Abstract
Although quantitative EEG parameters, such as spectral band powers, are sensitive to centrally acting drugs in dose- and time-related manners, changes of the EEG parameters are redundant. It is desirable to reduce multiple EEG parameters to a few components that can be manageable in a real space as well as be considered as parameters representing drug effects. We calculated factor loadings from normalized values of eight relative band powers (powers of 0.5, 1.0~2.0, 2.5~4.0, 4.5~5.5, 6.0~8.0, 8.5~12.0, 12.5~24.5, and 25~49.5 Hz bands expressed as ratios of the power of 0.5-49.5 Hz band) of EEG during pre-drug periods (11:00~12:00) by factor analysis and constructed a two-dimensional canonical space (reference canonical space) by canonical correlation analysis. Eight relative band powers of EEG produced by either physostigmine or yohimbine were reduced to two canonical scores in the reference canonical space. While changes of the band powers produced by physostigmine and yohimbine were too redundant to describe the difference between two drugs, locations of two drugs in the reference canonical space represented the difference between two drug's effects on EEG. Because the distance between two locations in the canonical space (Mahalanobis distance) indicates the magnitude of difference between two different sets of EEG parameters statistically, the canonical scores and the distance may be used to quantitatively and qualitatively describe the dose-dependent and time-dependent effects and also tell similarity and dissimilarity among effects. Then, the combination of power spectral analysis and statistical analysis may help to classify actions of centrally acting drugs.
Keywords: EEG, power spectral analysis, factor analysis, canonical correlation analysis, physostigmine, yohimbine
INTRODUCTION
Quantitative electroencephalography (qEEG) has been applied to the various purposes: to characterize psychotropic drug effects in human and animals (Herrmann, 1982), to develop sensitive biomarkers for diagnosing psychiatric disorders (John et al., 2007; Leiser et al., in press), disease progression (Luckhaus et al., 2008), treatment prognosis (Hansen et al., 2003), and to predict clinical efficacy of putative psychoactive substances (Knott et al., 1996; Parker et al., 2001; Galderisi, 2002). Particularly, it has been tried to develop methods for screening clinical effect of centrally acting drugs.
Drug-induced EEG changes have been used as an indicator of drug action. The qEEG parameters such as power spectrum and band powers are mostly used in evaluation of drug effects. There are several limitations in using the qEEG parameters for evaluation of drug effects (van Riezen and Glatt, 1993). Firstly, drug effects on multiple qEEG parameters can not be simply explained because many drugs produce an increase/decrease power of same bands and because the change of one band power show non-linear relationship with drug doses and also co-vary with those of neighboring bands. Secondly, drug effects are confounded with fluctuation of vigilance level such as sleep-wake cycles which affects EEG activity profoundly. Then, drug effects can not be separated from the changes of background activity.
To overcome the first limitation, multivariate statistical methods have been used to extract a few components which changes are mostly related to drug effects and reflect dose-effect relationship. For example, a set of band powers produced by standard drugs having already known mechanism or belong to certain classes, a reference data set, is fed as input data to the discriminant analysis and a few components which well described the classes of known drugs are extracted (Fairchild et al., 1980; Dimpfel and Decker, 1984). Then, a test drug is classified to a certain class by the rule constructed from the reference data set. Although it has been used widely for the classification of the psychoactive drugs, it requires a reference data set from standard psychoactive drugs having known clinical actions. If the data from additional standard drugs have been added to the reference data set, the classification rule should be modified. Different set of standard psychoactive drugs used in building the reference data set also gives different classification rule even though the same analysis steps and procedures are used. Then, to build a ubiquitous reference data set, a standard set of behavioral states which is independent to the sets of standard drugs, can be used.
To overcome the second limitation, vigilance controlling methods have been devised. For example, a treadmill running was used to maintain a certain level of vigilance during the recording (Krijzer et al., 1993). However, this and other vigilance controlling procedures can not keep the level of vigilance constant anyhow because of natural sleep pressure as well as drug effects such as sedation or stimulation which directly affect vigilance level (Lee, 1999). Here, we considered that the drug effects on the EEG include EEG changes from natural fluctuation and drug effects on EEG and interactions between them.
Therefore, we proposed band powers of EEG during natural sleep-wake cycles can be used as reference data set which is fed to multivariate statistics to produce a few components representing a state and the fluctuation itself of vigilance level is considered as basic components of a state. To maintain the average vigilance of reference data set to a certain level, we chose EEG signals during the same period (11:00~12:00) of the day before drug administration. Then, the reference canonical space was constructed by factor analysis and canonical correlation analysis and the EEG effects of physostigmine, an anticholinesterase to enhance cholinergic neurotransmission, and yohimbine, an alpha2-adrenergic receptor blocker to enhance adrenergic transmission, were compared in the canonical space whether their effects could be well differentiated.
MATERIALS AND METHODS
Animals
Eighteen male Sprague-Dawley rats (Samtaco, Osan, Korea) weighing 357~494 g (420±43 g) were used. Each two animals were housed in a plastic cage (28×42×18 cm) before surgery. Each animal was housed in a cage after surgery. They were maintained in a controlled environment throughout the study: 22~24℃ ambient temperature, 12:12 hour light-dark cycle (light on from 7:00 to 19:00), with a commercial food and tap water available ad libitum except on the day of recording. The rats were sacrificed by an overdose of CO2 inhalation after the completion of the experiment. Experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgery
Surgical procedure was similar to those in our previous study (Jang et al., 2009). In brief, epidural screw electrodes (tip diameter ~1.0 mm) for EEG recording were implanted under ketamine (75 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.) anesthesia. After the rats showed no movement in response to a tail-pinch, the rats were placed in a stereotaxic apparatus (David Kopf Instruments Inc., Tujunga, CA, USA). The midline scalp was locally anesthetized with 2% lidocaine, incised, and the soft tissue over skull surface was removed. Six small holes were drilled bilaterally in the frontal bone (2.5 mm anterior and 1.25 mm lateral to bregma), the parietal bone (6 mm posterior and 5 mm lateral to bregma) and the interparietal bone (3 mm posterior and 2 mm lateral to lambda) without perforating the dura mater using a low-speed drill (diameter 0.8 mm) under surgical microscope. Four stainless steel screws were inserted into the holes in the frontal and parietal bones for EEG recording from the frontal and parietal cortices, respectively. Two additional screws were inserted into the holes in the interparietal bones for reference and ground electrodes. The electrodes with connecting pins were arranged together in 3×2 matrices and fixed over the skull with dental cement. The rats were allowed at least 7 days to recover from the surgery before recording.
EEG recording
At the day of recording, two rats were placed in the recording chamber and connected to the swivel before 10:00. EEG signals were recorded continuously from 10:00 to 17:30. The 4 signals from the frontal and parietal cortices were recorded monopolarly with respect to the reference electrode. The signals were fed to the amplifier (AxoProbe 410 and CyberAmp 380, Axon Instruments Inc., Foster City, CA, USA), where they were amplified 10,000 times and filtered with low-pass 60 Hz and high-pass 1 Hz). They were saved to a PC by a data acquisition board (DigiData 1200A, Axon Instruments Inc., Foster City, CA, USA) at a sampling rate 200 Hz.
Drugs
Physostigmine sulfate 2H2O (Tocris, Ballwin, MO, USA) and yohimbine hydrochloride (Sigma Chemical Co., St. Louis, MO, USA) were purchased as commercial products. Physostigmine sulfate and yohimbine hydrochloride were freshly dissolved in distilled water before the first dosing. The administration volume was 0.5 or 1.0 ml/kg.
Treatment
The rats were randomly assigned to two groups (n=10 for physostigmine treatment, Phy; n=8 for yohimbine treatment, Yoh). Drugs were always administered at 12:00, 14:00 and 16:00±5 min with each of three doses: 0.2, 0.5 and 1.0 mg/kg physostigmine and 1, 2 and 5 mg/kg yohimbine intraperitoneally.
Power spectrum analysis
The raw EEG signals were visually inspected prior to analysis and 10-sec EEG epochs with artifacts were excluded from analysis. The EEG signals of 11:00~12:00 (Pre) before and eight 15-min segments (numbered with S1, S2, S3, S4, S5, S6, S7 and S8) after drug injections at 12:00, 14:00 and 16:00 were analyzed by power spectrum analysis. The EEG signals of±5 min around drug injection (beginning 5-min of the first and ending 5-min of the eighth segments) were excluded from analysis because of behavior disturbed by experimental procedure for injection. Ten power spectra were calculated by using a fast Fourier transform from 10 Hanning-windowed 2-sec FFT epochs (1-sec overlap) of one 10-sec EEG epoch and then averaged to a power spectrum.
All the power spectra from one animal were normalized with average total power (0.5~49.5 Hz) of its Pre values. The spectrum was divided to 8 frequency bands (1, 0.5; 2, 1.0~2.0; 3, 2.5~4.0; 4, 4.5~5.5; 5, 6.0~8.0; 6, 8.5~12.0; 7, 12.5~24.5; and 8, 25~49.5 Hz). The relative band powers were expressed as ratios of the absolute powers of 8 frequency bands to the total power. The absolute band powers were expressed as log10 of powers of 5 frequency bands (DE, 0.5~4.0; TH, 4.5~5.5, 6.0~8.0; AL, 8.5~12.0; BE, 12.5~24.5; GA 25~49.5; and TO, 0.5~49.5 Hz). All the calculations were performed by custom-made programs using MatLab (MathWorks Inc., Natick, MA, USA).
Statistical analysis
Data were expressed as mean for power spectra and Mahalanobis distance, mean ±0.5×standard deviation (SD) for the absolute band powers, mean±standard error of mean (SEM) for the relative band powers and mean±95% confidence limit of mean for canonical components. The power spectra were compared with multiple t-tests every frequency bin between each drug dose and Pre. The absolute band powers were analyzed by a repeated-measure ANOVA and a Tukey test for time. The eight relative band powers of all epochs of Pre were used as input variables for a factor analysis and a canonical correlation analysis. Resultant factor loadings of variables and eigenvectors were used to calculate two-dimensional canonical scores of all the EEG epochs of the recordings. The canonical components were compared by an ANOVA and a Tukey test with times and confidence intervals of the mean were compared among drugs and dosages. The Mahalanobis distance (McLachlan, 1999) which is expressed by square of the distance between two locations in the canonical space, was calculated from two canonical variables. All the calculations were performed by custom-made programs using MatLab (MathWorks Inc., Natick, MA, USA).
RESULTS
Power spectra
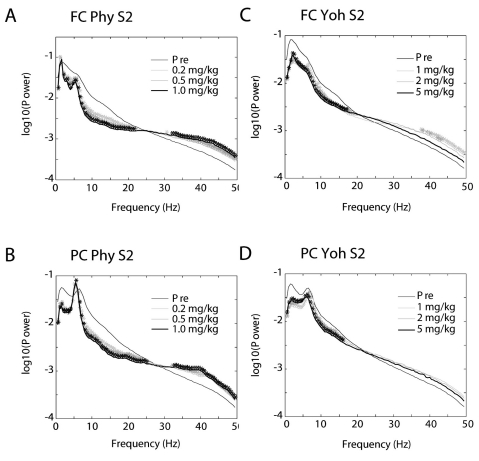
Significant changes in powers at each frequency bin of the mean spectra were produced by 3 doses of physostigmine (Phy, 0.2, 0.5 and 1.0 mg/kg) and of yohimbine (Yoh, 1, 2 and 5 mg/kg) (Fig. 1). No significant difference was observed between doses in both drugs. Maximum effect was observed by the highest dose of physostigmine, while it was observed by the lowest dose of yohimbine. Shown in maximal effect of physostigmine, powers were significantly decreased at the lower frequencies below 22 Hz in the frontal cortex and 25 Hz in the parietal cortex, and they were increased at the higher frequencies above 32 Hz in the frontal and parietal cortices (Fig. 1A, B). In contrast, shown in maximal effect of yohimbine, powers were significantly decreased at the lower frequencies below 20 Hz in the frontal cortex and 17 Hz in the parietal cortex, and they were increased at the higher frequencies above 37 Hz only in the frontal cortex (Fig. 1C, D).
Fig. 1.
Power spectra of EEG from the frontal cortex (A, C) and the parietal (B, D) cortex of the second segment (S2, 15~30 min) after each dose of drugs. Significant changes in powers at each frequency bin of the mean spectra were produced by 3 doses of physostigmine (Phy: 0.2, 0.5 and 1.0 mg/kg) (A, B) and of yohimbine (Yoh: 1, 2 and 5 mg/kg) (C, D). *p<0.05, significantly different from the Pre. FC: frontal cortex, PC: parietal cortex, S2: the 2nd segment (15~30 min after a dose).
Absolute band powers
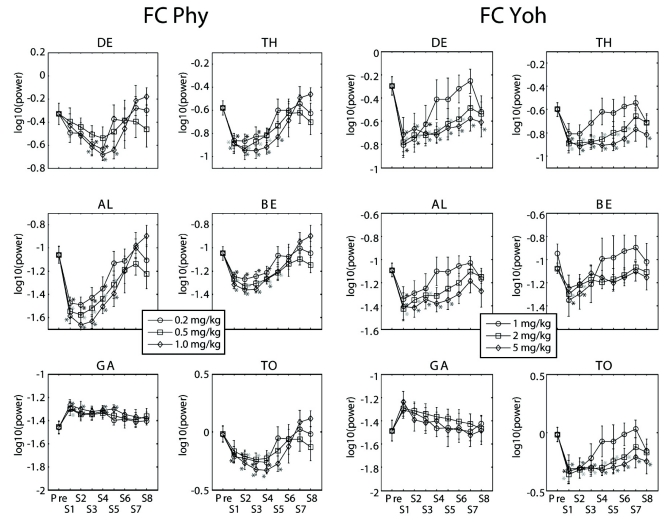
All doses of physostigmine produced decreases of all other band powers but GA band power which was increased, while significant changes were observed differently by some doses of yohimbine according to the bands in both the frontal and parietal cortices (Figs. 2, 3). In the frontal cortex (Fig. 2 Left), significant change was clearly shown from the first segment of 5~15 min after the highest dose injection in most bands (TH, AL, BE and GA), but was delayed in DE band from the third segment of 30~45 min after injection. This lasted in a range of 45~75 min. Most band powers seemed to be changed proportion to the doses. In the frontal cortex (Fig. 2 Right), the highest dose of yohimbine induced decreases of power of some bands (DE, TH, AL and TO) with longer duration while the intermediate dose induced larger magnitude but shorter duration of decrease in DE, TH, AL, BE and TO band powers. Significant changes in DE, TH and TO bands lasted for approximately 120 min from the start after drug injection to the end of recording at the largest dose.
Fig. 2.
Time trends of the absolute band powers of EEG fromt the frontal cortex (FC). All doses of physostigmine (Phy: 0.2, 0.5 and 1 mg/kg, i.p.) produced decreases of all other band powers but GA band power which was increased. In contrast, significant changes were observed differently by some doses of yohimbine (Yoh: 1, 2 and 5 mg/kg, i.p.) according to the bands. *p<0.05, significantly different from the Pre. DE: delta, TH: theta, AL: alpha, BE: beta, GA: gamma, and TO: total power, Pre: a period of 11:00~12:00, S1 to S8: the first to 8th segments (every 15 min period after a dose).
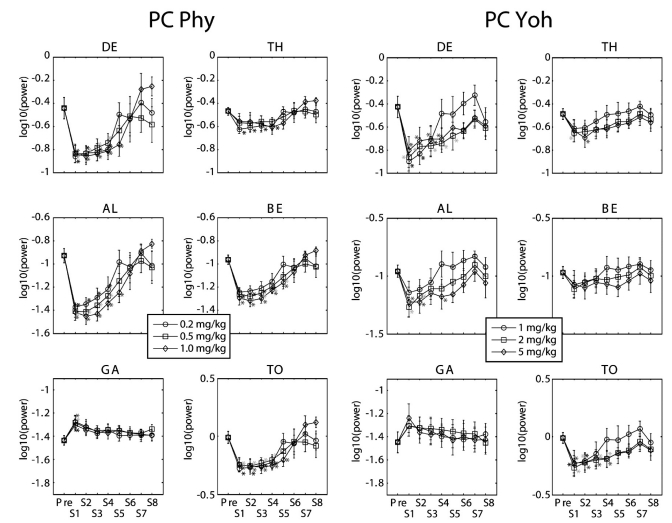
Fig. 3.
Time trends of the absolute band powers of EEG fromt the parietal cortex (PC). Note that all doses of physostigmine (Phy: 0.2, 0.5 and 1 mg/kg, i.p.) produced decreases of all other band powers but GA band power which was increased. In contrast, note that significant changes were observed differently by some doses of yohimbine (Yoh: 1, 2 and 5 mg/kg, i.p.) according to the bands. *p<0.05, significantly different from the Pre. DE: delta, TH: theta, AL: alpha, BE: beta, GA: gamma, and TO: total power, Pre: a period of 11:00~12:00, S1 to S8: the first to 8th segments (every 15 min period after a dose).
In the parietal cortex, similar changes as the frontal cortex were observed (Fig. 3 Left). Different from the frontal cortex, DE band power changed at the start after drug injection and TH band power did not significantly decreased after injection with the highest dose. TH band power was significantly decreased with the lowest dose of physostigmine. Significant changes with the largest dose of yohimbine were observed in DE, TH, AL and TO and lasted 15 to 60 min depending on the bands which is shorter than the duration in the frontal cortex (Fig. 3 Right).
Projections to the reference canonical space of the EEG from the cortex of the same group
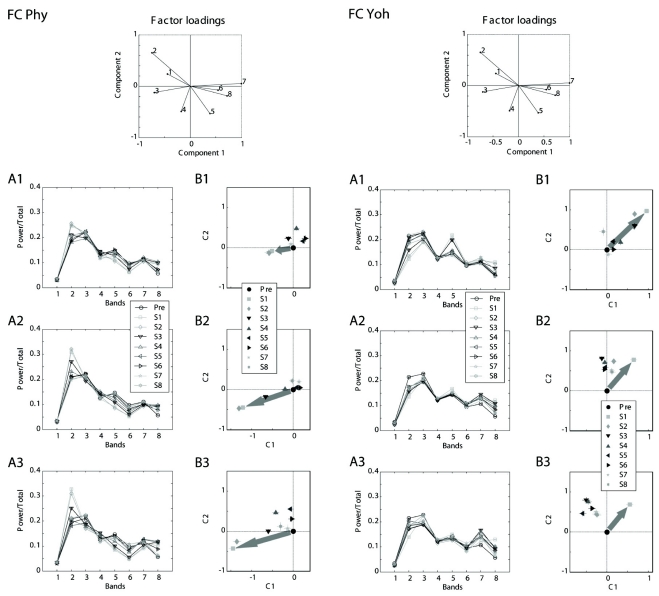
Despite some similarity and other dissimilarity between physostigmine and yohimbine were mentioned, evaluation of the difference of the overall drug effects was impossible due to irregularity and complexity of the changes among multiple band powers. Therefore, the factor analysis based on the effects observed in the 8 relative band powers in each cortex during one-hour pre-drug period (Pre) resulted in factor loadings to calculate common factors which may represent drug effects on the EEG and were used to measure the magnitude of the effects, and then canonical correlation analysis resulted in eigenvector for the two-dimensional reference canonical space.
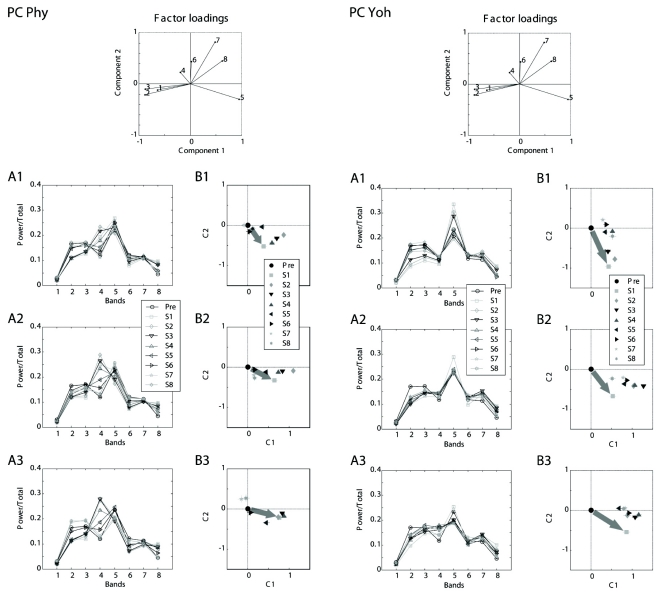
The data sets of relative band powers fed to the multivariate statistics were shown on the left panels of Figs. 4 and 5. Corresponding projection on the two-dimensional canonical space for each cortex were depicted on the right panels of Figs 4 and 5. The mark of the center (0, 0) of each graph is the location of Pre and others represented at 15-min segments after one dose of drug injection.
Fig. 4.
Projections to the reference canonical space of the EEG from the frontal cortex. The data sets of relative band powers fed to the multivariate statistics were shown on A1 to A3. Corresponding projection on the two-dimensional canonical space for the frontal cortex were depicted on B1 to B3. The mark of the center (0, 0) of each graph is the location of Pre and other symbol represents each of eight 15-min segments after one dose of drug injection. A1 and B1. low dose (Phy: 0.2 mg/kg and Yoh: 1 mg/kg); A2 and B2. intermediate dose (Phy: 0.5 mg/kg and Yoh: 2 mg/kg); and A3 and B3. high dose (Phy: 1 mg/kg and Yoh: 5 mg/kg). Large arrows on B1 to B3 display the direction and distance of projection of the first segment from the center. FC: frontal cortex, Phy: physostigmine, 1 to 8: eight bands, Pre: a period of 11:00~12:00, S1 to S8: the first to 8th segments (every 15 min period after a dose), C1 and C2: canonical variables.
Fig. 5.
Projections to the reference canonical space of the EEG from the parietal cortex. The data sets of relative band powers fed to the multivariate statistics were shown on A1 to A3. Corresponding projection on the two-dimensional canonical space for the parietal cortex were depicted on B1 to B3. The mark of the center (0, 0) of each graph is the location of Pre and other symbol represents each of eight 15-min segments after one dose of drug injection. A1 and B1. low dose (Phy: 0.2 mg/kg and Yoh: 1 mg/kg); A2 and B2. intermediate dose (Phy: 0.5 mg/kg and Yoh: 2 mg/kg); and A3 and B3. high dose (Phy: 1 mg/kg and Yoh: 5 mg/kg). Large arrows on B1 to B3 display the direction and distance of projection of the first segment from the center. PC: parietal cortex, Phy: physostigmine, 1 to 8: eight bands, Pre: a period of 11:00~12:00, S1 to S8: the first to 8th segments (every 15 min period after a dose), C1 and C2: canonical variables.
In the frontal cortex, the projections of the canonical variables after all doses of physostigmine injection started at the location down and left from the center of the space and then returned to the center with times (Fig. 4 Left B1 to B3). In contrast, the projections after the higher doses of yohimbine (2 and 5 mg/kg) started at the location up and right from the center and did not returned to the center but ended at the location up from the center or up and slightly left from the center (Fig. 4 Right B2 and B3). The projections after the lowest dose (1 mg/kg) returned to the center (Fig. 4B1).
In the parietal cortex, the projections started at the location right and slightly down from the center and then returned to the center with times (Fig. 5 Left B1 to B3). The projections after all doses of yohimbine started at the location down or down and left from the center and did not returned to the center with times but ended at the location down and left from the center (Fig. 5 Right B1 to B3).
Mahalanobis distances
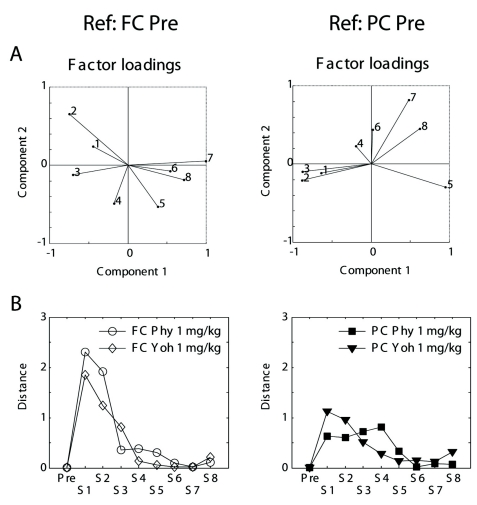
The Mahalanobis distance is expressed by square of the distance between two locations and indicates dissimilarity of the states, that is, projection to the reference canonical space of the EEG parameters after drug injection. In the reference canonical space constructed by the EEG parameters in the frontal cortex before drug administration, the projections after 1 mg/kg physostigmine or 1 mg/kg yohimbine were presented. Maximal distances of projections of frontal EEG were at S1 in both physostigmine and yohimbine (Fig 6A Left). However, maximal distances of projections of parietal EEG were at S1 in yohimbine but at S4 in physostigmine (Fig. 6B Left).
Fig. 6.
(A) Factor loadings for the reference canonical space for the frontal cortex (left) and for the parietal cortex (right). (B) Time trend of Mahalanobis distance in the reference canonical space for the frontal cortex (left) and for the parietal cortex (right) before and after drug administration. Maximal distances of projections of frontal EEG were at S1 in both physostigmine and yohimbine. However, maximal distances of projections of parietal EEG were at S1 in yohimbine but at S4 in physostigmine. Ref: reference, FC: frontal cortex, PC: parietal cortex, 1 to 8:eight bands, Pre: a period of 11:00~12:00, S1 to S8: the first to 8th segments (every 15 min period after a dose), C1 and C2: canonical variables.
DISCUSSION
The present study demonstrates that the relative band powers of EEG during natural sleep-wake cycles were used as a reference data set representing standard states including all natural levels of vigilance and the resulted reference canonical space was well used for differentiating psychotropic agents and for time trend of their actions. Thus, the EEG effects of physostigmine, an anticholinesterase to enhance cholinergic neurotransmission, and those of yohimbine, an alpha2-adrenergic receptor blocker to enhance adrenergic neurotransmission, were differentiated quantitatively and qualitatively by the location in the canonical space, that is, distance and direction from its center.
In general, it is believed that the action of psychotropic drugs on the specific neurotransmitter system(s) or receptors induces specific changes in power spectral profile. In this study, physostigmine induced a spectral profile different from one of pre-drug state and also from yohimbine in some frequency bins but not in others as power at each frequency bin of power spectrum was compared. Some characteristics such as significant power difference over a wider range observed after physostigmine than yohimbine, prominent theta peak disclosed by decreased powers around the peak, no significant power differences over the higher frequency ranges in yohimbine even significant power increases observed in lower dose of yohimbine in the frontal cortex, and etc., were apparent in visual inspection. But the description of drug induced change or recording regions was only possible verbosely in every frequency bin.
After data reduction from the power spectrum to the absolute powers over some specific frequency bands which are divided by empirically or statistically (Herrmann, 1982), the simpler description of drug effects was possible. For example, the peak time of the band powers are different from different drugs on different cortex. This may indicate temporal pattern of pharmacokinetic characteristic of the drugs but still does not say direct relationship between single band powers and drug concentration on the action site. Some researchers use specific EEG parameters, such as beta spindle activity or beta power with benzodiazepines action as indicator of drug concentration in action site (Mandema et al., 1991). Other researchers use a delta band and theta band during specific sleep state as indicator of different action mechanism on the receptors (Xi and Chase, 2008). However, there are subjective and empirical selection of the EEG parameters correlated with pharmacokinetic/pharmacodynamic actions (Bol et al., 2000).
Therefore, we used multivariate statistics whether we can describe the characteristics of drug action potency and temporal trend with simplicity and quantitatively. We used the relative band powers for the input to multivariate statistics because they are less dependent to the recording setup such as amplifier gain and electrode impedance than the absolute band powers. Even though information of magnitude of power may be removed, the power distribution profile of EEG induced by drugs was conserved. Then, we used factor analysis and canonical correlation analysis in order to extract from 8 relative band powers to two meaningful parameters which can be easily interpreted by human brain. We showed that the action at a time can be projected on the canonical space constructed from a reference data set. As the reference data set, the relative band powers of epochs of EEG from the cortex during pre-drug period of same time period of the day (11:00~12:00) were used. It includes all natural levels of vigilance, but the state as a whole was not different among animals (data not shown). That is, the same period of the day included all the natural sleep-wake states with consistent proportions of sleep-wake states among animal. Then, it could be standard classes for the reference data set. Between different cortices, there was different factor loading for the canonical variables, which may reflect the different brain state between the brain regions.
In this study, we did not used standard psychotropic drugs belong to several known classes as reference data sets that have been used in classification of psychotropic drugs by discriminant analysis with predefined classes (Itil et al., 1979; Ruigt et al., 1993; Dimpfel, 2003). Fairchild et al. (1975) calculated sleep-dependent canonical components and residuals canonical correlation analysis from EEG of representative segments of visually classified sleep stages, which was used as dependant variable, and then calculated two sleep-independent canonical variables from the residuals. Instead, we used factor analysis to calculate factor loadings from the data sets of the relative band powers of all the epochs during normal sleep-wake cycles, in which all the epochs were considered as all different classes or states, and then canonical correlation analysis to calculate canonical variables from drug induced EEG profile. This procedure is not affected by existence of very few standard drugs in predefined classes. Some studies have used different multivariate statistical analysis such as cluster analysis on the data without predefining the class to classify psychiatric disorders (John et al., 1983; John et al., 1992). The multivariate statistical analysis not requiring predefined class can be more realistic procedure to investigate the correspondence between the definable classes and psychotropic drugs and to find new classes.
Since different drug actions on specific receptors and neurotransmissions result in different EEG changes and then they result in different projection locations, it is possible to suppose that the location in the canonical space represent a brain functional state. And a distance in the canonical space, Mahalanobis distance, indicates a magnitude of the difference between two states (McLachlan, 1999). After physostigmine administration, the farthest projections from the center in the reference canonical space for the frontal cortex were dose-dependant and the projection of the segments became closer to the center with time after dose. Similarly, the farthest projections in the space for the parietal cortex were also dose dependant and the projection returned to the center with time. The direction of the farthest projections was left and down from the center in the space for the frontal cortex while were right from the center in the space for the parietal cortex. This does not explain what are the different action on the different cortex but may still indicate there is difference in action.
In contrast, the pattern of projections in the space after yohimbine was different from that of physostigmine. The farthest projections in the space for the frontal cortex were up and right from the center similar to the physostigmine, but the farthest among them was observed at lowest dose of yohimbine not in higher doses. The projections did not return to the center but moved to the up and right from the center. In the space for the parietal cortex, the farthest projection was observed at lowest dose of yohimbine and the projections did not return to the center but moved to left from the center. Three doses were administered cumulatively and then pre-exposure to drug may affect the sensitivity of the neurotransmission. These results may indicate that pre-exposure to physostigmine does not modify the sensitivity of the cholinergic neurotransmission but pre-exposure to yohimbine does modify the sensitivity of the adrenergic neurotransmission.
If we accept that a location in the canonical space represents a brain functional state and that a distance between two locations indicates magnitude of a difference between two states, we could differentially describe the effects of physostigmine and yohimbine. In the reference canonical space of the frontal cortex, physostigmine changed the normal functional state to a functional state of low and left location while yohimbine changed to a state of up and right location. In the reference canonical space of the parietal cortex, both physostigmine and yohimbine changed from the normal functional state to a state of low and right location. It may be interpreted that two drugs produced different functional states on the frontal cortex but similar functional state on the parietal cortex. However, these results can not show what mechanism of action affect differentially on the different cortex.
In this study, it may be possible that the magnitude of changes produced by different drugs can be compared with Mahalanobis distances calculated in the reference canonical space. The effect of 1 mg/kg of physostigmine is similar to that of 1 mg/kg yohimbine on the frontal cortex and the parietal cortex. Both drugs produce maximal effect at the first segment after drugs on the frontal cortex. In contrast, physostigmine produces maximal effect at the first segment after drug but yohimbine produces maximal effect at the 4-th segment after drug. Both drugs have bigger effects on the frontal cortex than the parietal cortex. Temporal changes can also be compared.
Taken together, these results suggest that the EEG effects of physostigmine and those of yohimbine can be compared quantitatively and qualitatively by the distance and direction from its center in the reference canonical space.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the NRF funded by the Ministry of Education, Science and Technology (2010-0022362).
References
- 1.Bol CJ, Vogelaar JP, Tang JP, Mandema JW. Quantification of pharmacodynamic interactions between dexmedetomidine and midazolam in the rat. J Pharmacol Exp Ther. 2000;294:347–355. [PubMed] [Google Scholar]
- 2.Dimpfel W. Preclinical data base of pharmaco-specific rat EEG fingerprints (tele-stereo-EEG) Eur J Med Res. 2003;8:199–207. [PubMed] [Google Scholar]
- 3.Dimpfel W, Decker H. Classification of drugs by stereotactic recording of focal brain activity in the rat (stereo-EEG) Neuropsychobiology. 1984;12:188–195. doi: 10.1159/000118135. [DOI] [PubMed] [Google Scholar]
- 4.Fairchild MD, Jenden DJ, Mickey MR. An application of long-term frequency analysis in measuring drug-specific alterations in the EEG of the cat. Electroencephalogr Clin Neurophysiol. 1975;38:337–348. doi: 10.1016/0013-4694(75)90258-8. [DOI] [PubMed] [Google Scholar]
- 5.Fairchild MD, Jenden DJ, Mickey MR, Yale C. The quantitative measurement of changes in EEG frequency spectra produced in the cat by sedative-hypnotics and neuroleptics. Electroencephalogr Clin Neurophysiol. 1980;49:382–390. doi: 10.1016/0013-4694(80)90234-5. [DOI] [PubMed] [Google Scholar]
- 6.Galderisi S. Clinical applications of pharmaco-EEG in psychiatry: the prediction of response to treatment with antipsychotics. Methods Find Exp Clin Pharmacol. 2002;24(Suppl C):85–89. [PubMed] [Google Scholar]
- 7.Hansen ES, Prichep LS, Bolwig TG, John ER. Quantitative electroencephalography in OCD patients treated with paroxetine. Clin Electroencephalogr. 2003;34:70–74. doi: 10.1177/155005940303400205. [DOI] [PubMed] [Google Scholar]
- 8.Herrmann WM. Electroencephalography in drug research. Gustav Fischer Verlag; 1982. [Google Scholar]
- 9.Itil TM, Shapiro DM, Herrmann WM, Schulz W, Morgan V. HZI systems for EEG parametrization and classification of psychotropic drugs. Pharmakopsychiatr Neuropsychopharmakol. 1979;12:4–19. doi: 10.1055/s-0028-1094590. [DOI] [PubMed] [Google Scholar]
- 10.Jang HS, Kim JY, Kim SH, Lee MG. Role of dopamine receptors on electroencephalographic changes produced by repetitive apomorphine treatments in rats. Korean J Physiol Pharmacol. 2009;13:147–151. doi: 10.4196/kjpp.2009.13.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.John ER, Prichep L, Ahn H, Easton P, Fridman J, Kaye H. Neurometric evaluation of cognitive dysfunctions and neurological disorders in children. Prog Neurobiol. 1983;21:239–290. doi: 10.1016/0301-0082(83)90014-x. [DOI] [PubMed] [Google Scholar]
- 12.John ER, Prichep LS, Almas M. Subtyping of psychiatric patients by cluster analysis of QEEG. Brain Topogr. 1992;4:321–326. doi: 10.1007/BF01135569. [DOI] [PubMed] [Google Scholar]
- 13.John ER, Prichep LS, Winterer G, Herrmann WM, diMichele F, Halper J, Bolwig TG, Cancro R. Electrophysiological subtypes of psychotic states. Acta Psychiatr Scand. 2007;116:17–35. doi: 10.1111/j.1600-0447.2006.00983.x. [DOI] [PubMed] [Google Scholar]
- 14.Knott VJ, Telner JI, Lapierre YD, Browne M, Horn ER. Quantitative EEG in the prediction of antidepressant response to imipramine. J Affect Disord. 1996;39:175–184. doi: 10.1016/0165-0327(96)00003-1. [DOI] [PubMed] [Google Scholar]
- 15.Krijzer F, Koopman P, Olivier B. Classification of psychotropic drugs based on pharmaco-electrocorticographic studies in vigilance-controlled rats. Neuropsychobiology. 1993;28:122–137. doi: 10.1159/000119015. [DOI] [PubMed] [Google Scholar]
- 16.Lee MG. Pharmacodynamic interactions of diazepam and flumazenil on cortical EEG in rats. J Appl Pharmacol. 1999;7:242–248. [Google Scholar]
- 17.Leiser SC, Dunlop J, Bowlby MR, Devilbiss DM. Aligning strategies for using EEG as a surrogate biomarker: a review of preclinical and clinical research. Biochem Pharmacol. 2010 doi: 10.1016/j.bcp.2010.10.002. (in press) [DOI] [PubMed] [Google Scholar]
- 18.Luckhaus C, Grass-Kapanke B, Blaeser I, Ihl R, Supprian T, Winterer G, Zielasek J, Brinkmeyer J. Quantitative EEG in progressing vs stable mild cognitive impairment (MCI): results of a 1-year follow-up study. Int J Geriatr Psychiatry. 2008;23:1148–1155. doi: 10.1002/gps.2042. [DOI] [PubMed] [Google Scholar]
- 19.Mandema JW, Sansom LN, Dios-Vieitez MC, Hollander-Jansen M, Danhof M. Pharmacokinetic-pharmacodynamic modeling of the electroencephalographic effects of benzodiazepines. Correlation with receptor binding and anticonvulsant activity. J Pharmacol Exp Ther. 1991;257:472–478. [PubMed] [Google Scholar]
- 20.McLachlan GJ. Mahalanobis distance. Resonance. 1999;4:20–26. [Google Scholar]
- 21.Parker TJ, Della Pasqua OE, Loizillon E, Chezaubernard C, Jochemsen R, Danhof M. Pharmacokinetic-pharmacodynamic modelling in the early development phase of anti-psychotics: a comparison of the effects of clozapine, S 16924 and S 18327 in the EEG model in rats. Br J Pharmacol. 2001;132:151–158. doi: 10.1038/sj.bjp.0703791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruigt GS, Engelen S, Gerrits A, Verbon F. Computer-based prediction of psychotropic drug classes based on a discriminant analysis of drug effects on rat sleep. Neuropsychobiology. 1993;28:138–153. doi: 10.1159/000119016. [DOI] [PubMed] [Google Scholar]
- 23.van Riezen H, Glatt AF. Introduction and history of the use of electroencephalography in animal drug studies. Neuropsychobiology. 1993;28:118–121. doi: 10.1159/000119014. [DOI] [PubMed] [Google Scholar]
- 24.Xi M, Chase MH. Effects of eszopiclone and zolpidem on sleep and waking states in the adult guinea pig. Sleep. 2008;31:1043–1051. [PMC free article] [PubMed] [Google Scholar]