monoclonal antibodies produced by immunization with oligomeric simian immunodeficiency virus envelope protein. J. Virol. 74:7922-7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gram, G. J., A. Hemming, A. Bolmstedt, B. Jansson, S. Olofsson, L. Akerblom, J. O. Nielsen, and J. E. Hansen. 1994. Identification of an N-linked glycan in the V1-loop of HIV-1 gp120 influencing neutralization by anti-V3 antibodies and soluble CD4. Arch. Virol. 139:253-261. [DOI] [PubMed] [Google Scholar]
- 17.Howe, L., C. Leroux, C. J. Issel, and R. C. Montelaro. 2002. Equine infectious anemia virus envelope evolution in vivo during persistent infection progressively increases resistance to in vitro serum antibody neutralization as a dominant phenotype. J. Virol. 76:10588-10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoxie, J. A. 1991. Hypothetical assignment of intrachain disulfide bonds for HIV-2 and SIV envelope glycoproteins. AIDS Res. Hum. Retrovir. 7:495-499. [DOI] [PubMed] [Google Scholar]
- 19.Huso, D. L., O. Narayan, and G. W. Hart. 1988. Sialic acids on the surface of caprine arthritis-encephalitis virus define the biological properties of the virus. J. Virol. 62:1974-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, R. P., J. D. Lifson, S. C. Czajak, K. S. Cole, K. H. Manson, R. Glickman, J. Yang, D. C. Montefiori, R. Montelaro, M. S. Wyand, and R. C. Desrosiers. 1999. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J. Virol. 73:4952-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, W. E., J. Morgan, J. Reitter, B. A. Puffer, S. Czajak, R. W. Doms, and R. C. Desrosiers. 2002. A replication-competent, neutralization-sensitive variant of simian immunodeficiency virus lacking 100 amino acids of envelope. J. Virol. 76:2075-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolchinsky, P., E. Kiprilov, P. Bartle, R. Rubinstein, and J. Sodroski. 2001. Loss of a single N-linked glycan allows CD4-independent human immunodeficiency virus type 1 infection by altering the position of the gp120 V1/V2 variable loops. J. Virol. 75:3435-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krachmarov, C. P., S. C. Kayman, W. J. Honnen, O. Trochev, and A. Pinter. 2001. V3-specific polyclonal antibodies affinity purified from sera of infected humans effectively neutralize primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 17:1737-1748. [DOI] [PubMed] [Google Scholar]
- 24.Kraiselburd, E. N., and J. V. Torres. 1995. Properties of virus-like particles produced by SIV-chronically infected human cell clones. Cell. Mol. Biol. 41(Suppl. 1):S41-S52. [PubMed] [Google Scholar]
- 25.Lapham, C. K., J. Ouyang, B. Chandrasekhar, N. Y. Nguyen, D. S. Dimitrov, and H. Golding. 1996. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science 274:602-605. [DOI] [PubMed] [Google Scholar]
- 26.Lee, W.-R., W.-J. Syu, B. Du, M. Matsuda, S. Tan, A. Wolf, M. Essex, and T.-H. Lee. 1992. Nonrandom distribution of gp120 N-linked glycosylation sites important for infectivity of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 89:2213-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonard, C., M. W. Spellman, R. Riddle, R. J. Harris, J. N. Thomas, and T. J. Gregory. 1990. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J. Biol. Chem. 265:10373-10382. [PubMed] [Google Scholar]
- 28.Li, Y., J. J. M. Bergeron, L. Luo, W. J. Ou, D. Y. Thomas, and C. Y. Kang. 1996. Effects of inefficient cleavage of the signal sequence of HIV-1 gp120 on its association with calnexin, folding and intracellular transport. Proc. Natl. Acad. Sci. USA 93:9606-9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, Y., L. Luo, N. Rasool, and C. Y. Kang. 1993. Glycosylation is necessary for the correct folding of human immunodeficiency virus gp120 in CD4 binding. J. Virol. 67:584-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, Y., L. Luo, D. Y. Thomas, and C. Y. Kang. 1994. Control of expression, glycosylation and secretion of HIV-1 gp120 by homologous and heterologous signal sequences. Virology 204:266-278. [DOI] [PubMed] [Google Scholar]
- 31.Losman, B., A. Bolmstedt, K. Schonning, A. Bjorndal, C. Westin, E. M. Fenyo, and S. Olofsson. 2001. Protection of neutralization epitopes in the V3 loop of oligomeric human immunodeficiency virus type 1 glycoproteins 120 by N-linked oligosaccharides in the V1 region. AIDS Res. Hum. Retrovir. 17:1067-1076. [DOI] [PubMed] [Google Scholar]
- 32.Montefiori, D. C., M. Altfield, P. K. Lee, M. Bilska, J. Zhou, M. N. Johnston, F. Gao, B. D. Walker, and E. S. Rosenberg. 2003. Viremia control despite escape from a rapid and potent autologous neutralizing antibody response after therapy cessation in an HIV-1-infected individual. J. Immunol. 170:3906-3914. [DOI] [PubMed] [Google Scholar]
- 33.Montelaro, R. C., K. S. Cole, and S. A. Hammond. 1998. Maturation of immune responses to lentivirus infection: implications for AIDS vaccine development. AIDS Res. Hum. Retrovir. 14:S255-S259. [PubMed] [Google Scholar]
- 34.Morikawa, Y., J. P. Moore, E. Fenouillet, and I. M. Jones. 1992. Complementation of human immunodeficiency virus glycoprotein mutations in trans. J. Gen. Virol. 73:1907-1913. [DOI] [PubMed] [Google Scholar]
- 35.Myers, G., B. Foley, J. W. Mellors, B. Korber, K.-T. Jeang, and S. Wain-Hobson. 1996. A compilation and analysis of nucleic acid and amino acid sequences. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 36.Nara, P. L., L. Smit, N. Dunlop, W. Hatch, M. Merges, D. Waters, J. Kelliher, R. C. Gallo, P. J. Fischinger, and J. Goudsmit. 1990. Emergence of viruses resistant to neutralization by V3-specific antibodies in experimental human immunodeficiency virus type 1 IIIB infection of chimpanzees. J. Virol. 64:3779-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogert, R. A., M. K. Lee, W. Ross, A. Buckler-White, M. A. Martin, and M. W. Cho. 2001. N-linked glycosylation sites adjacent to and within the V1/V2 and V3 loops of dualtropic human immunodeficiency virus type 1 isolate DH12 gp120 affect coreceptor usage and cellular tropism. J. Virol. 75:5998-6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollakis, G., S. Kang, A. Kliphuis, M. I. Chalaby, J. Goudsmit, and W. A. Paxton. 2001. N-linked glycosylation of the HIV-1 type 1 gp120 envelope glycoproteins as a major determinant of CCR5 and CXCR4 coreceptor utilization. J. Biol. Chem. 276:13433-13441. [DOI] [PubMed] [Google Scholar]
- 39.Puffer, B. A., S. Pohlmann, A. L. Edinger, D. Carlin, M. D. Sanchez, J. Reitter, D. D. Watry, H. S. Foz, R. C. Desrosiers, and R. W. Doms. 2002. CD4 independence of simian immunodeficiency virus Envs is associated with macrophage tropism, neutralization sensitivity, and attenuated pathogenicity. J. Virol. 76:2595-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quinones-Koch, M. I., L. Buonocore, and J. Rose. 2002. Role of N-linked glycans in a human immunodeficiency virus envelope glycoprotein: effects on protein function and the neutralizing antibody response. J. Virol. 76:4199-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reitter, J. N., and R. C. Desrosiers. 1998. Identification of replication-competent strains of simian immunodeficiency virus lacking multiple attachment sites for N-linked carbohydrates in variable regions 1 and 2 of the surface envelope glycoprotein. J. Virol. 72:5399-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reitter, J. N., R. E. Means, and R. C. Desrosiers. 1998. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 4:679-684. [DOI] [PubMed] [Google Scholar]
- 43.Robinson, J. E., K. S. Cole, D. H. Elliott, H. Lam, A. M. Amedee, R. Means, R. C. Desrosiers, J. Clements, R. C. Montelaro, and M. Murphey-Corb. 1998. Production and characterization of SIV envelope-specific rhesus monoclonal antibodies from a macaque asymptomatically infected with a live SIV vaccine. AIDS Res. Hum. Retrovir. 14:1253-1262. [DOI] [PubMed] [Google Scholar]
- 44.Sarkar, S., V. Kalia, M. Murphey-Corb, and R. C. Montelaro. 2002. Detailed analysis of CD4+ Th responses to envelope and Gag proteins of simian immunodeficiency virus reveals an exclusion of broadly reactive Th epitopes from the glycosylated regions of envelope. J. Immunol. 168:4001-4011. [DOI] [PubMed] [Google Scholar]
- 45.Schonning, D., B. Jansson, S. Olofsson, and J.-E. S. Hansen. 1996. Rapid selection for an N-linked oligosaccharide by monoclonal antibodies directed against the V3 loop of human immunodeficiency virus type 1. J. Gen. Virol. 77:753-758. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan, N., Y. Sun, Q. Sattentau, M. Thali, D. Wu, G. Denisova, J. Gershoni, J. Robinson, J. Moore, and J. Sodroski. 1998. CD4-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J. Virol. 72:4694-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trkola, A., T. Dragic, J. Arthos, J. M. Binley, W. C. Olson, G. P. Allaway, C. Cheng-Mayer, J. Robinson, P. J. Maddon, and J. P. Moore. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384:184-187. [DOI] [PubMed] [Google Scholar]
- 48.Van Rompay, K. K., C. J. Berardi, S. Dillard-Telm, R. P. Tarara, D. R. Canfield, C. R. Valverde, D. C. Montefiori, K. S. Cole, R. C. Montelaro, C. J. Miller, and M. L. Marthas. 1998. Passive immunization of newborn rhesus macaques prevents oral simian immunodeficiency virus infection. J. Infect. Dis. 177:1247-1259. [DOI] [PubMed] [Google Scholar]
- 49.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzales, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 432:307-312. [DOI] [PubMed] [Google Scholar]
- 50.Wu, L., N. P. Gerard, R. Wyatt, H. Choe, C. Parolin, N. Ruffing, A. Borsetti, A. A. Cardoso, E. Desjardin, W. Newman, C. Gerard, and J. Sodroski. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384:179-183. [DOI] [PubMed] [Google Scholar]
- 51.Wyatt, R., P. D. Kwong, E. Desjardins, R. W. Sweet, J. Robinson, W. E. Hendrickson, and J. G. Sodroski. 1998. The antigenic structure of the HIV-1 gp120 envelope glycoprotein. Nature 280:705-711. [DOI] [PubMed] [Google Scholar]