Abstract
Monoamine oxidase inhibitors (MAO-I) belong to the earliest drugs tried in Parkinson's disease (PD). They have been used with or without levodopa (L-DOPA). Non-selective MAO-I due to their side-effect/adverse reaction profile, like tranylcypromine have limited use in the treatment of depression in PD, while selective, reversible MAO-A inhibitors are recommended due to their easier clinical handling. For the treatment of akinesia and motor fluctuations selective irreversible MAO-B inhibitors selegiline and rasagiline are recommended. They are safe and well tolerated at the recommended daily doses. Their main differences are related to (1) metabolism, (2) interaction with CYP-enzymes and (3) quantitative properties at the molecular biological/genetic level. Rasagiline is more potent in clinical practise and has a hypothesis driven more favourable side effect/adverse reaction profile due to its metabolism to aminoindan. Both selegiline and rasagiline have a neuroprotective and neurorestaurative potential. A head-to head clinical trial would be of utmost interest from both the clinical outcome and a hypothesis-driven point of view. Selegiline is available as tablet and melting tablet for PD and as transdermal selegiline for depression, while rasagiline is marketed as tablet for PD. In general, the clinical use of MAO-I nowadays is underestimated. There should be more efforts to evaluate their clinical potency as antidepressants and antidementive drugs in addition to the final proof of their disease-modifying potential. In line with this are recent innovative developments of MAO-I plus inhibition of acetylcholine esterase for Alzheimer's disease as well as combined MAO-I and iron chelation for PD.
Keywords: selegiline, rasagiline, moclobemide, phenelzine, tranylcypromine
INTRODUCTION
Monoamine oxidase (MAO) is an important enzyme to metabolize in vivo endogenous and diet-derived biogenic amines via oxidative deamination. Major substrates are noradrenaline, adrenaline, dopamine, β-phenylethylamine (PEA) and serotonin. These substrates are underlying in the biochemical pathology of "depression" and Parkinson's disease (PD). The deficiency of serotonin, noradrenaline and dopamine builds-up the hypothesis of "depression" while a loss of dopamine, noradrenaline and serotonin is the biochemical basis of degenerative processes underlying PD. Therefore, supplementation of deficient biogenic amine neurotransmitters with 3,4-dihydroxy-phenylalanin (L-DOPA) has been established as early as the late 50th and early 60th of the last century including the use of MAO-inhibitors. This class of psychopharmacological active compounds inhibits the break-down of biogenic amine neurotransmitters and thus increase their concentration in the synaptic cleft and at respective postsynaptic receptor sites. A mood elevating effect in patients with tuberculosis after treatment with iproniazid was first described by Kline (1958). In PD the first reports were published by Sano (1960; Foley et al., 2000) using iproniazid and pheniprazine alone or in combination with D,L-DOPA in a small number of patients, Degkwitz et al. (1960), who used iproniazid in combination with L-DOPA and in reserpin treated patients with schizophrenia and Birkmayer, Hornykiewicz and Bernheimer, who tried a variety of compounds like harmine, isocarboxazid and other MAO-I's (Bernheimer et al., 1961; Birkmayer and Hornykiewicz 1961; 1962; 1964) alone or in combination with L-DOPA in PD. The effects were mild or not existing when these MAO-I were given alone. However all theses early reports agree that MAO-I potentiated the effect of (D), L-DOPA but intensified also adverse reactions. Further examination and post mortem studies gave evidence that MAO-I given shortly before patients deaths were restoring the levels of noradrenaline and serotonin with no significant effect on the concentration of brain dopamine (Bernheimer et al., 1962; 1963). This data pointed to a combination therapy of L-DOPA and MAO-I already in the early 60th of the last century. An extensive description of detailed historical aspects of MAO and its inhibitors is given in the excellent overview on treatment strategies in PD by Foley (2001). A further break-through was the discovery of multiple forms of MAO, MAO-A and MAO-B, by Johnston (1968). MAO-A deaminates especially serotonin, noradrenaline and tyramine and is inhibited selectively at low concentrations (µM) of clorgyline while MAO-B is insensitive to clorgyline and in the human brain desaminates PEA and to a high degree dopamine (Glover et al., 1977). The first selective MAO-B-I was L-deprenyl (E-250, L-deprenyl, selegiline), synthezised by Zoltan Ecseri in 1962, patented as antidepressant in 1965, 1966 and developed by Jozsef Knoll as "psychic energizer (Knoll et al., 1965)." The combination of selegilines selective MAO-B-I properties and the short-lasting stimulant effect of one of its metabolites, metamphetamine, (later proved to be also a reversible MAO-inhibitor see also Foley 2001 for details of such early developments) was indeed a concept to put forward new antidepressant agents (Varga and Tringer, 1967). Knoll mentioned in his 1965 publication that selegiline does not increase motility and lowers blood pressure in experimental animals. Using tyramine (a MAO-A and -B substrate) it became evident that selegiline antagonizes the socalled "cheese-effect" (increase of blood pressure noteable especially after consumption of larger amounts of cheese in patients treated with unselective or MAO-A-inhibitors) (Knoll and Magyar, 1972). As dopamine in rodent brain is s preferred MAO-A substrate an effect of MAO-inhibitors on motility (see above) has not been observed. PD, therefore, was not the focus for using MAO-B-I as therapeutic strategy. The suggestion in late 1974 for selegilines use in PD and especially in treating ON-OFF symptoms by one of use (PR) was based on (1) the early and in principle beneficial effects of MAO-I when combined with L-DOPA (see above), (2) the effects of selegiline in antagonizing the cheese-effect and (3) the possibility that dopamine in humans might well be a substrate for MAO-B. Convincing Moussa Youdim about using selegiline for a first trial in PD patients in PD he agreed to provide Walther Birkmayer and myself (P.R.) with some grams of selegiline that he had obtained from Josef Knoll. The trial was showing a beneficial effect in the symptomology of PD (Birkmayer et al., 1975; 1977; Foley, 2001; for historical notes see Riederer 2004; Youdim, 2006). Later (Birkmayer et al., 1983; 1985) we provided evidence for selegiline having neuroprotective action, a concept that is still followed and seems to be proven by the follow-up propargylaminoderivative rasagiline as shown recently in the ADAGIO-study (Olanow et al., 2008; 2009).
The innovating basic research and beneficial actions of selegiline in PD were responsible for intensive MAO-I drug developments for both PD and "depression". In this review we present the major compounds currently being used as well as an outlook of further such developments.
MAO-I in clinical practise
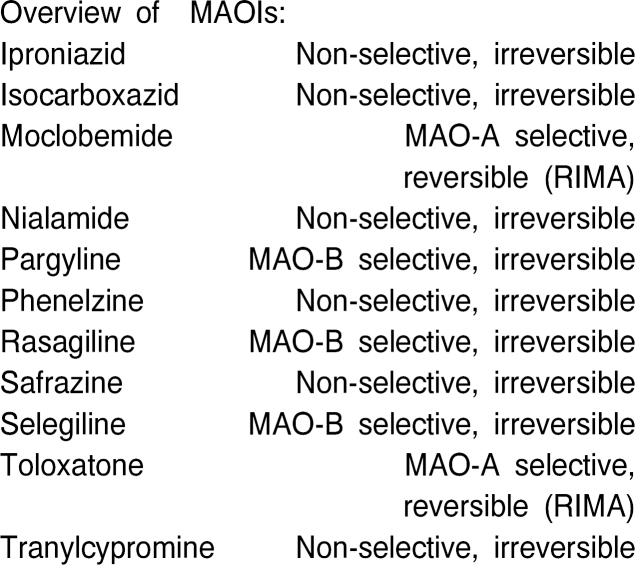
Monoamine oxidase (MAO) inhibitors (MAOIs) at present can be classified into 3 types:
Older, irreversible nonselective agents such as phenelzine and tranylcypromine
Irreversible, selective drugs (MAO-B-I's) such as selegiline and rasagiline
Reversible, selective MAO-A inhibitors (RIMAs = reversible inhibitors of MAO-A) such as moclobemide (Overviews: Szelenyi 1993; Laux et al., 1995)
The clinical indications and efficacy of the MAOIs are established for disorders as follows:
Parkinson's disease (PD)
Depressive disorders
Anxiety disorders (social phobia, panic disorder, PTSD)
Other potential therapeutic uses and indications can be smoking cessation, attention deficit hyperactivity disorder and cognitive deficits in dementia for moclobemide (Chan-Palay, 1992; Berlin et al., 1995).
INDICATIONS IN PARKINSON'S DISEASE
Treatment of motor symptoms
Both, selegiline and rasagiline (Table 1) are beneficial in treating motor symptoms in PD as monotherapy and in combination with L-DOPA and a decarboxylase inhibitor. Rasagiline is more effective in this regard as shown also in the daily dosis necessary for a symptomatic effect: 5~10 mg/day selegiline, 1 mg/day rasagiline. Long-term trials with selegiline point to the fact that 30~40% of the daily L-DOPA dosis can be spared when combined with the MAO-B-I (Szeleny, 1993; Lees et al., 1995; Myllylä et al., 1997). It is not far-fetched to assume, that PEA, which increases after selegiline treatment in brain tissue (Reynolds et al., 1978) and by this exerts dopamine release-promoting properties, contributes to dopamine's behavioural effects including improvement of motility (Foley, 2001; Gerlach et al., 2007; Riederer, 2009).
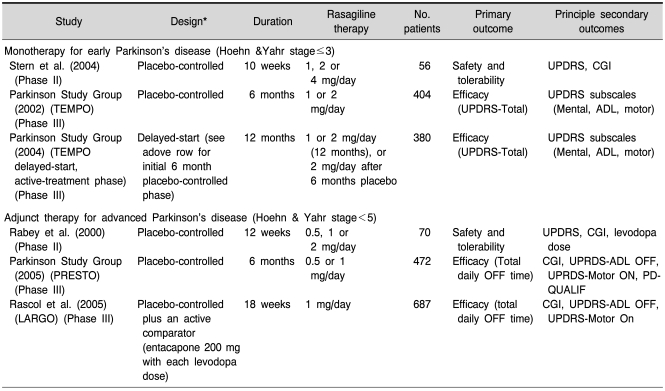
Table 1.
Monoamine oxidase inhibitors
As reviewed by Magyar et al., 2010; Weinreb et al., 2010; Naoi and Maruyama, 2010.
There is overwhelming evidence that selegiline has beneficial effects on motoricity and motorfluctuations as shown already in the first clinical trial (Birkmayer et al., 1975) and in follow-up clinical studies as summarized by Gerlach et al. (2007).
Rasagiline effects on motoricity are even stronger as shown by the TEMPO- (Parkinson Study Group, 2002; 2004), PRESTO- (Parkinson-Study-Group, 2005), LARGO- (Rascol et al., 2005) and ADAGIO- (Olanow et al., 2008; 2009) studies.
In all these and additional clinical studies (eg Rabey et al., 2000; Thebault et al., 2004; Rascol, 2005; Biglan et al., 2006; Siderowf and Stern, 2006) it could be shown that rasagiline is well tolerated, safe, improves motor symptoms, prevents motor complications in PD, has beneficial effects on quality of life parameters, is effective as monotherapy or in adjunctions to L-DOPA-therapy, is beneficial in early and late stages of PD, is safe when combined with all other PD-relevant therapies including COMT-inhibitors and may have disease-modifying properties.
Neuroprotection and disease-modification
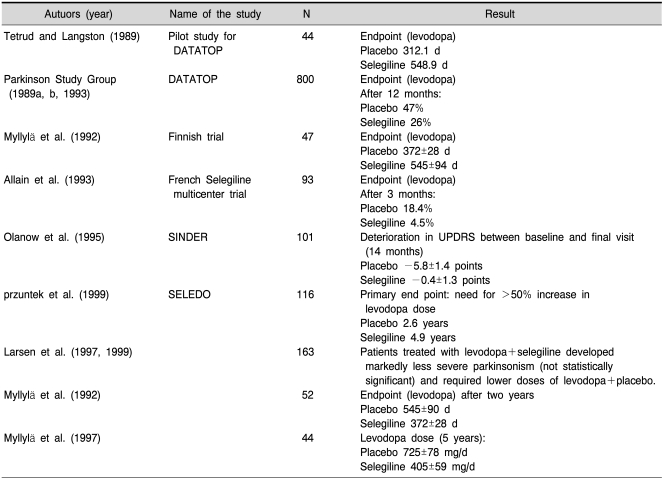
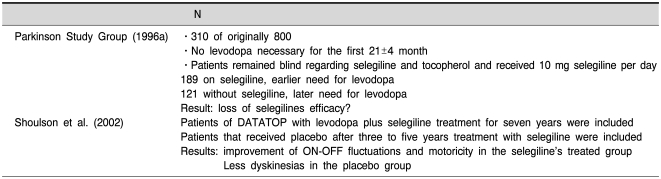
Selegiline and rasagiline are "suizide-inhibitors" which inhibit the enzyme irreversibly and for a rather long time. Therefore only newly synthesized enzyme will recover MAO activity sufficient to metabolize its substrates in an adequate amount. Positron-emission tomography (PET) studies have shown a biological half-life time (HLT) of MAO-B recovery after selegiline- (Fowler et al., 1994) or rasagiline- (Freedman et al., 2005) induced blockade of about 30~40 days. As described earlier (Riederer and Lachenmayer, 2003; Gerlach et al., 2007) PET-studies are only in part suitable to detect the HLT of MAO-inhibitors as they only detect the distribution of radiolabeled inhibitor and its metabolites. In addition the exact kinetic properties underlying these PET-studies have been described only at random (Arnett et al., 1987). In contrast, measurement of PEA, the pure MAO-B substrate, in the urine of healthy individuals after selegiline dosing showed recovery (decline) of this amine concentration to normal values already 2~3 days after withdrawal of selegiline (Clarke et al., 2001). Such data agree with other pharmacological studies as described by Riederer and Lachenmayer (2003) and point to the conclusion that the HLT of MAO-B recovery after irreversible inhibition by selegiline is about 7 days. According to Green et al. (1977) an 80% inhibition of MAO-isoenzymes is necessary to increase concentration of biogenic amines significantly. This means that newly synthesized enzyme in the range of 20 to 30% is sufficient to lose any symptomatic behavioural effect after withdrawal of any MAO-inhibitor. In line with this are studies by Youdim and Tipton (2002) who could not detect selegiline-induced stereotyped behaviour due to PEA increase 4 days after withdrawal of selegiline. At this time MAO-B recovery was already 20%. Taking these findings into account a wash-out phase of two weeks or after selegiline/rasagiline withdrawal is sufficient to avoid any symptomatic effect of these MAO-I's. Therefore, the interpretations of the DATATOP-study and other follow-up trials to demonstrate a "neuroprotective" effect have to be reconsidered. If so, a neuroprotective effect of selegiline cannot be excluded (Riederer and Lachenmayer, 2003; Gerlach et al., 2007). Table 2 (taken in part from Riederer and Lachenmayer 2003 with permission) illustrates the major outcomes of the most important clinical long-term trials with selegiline. While treatment of patients in an early phase of PD with selegiline shows sufficient results regarding motoricity and disease-modification the unconclusive results of the DATATOP-follow-up clinical trials may demonstrate a bias in severity of patients grouping as given in the first follow-up study (Parkinson Study Group, 1996a) or that the patients have non-response based on disease-progression and/or accompanying diseases (Table 3). As there were no final conclusions about the neuroprotective effect of selegiline the development of more efficient clinical trial designs have been investigated including PET-controlled studies as shown with dopaminergic agonists. More recently the "delayed-start study design" has been developed as discussed by D'Agostino (2009). Rasagiline has been the first drug tested in such clinical trial. In fact the ADAGIO-study has given profound evidence for a disease-modifying effect of 1 mg/day rasagiline, while there was not such benefit at a 2 mg/day dosis (Table 4). Although - and in comparison to the negative outcome of a delayed-start-study with pramipexol (Schapira et al., 2009) - there is ample evidence for a significant disease-modification with low dosis rasagiline this clinical outcome has been questioned recently (Ahlskog and Uitti, 2010; Sampaio and Ferreira, 2010; Schwarzschild, 2010). Caslake et al. (2009) conclude in their Cochrane Database report: "MAO-B inhibitors are one option for the early treatment of PD although they have weaker symptomatic effects than levodopa and dopamine agonists. They may reduce the rate of motor fluctuations compared with initial levodopa therapy and may have fewer significant adverse effects than the older agonists but data are too few to provide reliable conclusions".
Table 2.
Nueroprotection in Parkinson's disease by selegline (prospective, randomized, double-blind, placebo-controlled studies)
From Riederer and Lachenmayer 2003.
Table 3.
Datatop follow-up-clinical studies
Table 4.
Summary of rasagiline clinical studies
*All studies were randomised, multi-centre, parallel-group and double-blind.
ADL: activities of daily living, CGI: clinical global impressions scale, LARGO: lasting effect in adjunct therapy with rasagiline given once daily, OFF: poor symptom response, ON: good symptom reponse, PD-QUALIF: parkinson's disease quality of life, PRESTO: Parkinson's rasagiline efficacy and safety in the treatment of OFF, TEMPO: trial of etanercept and methotrexate with radiographic patient outcomes, UPDRS-ADL: unified parkinson's disease rating scale-activities of daily living. From Rascol 2005.
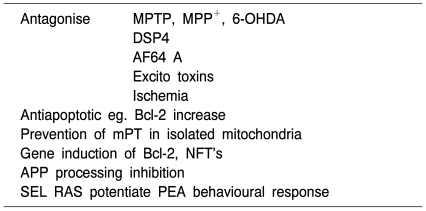
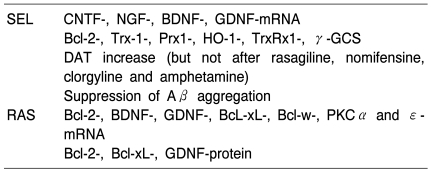
Neuroprotective, neurorestaurative and disease-modifying effects of selegiline and rasagiline are substantiated by in vitro-as well as in vivo experimental studies (Table 5). There is ample evidence that compounds attached to a propargylamino group induce molecular processes which act against cell death mechanisms like apoptosis, excitotoxicity and oxidative stress. These are non-MAO based mechanisms and they act not only on a functional level but rather on influencing gene expression and protein synthesis. These more recent discoveries are in line with the earlier assumption of neuroprotective/neurorestaurative/disease-modifying properties especially of this chemical class of compounds.
Table 5A.
Actions of propargylamine derived MAO-B-I
As reviewed by Magyar et al 2010, Weinreb et al 2010, Naoi and Maruyama 2010.
Depression in PD
With a prevalence of approximately 40% depression is considered to be the most frequent psychiatric manifestation in PD (Burn, 2002; McDonald and DeLong, 2003; Veazey et al., 2005). Particular clinical features of the depressive symptom profile in patients with PD have been described (Wermuth and Bech, 2006; Brand et al., 2007) and recommendations towards better recognition given (Burn 2002). About 50% of the patients meet the criteria for major depressive disorder (MDD), 50% have minor depression or dysthymia (Chaudhuri et al., 2006). Depression in PD is associated with increased disability and reduced quality of life, the impact of depressive symptoms in early PD especially should not be underestimated (Ravina et al., 2007).
MAOIs in depression
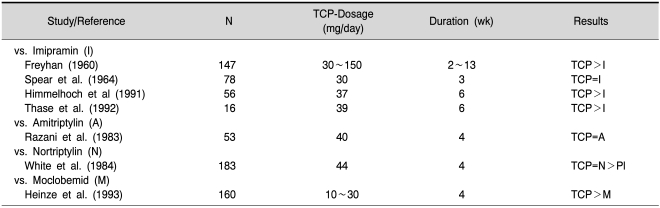
While early studies suggested that MAOIs were not as effective as other antidepressants, more recent studies have demonstrated that, when prescribed in adequate dosages, phenelzine and tranylcypromine are as effective as other antidepressants (Pare, 1985). Table 6 gives a summary of important controlled studies with tranylcypromine. In a recent study tranylcypromine, 60 mg daily, was found effective in the treatment of panic disorder and social anxiety disorder comorbidity (Nardi et al., 2010).
Table 6.
Randomised, double-blind controlled studies with tranylcypromine (TCP) in the treatment of depressive disorders (from Laux et al., 2002)
Legend: =indicates equivalent to, >indicates more effective than references in this table are given in detail in Laux et al., 2002.
The main indications for the classical irreversible MAOIs are subgroups of depression such as atypical depression, dysthymia or for patients who do not respond to reuptake inhibitors, so-called therapy resistant depressions (Nolen et al., 1994; Paykel, 1995; Bauer et al., 2002).
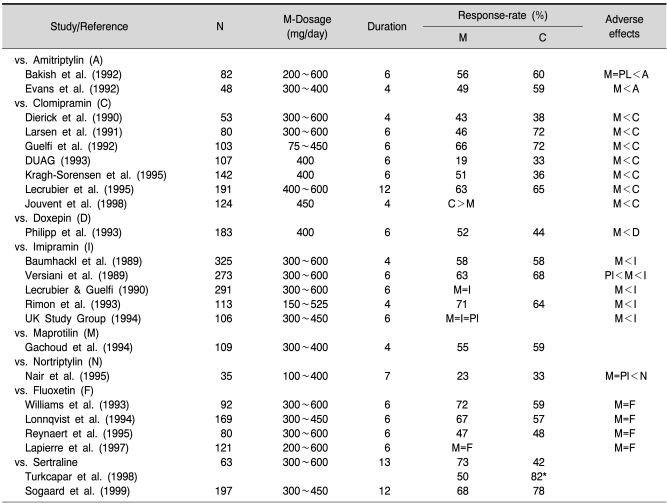
The therapeutic efficacy of moclobemide has been assessed in numerous controlled studies comparing it with placebo and established antidepressants (for reviews see Laux, 1989; Fitton et al., 1992; Fulton and Benfield, 1996; Laux et al., 2002). An overview of the most relevant trials is given in Table 7.
Table 7.
Randomised, double-blind controlled studies with moclobemide (M) in the treatment of depressive disorders (selection; references see Fulton and Benfield 1996; Laux et al., 2002)
C: comparator, =indicates equivalent to, >indicates more effective/better tolerated than. *melancholic subtype.
Large trials in patients with major depression have generally confirmed that the efficacy of moclobemide is equivalent to that of TCAs beside some negative studies. Moclobemide treatment was usually fixed up to 300, 400 or 600 mg/day. It should be noted, however, that the use of dosages of up to 900 mg/d has been established due to clinical experience nowadays particularly in patients with refractory depression (Guentert et al., 1995). A meta-analysis of inpatient studies indicated that higher dosages (>450 mg/day) of moclobemide are needed for full therapeutic effect in patients with severe depression (Angst et al., 1995).
Subgroup analyses of patients revealed moclobemide to be effective in patients with dysthymia or atypical depression especially (Lonnqvist et al., 1995).
Several trials have evaluated moclobemide alone or in combination with TCAs or SSRIs in the treatment of refractory depression (Bakish et al., 1995) reporting positive results. These data are limited and great caution is necessary because of the potential to induce the serotonin syndrome when combining moclobemide with serotoninergic drugs.
Uncontrolled long term follow-up studies demonstrated continued effectiveness of moclobemide over the treatment period of 1 year with more than 60% of patients having continued response (Fulton and Benfield, 1996). The role of MAOIs in maintenance treatment of depression still has to evaluated (Kennedy, 1997).
MAO-B inhibitors like selegiline in high dosage have been used in therapy-refractory depressions probably due to non-selective MAO effects (see review Laux, 1993). Recently, transdermal selegiline has been approved and released in the USA for treatment of major depression with a target dose of 6 mg/24 hours. This dosage and application overcomes the MAO-B selectivity and leads to MAO-A inhibition, seen to be necessary for antidepressive effects. In several controlled studies selegiline transdermal system exhibited significant treatment effects on MDD including core depression symptoms, vegetative symptoms and motor retardation (Frampton and Plosker, 2007; Robinson et al., 2007). A combination of selegiline and 5-hydroxytryptophan has been tested in a pilot study and proved antidepressant efficacy (Mendlewicz and Youdim, 1978).
Treatment with antidepressants and MAOIs in PD
The scientific knowledge about the treatment with antidepressant drugs among PD patients is nearly missing. A pharmaco-epidemiological study in Danmark showed that persons treated with antiparkinson drugs have higher frequency of antidepressant drug treatment than have controls (Brandt-Christensen et al., 2007). Most authors conclude, that recommendations for the optimal drug treatment of depression in PD are difficult to give (Burn, 2002). Serotonin selective reuptake inhibitors (SSRIs) are given frequently, the benefit of SSRI treatment in PD has not been established, however (Wermuth and Bech, 2006).
Thirty-seven patients with early PD have been treated successfully with tranylcypromine (TCP), parkinsonian symptoms improved slightly, follow-up after 1.5 years on average revealed only slight worsening (Fahn and Chouinard, 1998). No other studies have been reported with TCP so far.
Ten patients with PD have been treated successfully with moclobemide vs. moclobemide with selegiline under tyramine restriction (Steuer and Ballering, 1997). Sufficient data allowing conclusions are missing.
Dosage and administration
The recommended initial dosage of tranylcypromine is 10~20 mg/day, of moclobemide 300 to 450 mg/day, given in 2 to 3 divided doses. Subsequent dosage increase to a maximum of 60 mg/day, 900 mg/day respectively, are made as clinically indicated (Beckmann and Laux, 1991; Fitton et al., 1992). Clinical experience and studies in the last years clearly have shown the necessity of higher dosage of moclobemide pointing out the lack of early sufficient dose-finding studies. Recommended daily doses of phenelzine are 30 to 90 mg, of isocarboxazid 30 to 60 mg, respectively.
Dietary restrictions are essential for tranylcypromine (tyramine-rich food), unnecessary for moclobemide taken at the end of a meal. A 2 week wash-out period is required for switching between tranylcypromine and other classes of antidepressants, not between moclobemide and other antidepressants.
In patients with severe hepatic impairment, tranylcypromine and moclobemide dosages should be reduced by one-third to one-half in such patients (Atkinson and Ditman, 1965; Fitton et al., 1992).
The target dose of selegiline transdermal (not released in Europe so far) is 6 mg/24 hours.
Tolerability and safety
The most frequent adverse effects of irreversible MAOIs are orthostatic hypotension, sleep disturbances and nervousness/agitation (Remick et al., 1989). The mostly limiting factor in the use of these MAOIs is the potential for dangerous interactions with tyramine-rich foods and sympathomimetic and serotoninergic substances. Therefore, prescription is only possible to patients being strongly compliant with dietary restrictions. In the case of RIMAs like moclobemide there is no need for dietary restrictions. For "selegiline transdermal system" tyramine dietary restrictions are not needed. The incidence of the most frequently reported adverse effects from patients receiving MAOIs are summarised in Table 7.
Insomnia and impotency in men have been the most frequent side effects of TCP in patients with early PD (Fahn and Chouinard, 1998).
Unlike nonselective, irreversible MAOIs and tricyclics, moclobemide has little effect on the cardiovascular system and lacks anticholinergic properties associated with TCAs (Moll et al., 1994; Fulton and Benfield, 1996). In almost all controlled clinical studies comparing moclobemide with TCAs moclobemide showed clearly superior tolerability (Versiani et al., 1990; Fitton et al., 1992; Fulton and Benfield, 1996). Headache, insomnia and agitation were the only one side effects being reported more frequently with moclobemide compared to re-uptake inhibiting antidepressants. Dizziness and nausea have been noticed additionally. No systematic changes in blood pressure were observed with moclobemide, whereas increases in both blood pressure and pulse were recorded for tranylcypromine (Laux et al., 1996). Body weight gain has been observed with phenelzine and isocarboxazid, no clearcut cases with tranylcypromine or moclobemide have been reported (Cantu and Korek, 1988). Compared to SSRIs moclobemide showed fewer gastrointestinal adverse effects and sexual dysfunction have not been reported with moclobemide (Philipp et al., 1999).
Regarding cognitive functions data in favour of moclobemide compared to other antidepressants are available: Behavioural toxicity assessed by choice reaction time is very low or missing, psychomotor functions seem not be influenced negatively perhaps even improved (Hindmarch et al., 1992).
The principal side effects of selegiline transdermal were local dermal reactions and (dose-related) insomnia (Robinson and Amsterdam, 2008).
After ingestion of up to 20 g of moclobemide no fatalities were observed, so moclobemide can be considered as a 'safe' antidepressant (DeJonghe and Swinkels, 1992; Chen and Ruch, 1993). Several fatal overdoses have been reported when moclobemide was combined with serotoninergic antidepressants like citalopram, clomipramine or fluoxetine due to occurrence of a serotonin syndrome (Neuvonen et al., 1993). In contrast, irreversible MAOIs like tranylcypromine must be regarded as less safe regarding to the fatal toxicity index (Henry et al., 1995).
Side effects of selegiline and rasagiline
Selegiline is well tolerated. Side effects/adverse reactions like sleeplessness, nausea, vomiting, dizziness, dry mouth, orthostatic hypotension and dyskinesias have been all observed in the range of 2~5% of PD patients (Parkinson-Study-Group, 1993; Reichmann et al., 2002) which is comparable to placebo. Other side effects like headache, heart beating, dyspnoe, edema, confusion, micturition dysfunction, loss of appetite and anxiety are even more rare and below 2% (Reichmann et al., 2002).
Rasagiline
Adverse reactions as seen with other dopaminergic drugs, like nausea, vomiting, orthostatic hypotension, somnolence, hallucinations and dyskinesias are tolerable in most cases. Vomiting was noteable at 1 mg/day in the PRESTO-study, as was the occurrence of dyskinesias but such adverse reactions have not been described in other clinical trials like the LARGO-trial. Cognitive and behavioural symptoms of PD are not changed/worsened at 1 mg/day rasagiline (Elmer et al., 2006).
Although interactions may be suggested when MAO-B inhibitors are combined with COMT-inhibitors, such adverse reactions have not been reported to be of relevance. In fact rasagiline is effective and well tolerated in PD patients with L-DOPA induced motor fluctuations receiving entacapone (Elmer et al., 2006).
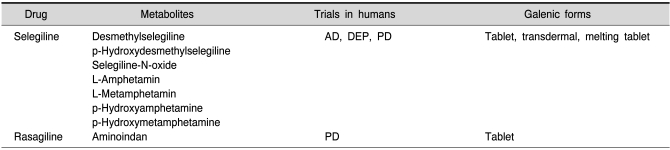
The cardiovascular risk in selegiline and rasagiline treated PD
Selegiline is metabolized mainly to desmethylselegiline, L-amphetamine and L-metamphetamine. Given these facts there was always a profound discussion about the contribution of these metabolites to selegiline's clinical symptomatic effects (Reynolds et al., 1978; Elsworth et al., 1982) and selegilines adverse reactions, predominantly of cardiovascular origin (Churchyard et al., 1997). L-Amphetamine has about a ten times lower activity on the peripheral sympathetic system compared to D-amphetamine. On the other hand, both are equipotent in blocking striatal dopamine uptake (Coyle and Snyder, 1969). A clinical trial of daily 10 mg selegiline vs. the calculated dosis and ratio of L-amphetamine and L-metamphetamine in PD patients came to the conclusion that selegiline did show antiakinetic efficacy while the metabolites did not (Elsworth et al., 1982). Therefore, selegiline is not acting via the amphetamine metabolites. Also, from the side effect profile as reported in the large selegiline based trials there is no evidence for enhanced cardiovascular risk (eg. Parkinson Study Group, 1989; 1993). This holds true also when selegiline is compared to treatment based on L-DOPA and dopaminergic receptor agonists. However, head-to head comparison is missing.
Nevertheless, to avoid further such discussion a sublingual galenic form of selegiline treatment has been developed named Zydis-selegiline (Clarke et al., 2003a; 2003b). This "melting-tablet" avoids a first-pass effect and therefore a significant break-down to L-amphetamine and L-metamphetamine in the range of 90%. A reduction of the daily dosis of 10 mg selegiline to 1, 25 mg selegiline is advised. The bioavailability of selegiline when given as "melting-tablet" is more homogene and better reproduceable compared to the peroral type of application (Clarke et al., 2003a; 2003b). In addition a transdermal galenic form of selegiline has been developed for the treatment of major depression again reducing selegilines first-pass biotransformation (Frampton and Plosker, 2007; Robinson et al., 2007).
Melting tablet and transdermal selegiline avoid first-pass metabolism, cause higher drug availability in MAO-B preferring organs and reduce the concentration of metabolites. Increased drug concentration may cause significant inhibition of both MAO-B and -A in brain but not in the periphery. This explains selegilines antidepressant activity when combined with 5-hydroxytryptophan without the cheese-effect and without the serotonin syndrome (Mendlewicz and Youdim, 1978).
Under clinical conditions selegiline is not more "toxic" than other dopaminergic treatment strategies. In fact the side effect and adverse reaction profile of selegiline has been evaluated as being well tolerated and "mild" compared to other PD treatment strategies, eg. orthostatic reactions have been noteable in only 3, 7% of patients (Reichmann et al., 2002).
It should be mentioned that L-amphetamine is in clinical use for the treatment of attention-deficit-hyperactivity syndrome (ADHD) without major side effects. In line with this are several studies as summarized by Reidenberg (1994) showing no abuse liability of selegiline.
The contribution of desmethylselegiline (DMS) to the effects of selegiline has been underestimated so far. DMS is a weak inhibitor of MAO-B and seems to have glutathione related properties (Heinonen, 1997; Heinonen et al., 1997).
In contrast to selegiline, rasagiline is not metabolized to amphetamine. The main metabolite of rasagiline is aminoindan. Therefore any cardiovascular responses of rasagiline are neglectible as shown in animal studies (Finberg et al., 2006) and in all clinical trials including the TEMPO- (Parkinson Study Group, 2002), PRESTO- (Parkinson Study Group, 2005), LARGO- (Rascol et al., 2005) and ADAGIO-study (Olanow et al., 2008; 2009).
In the recommended therapeutic dosis of 1 mg/day rasagiline does not potentiate the tyramine induced cheese-effect (de Marcaida et al., 2006). This is in agreement with earlier findings in rats and cats (Finberg et al., 1981; Chen and Swope, 2005; Finberg et al., 2006).
Drug interactions
Coadministration of SSRIs and other serotoninergic substances to tranylcypromine is contraindicated due to the risk of serotonin syndrome. With moclobemide great caution should be exercised with this combination. Severe, sometimes fatal, interactions between nonselective, irreversible MAOIs, moclobemide as well as selegiline and pethidine and dextromethorphan have been reported. Indirectly acting sympathomimetics (tyramine, ephedrine, pseudoephedrine) should be administered with caution in patients treated with MAOIs (Dingemanse, 1993).
The elimination of moclobemide is significantly reduced by cimetidine making dose adjustment necessary.
Although selegiline has been reported to have antidepressant actions when combined with 5-hydroxytryptophan (Mendlewicz and Youdim, 1978) MAO-B-inhibitors may not be safe enough to avoid the "serotonin-syndrome" when given in adjunct to serotonin-reuptake inhibitors (SSRIs) to treat depression and anxiety in PD patients. However, there is a low risk (0, 24%) to develop the "serotonin-syndrome" in selegiline treated patients (Richard et al., 1997). Also the results of a small number of PD patients treated with tricyclics and SSRIs plus rasagiline in the TEMPO-, PRESTO- and LARGO-studies do not give evidence for the "serotonin-syndrome", the population incidence of serotonin toxicity in those patients has a 9, 5% probability of being less than 1, 2% (Pannisset et al., 2007; Montgomery and Panisset, 2009). However, a clinical trial with a biostatistic power relevant to give a definite answer to the problem is missing. Therefore MAO-B inhibitors have not to be combined with drugs stimulating the serotoninergic system.
Meperidin plus selegiline has been reported to be dangerous as it causes severe hypertension (Zornberg et al., 1991).
Selegiline transdermal without tyramine restriction revealed no acute hypertensive reactions in trials, until more data are available, foods that are rich sources of tyramine should be avoided, however.
As described by Chen and Swope (2005) CYP1A2 inhibitors (cimetidine, fluvoxamine, ciprofloxacin) increase the area under curve of rasagiline, while CYP1A2 inducers like omeprazol decrease it, as it may decrease in heavy smokers.
Minor time-dependent mechanism-based inhibition of CYP2D6 has been described for selegiline and moclobemid in experimental designs; the significance in human beings remains to be investigated (Polasek et al., 2006). For selegiline interactions with CYP 3A4 and CYP 2E1 may be relevant too.
Expert commentary
While selective MAO-B-inhibitors demonstrate a significant benefit in PD and improve motoricity and motor fluctuations eventually causing disease-modification in general MAOIs to date play a subordinate therapeutic role in the treatment of depression in PD compared to SSRIs or reboxetine, a selective noradrenergic reuptake inhibitor. This attitude is primarily due to the risk of adverse effects and compliance problems (e.g. dietary restrictions, interaction problems) with MAOIs. Moclobemide according to its favourable adverse effect, interaction and toxicity profile can be used in depressed Parkinson patients when activating properties are necessary, especially.
Overall, the clinical use of MAOIs may be limited by the possible adverse effects of restlessness and insomnia - but see rasagiline. As far as long term and prophylactic treatments are concerned, the place of MAOIs still has to be verified as antidepressant or antidementive drugs in PD (Tariot et al., 1987; Sano et al., 1997; Filip and Kolibas, 1999; Sterling et al., 2002; Elmer et al., 2006; Carageorgiou et al., 2008). Controlled studies are urgent needed for final evaluation and recommendations.
Five-year view
It would be worthwile to perform clinical studies to demonstrate the capacity of MAO-B inhibitors as antidepressants and antidementive drugs. New substances like ladostigil (MAOA/B inhibitor; AchE-inhibitor) or M30 and HLA-20 (MAO-A/B inhibitor, iron-chelator) and M30P (MAO-A/B inhibitor, carbonate cholinesterase inhibitor) give future aspects of using MAO-I's in a variety of clinical indications (Weinreb et al., 2010). The pharmacological properties of aminoindan have to be elucidated as they may be neuroprotective (Bar-Am et al., 2010).
Key issues
Head-to-head clinical trials are necessary to demonstrate disease-modification using improved delayed-start-study designs with selegiline and rasagiline. Otherwise no objective comparison can be made between the neuroprotective/neurorestaurative properties of selegiline and rasagiline.
Aminoindan has to be tested in vivo in order to get insight into its anti-parkinsonian efficacy.
The many molecular biological and -genetic data evolved from in-vitro studies have to be confirmed in in-vivo experiments to prove relevance in human beings. That other MAO-Is, even reversible ones might well be important in neuroprotection/disease modification is a just ongoing important issue. In addition, the respective role of MAO-A has to be elucidated in more detail.
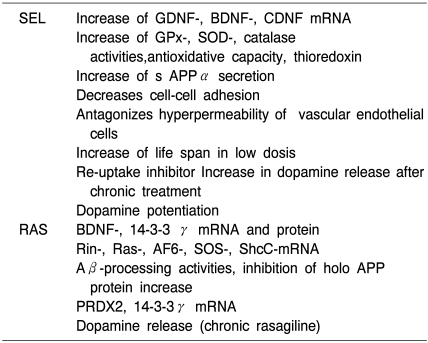
Table 5B.
Non-MAO related actions of selegiline and rasagiline on molecular paramters in vitro
As reviewed by Magyar et al 2010, Weinreb et al 2010, Naoi and Maruyama 2010.
Table 5C.
Non-MAO related actions of selegiline and rasagiline on molecular parameters in vivo
As reviewed by Magyar et al 2010, Weinreb et al 2010, Naoi and Maruyama 2010.
References
- 1.Ahlskog JE, Uitti RJ. Rasagiline, Parkinson neuroprotection and delayed-start trials. Neurology. 2010;74:1143–1148. doi: 10.1212/WNL.0b013e3181d7d8e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angst J, Amrein R, Stabl M. Moclobemide and tricyclic antidepressants in severe depression: meta-analysis and prospective studies. J Clin Psychopharmacol. 1995;15:16S–23S. doi: 10.1097/00004714-199508001-00004. [DOI] [PubMed] [Google Scholar]
- 3.Arnett CD, Fowler JS, MacGregor RR, Schlyer DJ, Wolf AP, Långström B, Halldin C. Turnover of brain monoamine oxidase measured in vivo by positron emission tomography using L-[11C]deprenyl. J Neurochem. 1987;49:522–527. doi: 10.1111/j.1471-4159.1987.tb02895.x. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson R, Ditman K. Tranylcypromine: a review. Clin Pharmacol Ther. 1965;6:631–655. doi: 10.1002/cpt196565631. [DOI] [PubMed] [Google Scholar]
- 5.Bakish D, Hooper C, West D. Moclobemide and specific serotonin re-uptkae inhibitor combination treatment of resistant anxiety and depressive disorders. Hum Psychopharmacol. 1995;10:105–109. [Google Scholar]
- 6.Ban TA, Healy D, Shorter E. Reflections on twentieth-century psychopharmacology. Budapest, Hungary: Animula, Publishing House; 2004. Vol 4. [Google Scholar]
- 7.Bar-Am O, Weinreb O, Amit T, Yoduim MBH. Regulation of Bcl-2 protein familiy proteins, neurotrophic fators, and APP processing in the neurorescue activity of progargylamine. FASEB J. 2005;19:1899–1901. doi: 10.1096/fj.05-3794fje. [DOI] [PubMed] [Google Scholar]
- 8.Bar-Am O, Weinreb O, Amit T, Youdim MBH. The neuroprotective mechanism of 1-(R)-aminoindan, the major metabolite of the anti-parkinsonian drug rasagiline. J Neurochem. 2010;112:1131–1137. doi: 10.1111/j.1471-4159.2009.06542.x. [DOI] [PubMed] [Google Scholar]
- 9.Bauer M, Whybrow P, Angst J, Versiani M, Möller HJ WFSBP Task Force. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for biological treatment of unipolar depressive disorders, part 1: Acute and continuation treatment of major depressive disorders. World J Biol Psychiatry. 2002;3:5–43. doi: 10.3109/15622970209150599. [DOI] [PubMed] [Google Scholar]
- 10.Beckmann H, Laux G. Aktuelles zur Therapie mit MAO-Hemmern. Krankenhauspsychiatrie. 1991;2:201–202. [Google Scholar]
- 11.Berlin I, Saïd S, Spreux-Varoquaux O, Launay JM, Olivares R, Millet V, Lecrubier Y, Puech AJ. A reversible monoamine oxidase A inhibitor (moclobemide) facilitates smoking cessation and abstinence in heavy, dependent smokers. Clin Pharmacol Ther. 1995;58:444–452. doi: 10.1016/0009-9236(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 12.Bernheimer H, Birkmayer W, Hornykiewicz O. Verhalten der monoaminoxydase im gehirn des menschen nach therapie mit monoaminoxydase-hemmern. Wr Klin Wschr. 1962;74:558–559. [Google Scholar]
- 13.Bernheimer H, Birkmayer W, Hornykiewicz O. Zur Biochemie des Parkinson-Syndromes des Menschen. Einflu β der Monoaminoxydase-Hemmer-Therapie auf die Konzentration des Dopamins, Noradrenalins und 5-Hydroxytryptamins im Gehirn. Klin Wschr. 1963;41:465. [Google Scholar]
- 14.Bernheimer H, Birkmayer W, Hornykiewicz O. Verteilung des 5-Hydroxytryptamins (Serotonin) im gehirn des menschen und sein verhalten bei patienten mit parkinson-syndrom. Klin Wschr. 1961;39:1056–1059. doi: 10.1007/BF01487648. [DOI] [PubMed] [Google Scholar]
- 15.Biglan KM, Schwid S, Eberly S, Blindauer K, Fahn S, Goren T, Kieburtz K, Oakes D, Plumb S, Siderowf A, Stern M, Shoulson I Parkinson Study Group. Rasagiline improves quality of life in patients with early Parkinson's disease. Mov Disord. 2006;21:616–623. doi: 10.1002/mds.20764. [DOI] [PubMed] [Google Scholar]
- 16.Birkmayer W, Hornykiewicz O. Der L-Dioxyphenylalanin (=L-DOPA). Effekte beim Parkinson-Syndrom des Menschen: Zur Pathogenese und Behandlung der Parkinson-Akinese. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1962;203:560–574. doi: 10.1007/BF00343235. [DOI] [PubMed] [Google Scholar]
- 17.Birkmayer W, Hornykiewicz O. Weitere experimentelle Untersuchungen über L-DOPA beim Parkinson-Syndrom und Reserpin-Parkinsonismus. Arch Psychiat u Zschr fd ges Neurol. 1964;206:367. doi: 10.1007/BF00341704. [DOI] [PubMed] [Google Scholar]
- 18.Birkmayer W, Hornykiewicz O. Der L-3,4-Dioxyphenylalanin (=DOPA)-Effekt bei der Parkinson-Akinese. Wr klin Wschr. 1961;73:787–788. [PubMed] [Google Scholar]
- 19.Birkmayer W, Knoll J, Riederer P, Youdim MB. (-)-Deprenyl leads to prolongation of L-dopa efficacy in Parkinson's disease. Mod Probl Pharmacopsychiatry. 1983;19:170–176. doi: 10.1159/000407513. [DOI] [PubMed] [Google Scholar]
- 20.Birkmayer W, Knoll J, Riederer P, Youdim MB, Hars V, Marton J. Increased life expectancy resulting from addition of L-deprenyl to Madopar® treatment in Parkinson's disease: a longterm study. J Neural Transm. 1985;64:113–127. doi: 10.1007/BF01245973. [DOI] [PubMed] [Google Scholar]
- 21.Birkmayer W, Riederer P, Abrozi L, Youdim MBH. Implications of combined treatment with "Madopar" and L-deprenil in Parkinson's disease. A long-term study. Lancet. 1977;1:439–443. doi: 10.1016/s0140-6736(77)91940-7. [DOI] [PubMed] [Google Scholar]
- 22.Birkmayer W, Riederer P, Youdim MBH, Linauer W. The potentiation of the anti akinetic effect after L-dopa treatment by an inhibitor of MAO-B, Deprenil. J Neural Transm. 1975;36:303–326. doi: 10.1007/BF01253131. [DOI] [PubMed] [Google Scholar]
- 23.Brand S, Dodel R, Hautzinger M, Gründer G, Althaus A, Schneider F. Depression in Parkinson's disease. Assessment and treatment. Nervenarzt. 2007;78:715–728. doi: 10.1007/s00115-007-2292-2. [DOI] [PubMed] [Google Scholar]
- 24.Brandt-Christensen M, Kvist K, Nilsson FM, Andersen PK, Kessing LV. Treatment with antiparkinson and antidepressant drugs: a register-based, pharmaco-epidemiological study. Mov Disord. 2007;22:2037–2042. doi: 10.1002/mds.21472. [DOI] [PubMed] [Google Scholar]
- 25.Burn DJ. Beyond the iron mask: towards better recognition and treatment of depression associated with Parkinson's disease. Mov Disord. 2002;17:445–454. doi: 10.1002/mds.10114. [DOI] [PubMed] [Google Scholar]
- 26.Cantu T, Korek J. Monoamine oxidase inhibitors and weight gain. Drug Intell Clin Pharm. 1988;22:755–759. doi: 10.1177/106002808802201002. [DOI] [PubMed] [Google Scholar]
- 27.Carageorgiou H, Sideris AC, Mesari I, Liakou CI, Tskiris S. The effects of rivastigmine plus selegiline on brain acetylcholinesterase, (Na+K+)-, Mg2+-ATPase activities, antioxidant status, and learning performance of aged rats. Neuropsychiatr Dis Treat. 2008;4:687–699. doi: 10.2147/ndt.s3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caslake R, Macleod A, Ives N, Stowe R, Counsell C. Monoamine oxidase B inhibitors versus other dopaminergic agents in early Parkinsons disease. Cochrane Database Syst Rev. 2009;(4):CD006661. doi: 10.1002/14651858.CD006661.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Chan-Palay V. Depression and senile dementia of the Alzheimer type: a role for moclobemide. Psychopharmacology (Berl) 1992;106:S137–S139. doi: 10.1007/BF02246259. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhuri KR, Healy DG, Schapira AH. Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol. 2006;5:235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 31.Chen DT, Ruch R. Safety of moclobemide in clinical use. Clin Neuropharmacol. 1993;16:S63–S68. [PubMed] [Google Scholar]
- 32.Chen JJ, Swope DM. Clinical Pharmacology of Rasagiline: A Novel, Second-Generation Propargylamine for the Treatment of Parkinson Disease. J Clin Pharmacol. 2005;45:878–894. doi: 10.1177/0091270005277935. [DOI] [PubMed] [Google Scholar]
- 33.Churchyard A, Mathias CJ, Boonkongchuen P, Lees AJ. Autonomic effects of selegiline: possible cardiovascular toxicity in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1997;63:228–234. doi: 10.1136/jnnp.63.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarke A. Xilopar - The evolution of selegiline. Satelliten-symposium "Neuroprotection beim Parkinson-Syndrom - Back to the future?"; Kongress der Deutschen Gesellschaft für Neurologie; 22. September; Aachen. 2001. (Nervenheilkunde 10/2001) [Google Scholar]
- 35.Clarke A, Brewer F, Johnson ES, Mallard N, Hartig F, Taylor S, Corn TH. A new formulation of selegiline: improved bioavailability and selectivity of MAO-B inhibition. J Neural Transm. 2003a;110:1241–1255. doi: 10.1007/s00702-003-0036-4. [DOI] [PubMed] [Google Scholar]
- 36.Clarke A, Johnson ES, Mallard N, Corn TH, Johnston A, Boyce M, Warrington S, MacMahon DG. A new low-dose formulation of selegiline: clinical efficacy, patient preferene and selectivity for MAO-B inhibition. J Neural Transm. 2003b;110:1257–1271. doi: 10.1007/s00702-003-0042-6. [DOI] [PubMed] [Google Scholar]
- 37.Coyle JT, Snyder SH. Antiparkinsonian drugs: inhibition of dopamine uptake in the corpus striatum as a possible mechanism of action. Science. 1969;166:899. doi: 10.1126/science.166.3907.899. [DOI] [PubMed] [Google Scholar]
- 38.D'Agostino RB. The delayed-start study design. N Engl J Med. 2009;361:1304–1306. doi: 10.1056/NEJMsm0904209. [DOI] [PubMed] [Google Scholar]
- 39.Degkwitz R, Frowein R, Kulenkampff C, Mohs U. über die Wirkungen des L-DOPA beim Menschen und deren Beeinflussung durch Reserpin, Iproniazid und Vitamin B6. Klin Wschr. 1960;38:120. [Google Scholar]
- 40.DeJonghe F, Swinkels J. The safety of antidepressants. Drugs. 1992;41:40–47. doi: 10.2165/00003495-199200432-00007. [DOI] [PubMed] [Google Scholar]
- 41.DeMarcaida JA, Schwid SR, White WB, Blindauer K, Fahn S, Kieburtz K, Stern M, Shoulson I Parkinson Study Group TEMPO; PRESTO Tyramine Sub study Investigators and Coordinators. Effects of tyramine administration in parkinson's disease patients treated with selective mao-b inhibitor rasagiline. Mov Disord. 2006;21:1716–1721. doi: 10.1002/mds.21048. [DOI] [PubMed] [Google Scholar]
- 42.Dingemanse J. An update of recent moclobemide interaction data. Int Clin Psychopharmacol. 1993;7:167–180. doi: 10.1097/00004850-199300730-00008. [DOI] [PubMed] [Google Scholar]
- 43.Elmer L, Schwid S, Eberly S, Goetz C, Fahn S, Kieburtz K, Oakes D, Blindauer K, Salzman P, Oren S, Prisco UL, Stern M, Shoulson I Parkinson Study Group TEMPO; PRESTO Investigators. Rasagiline-associated motor improvement in PD occurs without worsening of cognitive and behavioral symptoms. J Neurol Sci. 2006;248:78–83. doi: 10.1016/j.jns.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 44.Elsworth JD, Glover V, Reynolds GP, Sandler M, Lees AJ, Phuapradit P, Shaw KM, Stern GM, Kumar P. Deprenyl administration in man: a selective monoamine oxidase B inhibitor without the "cheese effect". Psychopharmacology (Berl) 1978;57:33–38. doi: 10.1007/BF00426954. [DOI] [PubMed] [Google Scholar]
- 45.Elsworth JD, Sandler M, Lees AJ, Ward C, Stern GM. The contributuion of amphetamine metabolites of (-)-deprenyls to its antiparkinsonian properties. J Neural Transm. 1982;54:105–110. doi: 10.1007/BF01249283. [DOI] [PubMed] [Google Scholar]
- 46.Fahn S, Chouinard S. Experience with tranylcypromine in early Parkinson's disease. J Neural Transm Suppl. 1998;52:49–61. doi: 10.1007/978-3-7091-6499-0_6. [DOI] [PubMed] [Google Scholar]
- 47.Filip V, Kolibas E. Selegiline in the treatment of Alzheimer's disease: a long-term randomized placebo-controlled trial. Czech and Slovak senile dementia of Alzheimer type study group. J Psychiatry Neurosci. 1999;24:234–243. [PMC free article] [PubMed] [Google Scholar]
- 48.Finberg JP, Gross A, Bar-Am O, Friedman R, Loboda Y, Youdim MB. Cardiovascular responses to combined treatment with selective monoamine oxidase type B inhibitors and L-DOPA in the rat. Br J Pharmacol. 2006;149:647–656. doi: 10.1038/sj.bjp.0706908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finberg JP, Takeshima T, Johnston JM, Commissiong JW. Increased survival of dopaminergic neurons by rasagiline, a monoamine oxidase B inhibitor. Neuroreport. 1998;9:703–707. doi: 10.1097/00001756-199803090-00026. [DOI] [PubMed] [Google Scholar]
- 50.Finberg JP, Tenne M, Youdim MB. Tyramine antagonistic properties of AGN 1135, an irreversible inhibitor of monoamine oxidase type B. Br J Pharmacol. 1981;73:65–74. doi: 10.1111/j.1476-5381.1981.tb16772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fitton A, Faulds D, Goa K. Moclobemide: a review of its pharmacological properties and therapeutic use in depressive illness. Drugs. 1992;43:561–596. doi: 10.2165/00003495-199243040-00009. [DOI] [PubMed] [Google Scholar]
- 52.Foley P. Beans, roots and leaves. A history of the chemical therapy of parkinsonism. 2001. [PubMed] [Google Scholar]
- 53.Foley P, Mizuno Y, Nagatsu T, Sano A, Youdim MBH, McGeer P, McGeer E, Riederer P. The L-DOPA story - an early Japanese contribution. Elsevier: Parkinsonism and Related Disorders. Mov Dis. 2000;6:1. [Google Scholar]
- 54.Fowler JS, Volkow ND, Logan J, Wang GJ, MacGregor RR, Schyler D, Wolf AP, Pappas N, Alexoff D, Shea C, et al. Slow recovery of human brain MAO B after L-deprenyl (Selegeline) withdrawal. Synapse. 1994;18:86–93. doi: 10.1002/syn.890180203. [DOI] [PubMed] [Google Scholar]
- 55.Frampton JE, Plosker GL. Selegiline transdermal system: in the treatment of major depressive disorder. Drugs. 2007;67:257–265. doi: 10.2165/00003495-200767020-00006. [DOI] [PubMed] [Google Scholar]
- 56.Freedman M, Rewilak D, Terri T, Cohen S, Gordon AS, Shandling M, Logan AG. L-deprenyl in Alzheimer's disease: cognitive and behavioral effects. Neurology. 1998;50:660–668. doi: 10.1212/wnl.50.3.660. [DOI] [PubMed] [Google Scholar]
- 57.Freedman NM, Mishani E, Krausz Y, Weininger J, Lester H, Blaugrund E, Ehrlich D, Chisin R. In Vivo Measurement of Brain Monoamine Oxidase B Occupancy by Rasagiline, Using 11C-L-Deprenyl and PET. J Nucl Med. 2005;46:1618–1624. [PubMed] [Google Scholar]
- 58.Fulton B, Benfield P, Moclobemide K. An update of its pharmacological properties and therapeutic use. Drugs. 1996;52:450–474. doi: 10.2165/00003495-199652030-00013. [DOI] [PubMed] [Google Scholar]
- 59.Gerlach M, Reichmann H, Riederer P. Die Parkinson-Krankheit. Grundlagen, Klinik, Therapie. 4. Auflage. New York: Springer Wien; 2007. [Google Scholar]
- 60.Glover V, Sandler M, Owen F, et al. Dopamine is a monoamine oxidase B substrate in men. Nature. 1977:80–81. doi: 10.1038/265080a0. [DOI] [PubMed] [Google Scholar]
- 61.Götz ME, Breithaupt W, Wautter J, Kupsch A, Scharz J, Oertel WH, Youdim MBH, Riederer P, Gerlach M. Chronic TVP-1012 (rasagiline) dose-activity response of monoamine oxidases A and B in the brain of the common marmoset. J Neural Transm Suppl. 1998;52:271–278. doi: 10.1007/978-3-7091-6499-0_27. [DOI] [PubMed] [Google Scholar]
- 62.Green AR, Mitchell BD, Tordoff AF, Youdim MBH. Evidence for dopamine deamination by both type A and type B monoamine oxidase in rat brain in vivo and for the degree of inhibition of enzyme necessary for increased functional activity of dopamine and 5-hydroytryptamine. Br J Pharmacol. 1977;60:343–349. doi: 10.1111/j.1476-5381.1977.tb07506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guentert TW, Banken L, Hilton S, Holford NH. Moclobemide: relationships between dose, drug concentration in plasma, and occurrence of adverse events. J Clin Psychopharmacol. 1995;15:84S–94S. doi: 10.1097/00004714-199508001-00014. [DOI] [PubMed] [Google Scholar]
- 64.Heikkila RE, Duvoisin RC, Finberg JPM, Youdim MBH. Prevention of MPTP-induced neurotoxicity by AGN-1133 and AGN-1135, selective inhibitors of monoamine oxidase-B. Eur J Pharmacol. 1985;116:313–317. doi: 10.1016/0014-2999(85)90168-2. [DOI] [PubMed] [Google Scholar]
- 65.Heinonen E. Long-term efficacy and safety of selegiline in the treatment of Parkinson's disease review; 12th Int. Symposium on Parkinson's Disease; 23.-26. March; London. 1997. [Google Scholar]
- 66.Heinonen EH, Anttila MI, Karnani HL, Nyman LM, Vuorinen JA, Pyykkö KA, Lammintausta RA. Desmethylselegiline, a metabolite of selegiline, is an irreversible inhibitor of monoamine oxidase type B in humans. J Clin Pharmacol. 1997;37:602–609. doi: 10.1002/j.1552-4604.1997.tb04342.x. [DOI] [PubMed] [Google Scholar]
- 67.Heinonen EH, Anttila MI, Lammintausta RAS. Pharmacokinetics and Clinical Pharmacology of Selegiline. In: Szelanyi I, editor. Inhibitors of monoamine oxidase B. Pharmacology and Clinical Use in Neurodegenerative Disorders. Basel/Switzerland: Birkhäuser Verlag; 1993. pp. 201–213. [Google Scholar]
- 68.Heinonen EH, Myllylä V. Safety of selegiline (deprenyl) in the treatment of Parkinson's disease. Drug Saf. 1998;19:11–22. doi: 10.2165/00002018-199819010-00002. [DOI] [PubMed] [Google Scholar]
- 69.Henry JA, Alexander C, Sener E. Relative mortality from overdose of antidepressants. BMJ. 1995;310:221–224. doi: 10.1136/bmj.310.6974.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hindmarch I, Alford C, Barwell F. Measuring the side-effects of psychotropics: the behavioural toxicity of antidepressants. J Psychopharmacol. 1992;6:198–203. doi: 10.1177/026988119200600212. [DOI] [PubMed] [Google Scholar]
- 71.Johnston JP. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968;17:1285–1297. doi: 10.1016/0006-2952(68)90066-x. [DOI] [PubMed] [Google Scholar]
- 72.Kennedy SH. Continuation and maintenance treatments in major depression: the neglected role of monoamine oxidase inhibitors. J Psychiatry Neurosci. 1997;22:127–131. [PMC free article] [PubMed] [Google Scholar]
- 73.Kline NS. Clinical experience with iproniazid (marsilid) J Clin Exp Psychopathol. 1958;19:72–78. [PubMed] [Google Scholar]
- 74.Knoll J. The striatal dopamine dependency of life span in male rats: longevity study with (-)-deprenyl. Mech Ageing Dev. 1988;46:237–262. doi: 10.1016/0047-6374(88)90128-5. [DOI] [PubMed] [Google Scholar]
- 75.Knoll J, Ecseri Z, Kelemen K, Nievel J, Knoll B. Phenylisopropylmenthylpropinylamine (E250), a new spectrum psychic energizer. Arch Int Pharmacodyn Ther. 1965;155:154–164. [PubMed] [Google Scholar]
- 76.Knoll J, Magyar K. Some puzzling pharmacological effects of monoamine oxidase inhibitors. Adv Biochem Psychopharmacol. 1972;5:393–408. [PubMed] [Google Scholar]
- 77.Kornhuber J, Konradi C, Mack-Burkhardt F, Riederer P, Heinsen H, Beckmann H. Ontogenesis of monoamine oxidase-A and -B in the human brain frontal cortex. Brain Res. 1989;499:81–86. doi: 10.1016/0006-8993(89)91136-0. [DOI] [PubMed] [Google Scholar]
- 78.Kupsch A, Sautter J, Gotz M, Breithaput W, Schwarz J, Youdim MBH, Riederer P, Gerlach M, Oertel WH. Monoamine oxidase-inhibition and MPTP-induced neurotoxicity in the non -human primate: comparison of rasagline (TVP 1012) with selegiline. J Neural Transm. 2001;108:985–1009. doi: 10.1007/s007020170018. [DOI] [PubMed] [Google Scholar]
- 79.Lamensdorf I, Porat S, Simantov R, Finberg JP. Effect of low-dose treatment with selegiline on dopamine transporter (DAT) expression and amphetamine-induced dopamine release in vivo. Br J Pharmacol. 1999;126:997–1002. doi: 10.1038/sj.bjp.0702389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laux G. Do MAO-B inhibitors have any role in the treatment of depression? In: Szelenyi I, editor. Inhibitors of monoamine oxidase B. Birkhäuser: Basel; 1993. pp. 319–326. [Google Scholar]
- 81.Laux G. Moclobemide in the treatment of depression--an overview. Psychiatr Prax. 1989;16:37–40. [PubMed] [Google Scholar]
- 82.Laux G, Becker T, Müller U. Monoamin-Oxidase- Hemmer. Klinik. In: Riederer P, Laux G, Pöldinger W, editors. Neuro-Psychopharmaka. Wien: Springer; 2002. pp. 489–518. [Google Scholar]
- 83.Laux G, Philipp M, Kohnen R. Hypertension with moclobemide. Lancet. 1996;347:1330. doi: 10.1016/s0140-6736(96)90975-7. [DOI] [PubMed] [Google Scholar]
- 84.Laux G, Volz HP, Müller HJ. Newer and older monoamine oxidase inhibitors. A comparative profile. CNS Drugs. 1995;3:145–158. [Google Scholar]
- 85.Lees AJ the PDRG-UK. Comparison of therapeutic effects and mortality data of levodopa and levodopa combined with selegiline in patients with early, mild Parkinson's disease. BMJ. 1995;311:1602–1607. doi: 10.1136/bmj.311.7020.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Levites Y, Amit T, Mandel S, Youdim MBH. Neuroprotection and neurorescue against Aβ toxicity and PKC-dependent release of non-amyloidogenic solubleprecursor protein by green tea polyphenol (-)-epigallocatehin-3-gallate. FASEB J. 2003;17:952–954. doi: 10.1096/fj.02-0881fje. [DOI] [PubMed] [Google Scholar]
- 87.Lonnqvist J, Sihvo S, Syvalahti E, Sintonen H, Kiviruusu O, Pitkanen H. Moclobemide and fluoxetine in the prevention of relapses following acute treatment of depression. Acta Psychiatr Scand. 1995;91:189–194. doi: 10.1111/j.1600-0447.1995.tb09765.x. [DOI] [PubMed] [Google Scholar]
- 88.Magyar K, Szende B, Jenei V, Tabi T, Palfi M, Szökö E. R-deprenyl: pharmacological spectrum of its activity. Neurochem Res. 2010;35:1922–1932. doi: 10.1007/s11064-010-0238-8. [DOI] [PubMed] [Google Scholar]
- 89.Magyar K, Szende B, Lemgyel J, Tekes K. The pharmacology of B-type selective monoamine oxidase inhibitors: milestones in (-)-deprenyl research. J Neural Transm Suppl. 1996;48:29–43. doi: 10.1007/978-3-7091-7494-4_4. [DOI] [PubMed] [Google Scholar]
- 90.McDonald R, DeLong MR. Prevalence, etiology, and treatment of depression in Parkinson's disease. Biol Psychiatry. 2003;54:363–375. doi: 10.1016/s0006-3223(03)00530-4. [DOI] [PubMed] [Google Scholar]
- 91.Mendlewicz J, Youdim MBH. Anti-depressant potentiation of 5-hydroxytrytophan by l-deprenyl, a monoamine oxidase type B inhibitor. J Neural Transm. 1978;43:279–286. doi: 10.1007/BF01246965. [DOI] [PubMed] [Google Scholar]
- 92.Moll E, Neumann N, Schmid-Burgk W, Stabl M, Amrein R. Safety and efficacy during long-term treatment with moclobemide. Clin Neuropharmacol. 1994;17:S74–S87. doi: 10.1097/00002826-199417001-00009. [DOI] [PubMed] [Google Scholar]
- 93.Montgomery EB, Panisset JM. Retrospective statistical analysis of the incidence of serotonin toxicity in patients taking rasagiline and anti-depressants in clinical trials. Mov Dis. 2009;24:359. [Google Scholar]
- 94.Myllylä VV, Sotaniemi KA, Hakulinen P, Maki-Ikola O, Heinonen EH. Selegiline as the primary treatment of Parkinson's disease - a longterm double-blind study. Acta Neurol Scand. 1997;95:211–218. doi: 10.1111/j.1600-0404.1997.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 95.Mytilineou C, Leonardi EK, Radcliffe P, Heinonen EH, Han SK, Werner P, Cohen G, Olanow CW. Deprenyls and desmethylselegiline protect mesencephalic neurons from toxicity induced by glutathione depletion. J Pharmacol Exp Ther. 1998;284:700–706. [PubMed] [Google Scholar]
- 96.Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Neuroprotective effects of brain -derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Naoi M, Maruyama W. Functional mechanism of neuroprotection by inhibitors of type B monoamine oxidase in Parkinson's disease. Expert Rev Neurother. 2009;9:1233–1250. doi: 10.1586/ern.09.68. [DOI] [PubMed] [Google Scholar]
- 98.Naoi M, Maruyama W. Monoamine oxidase inhibitors as neuroprotective agents in age-dependent. Curr Pharm Des. 2010 doi: 10.2174/138161210793176527. in press. [DOI] [PubMed] [Google Scholar]
- 99.Nardi AE, Lopes FL, Valenca AM, Freire RC, Nascimento I, Veras AB, Mezzasalma MA, de-Melo-Neto VL, Soares-Filho GL, King AL, Grivet LO, Rassi A, Versiani M. Double-blind comparison of 30 and 60 mg tranylcypromine daily in patients with panic disorder comorbid with social anxiety disorder. Psychiatry Res. 2010;175:260–265. doi: 10.1016/j.psychres.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 100.Neuvonen P, Pohjola-Sintonen S, Tacke U. Five fatal cases of serotonin syndrome after moclobemide-citalopram or moclobemide-clomipramine overdoses. Lancet. 1993;342:1419. doi: 10.1016/0140-6736(93)92774-n. [DOI] [PubMed] [Google Scholar]
- 101.Nolen W, Hoencamp I, Haffmans PMJ. Classical and selective monoamine oxidase inhibitors in refractory major depression. In: Nolen W, Zohar J, Roose SP, editors. Refractory depression: current strategies and future directions. New York: Wiley; 1994. [Google Scholar]
- 102.Norman TR, Burrows GD. A risk-benefit assessment of moclobemide in the treatment of depressive disorders. Drug Saf. 1995;12:46–54. doi: 10.2165/00002018-199512010-00004. [DOI] [PubMed] [Google Scholar]
- 103.Olanow CW, Hauser RA, Jankovic J, Langston W, Lang A, Poewe W, Tolosa E, Stocchi F, Melamed E, Eyal E, Rascol O. A randomized, double-blind, placebo-controlled, delayed start study to assess rasagiline as a disease modifying therapy in parkinson's disease (The ADAGIO study): rationale, design, and baseline characteristics. Mov Disord. 2008;23:2194–2201. doi: 10.1002/mds.22218. [DOI] [PubMed] [Google Scholar]
- 104.Olanow WC, Rascol O, Hauser R, Feigin PD, Jankovic J, Lang A, Langston W, Melamed E, Poewe W, Stocchi F, Tolosa E the ADABIO Study Investigators. A double-blind, delayed-start trial of rasagiline in parkinson's disease. N Engl J Med. 2009;361:1268–1278. doi: 10.1056/NEJMoa0809335. [DOI] [PubMed] [Google Scholar]
- 105.Panisset M, Schwied S, Ondo W. Safety of concomitant therapy with rasagiline and antidepressants in parkinson's diesease. Mov Dis. 2007;22:340. [Google Scholar]
- 106.Pare C. The present status of monoamine oxidase inhibitors. Br J Psychiatry. 1985;146:576–584. doi: 10.1192/bjp.146.6.576. [DOI] [PubMed] [Google Scholar]
- 107.Parkinson Study Group. A controlled, randomized, delayed-start study of rasagiline in early Parkinson disease. Arch Neurol. 2004;61:561–566. doi: 10.1001/archneur.61.4.561. [DOI] [PubMed] [Google Scholar]
- 108.Parkinson Study Group. A controlled trial of rasagiline in early Parkinson disease: the TEMPO Study. Arch Neurol. 2002;59:1937–1943. doi: 10.1001/archneur.59.12.1937. [DOI] [PubMed] [Google Scholar]
- 109.Parkinson Study Group. A randomized placebo-controlled trial of rasagiline in levodopa-treated patients with Parkinson disease and motor fluctuations: the PRESTO study. Arch Neurol. 2005;62:241–248. doi: 10.1001/archneur.62.2.241. [DOI] [PubMed] [Google Scholar]
- 110.Parkinson Study Group. Effect of deprenyl on the progression of disabilty in early Parkinson's disease. N Engl J Med. 1989;321:1364–1371. doi: 10.1056/NEJM198911163212004. [DOI] [PubMed] [Google Scholar]
- 111.Parkinson Study Group. Effects of tocopherol and deprenyl on the progression of disability in early Parkinson's disease. N Engl J Med. 1993;328:176–183. doi: 10.1056/NEJM199301213280305. [DOI] [PubMed] [Google Scholar]
- 112.Parkinson Study Group. Impact of deprenyl and tocopherol treatment on Parkinson's disease in DATATOP patients requiring levodopa. Ann Neurol. 1996b;39:37–45. doi: 10.1002/ana.410390107. [DOI] [PubMed] [Google Scholar]
- 113.Parkinson Study Group. Impact of deprenyl and tocopherol treatment on Parkinson's disease in DATATOP subjects not requiring levodopa. Ann Neurol. 1996a;39:29–36. doi: 10.1002/ana.410390106. [DOI] [PubMed] [Google Scholar]
- 114.Paykel ES. Clinical efficacy of reversible and selective inhibitors of monoamine oxidase A in major depression. Acta Psychiatr Scand Suppl. 1995;386:22–27. doi: 10.1111/j.1600-0447.1995.tb05920.x. [DOI] [PubMed] [Google Scholar]
- 115.Philipp M, Delini-Stula A, Baier D, Kohnen R, Scholz H, Laux G. Assessment of sexual dysfunction in depressed patients and reporting attitudes in routine daily practice: results of the postmarketing observational studies with moclobemide, a reversible MAO-A inhibitor. Int J Psychiatry Clin Practice. 1999;3:257–264. doi: 10.3109/13651509909068393. [DOI] [PubMed] [Google Scholar]
- 116.Polasek TM, Elliot DJ, Somogyi AA, Gillam EM, Lewis BC, Miners JO. An evaluation of potential mechanism-based inactivation of human drug metabolizing cytochromes P340 by monoamine oxidase inhibitors, including isoniazid. Br J Clin Pharmacol. 2006;61:570–584. doi: 10.1111/j.1365-2125.2006.02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rabey JM, Sagi I, Huberman M, Melamed E, Korczyn A, Giladi N, Inzelberg R, Djaldetti R, Klein C, Berecz G Rasagiline Study Group. Rasagiline mesylate, a new mao-b inhibitor for the treatment of parkinson's disease: a double-blind study as adjunctive therapy to levodopa. Clin Neuropharmacol. 2000;23:324–330. doi: 10.1097/00002826-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 118.Rascol O. Rasagiline in the pharmacotherapy of Parkinson's disease - a review. Expert Opin Pharmacother. 2005;6:2061–2075. doi: 10.1517/14656566.6.12.2061. [DOI] [PubMed] [Google Scholar]
- 119.Rascol O, Brooks DJ, Melamed E, Oertel W, Poewe W, Stocchi F, Tolosa E the LARGO study group. Rasagiline as an adjunct to levodopa in patients with Parkinson's disease and motor fluctuations (LARGO, Lasting effect in Adjunct therapy with Rasagiline Given Once daily, study): a randomised, double-blind, parallel-group trial. Lancet. 2005;365:947–954. doi: 10.1016/S0140-6736(05)71083-7. [DOI] [PubMed] [Google Scholar]
- 120.Ravina B, Camicioli R, Como PG, Marsh L, Jankovic J, Weintraub D, Elm J. The impact of depressive symptoms in early Parkinson disease. Neurology. 2007;69:342–347. doi: 10.1212/01.wnl.0000268695.63392.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reichmann H, Sommer U, Engfer A. Nebenwirkungsprofil von Parkinson-Medikamenten. Stuttgart: Thieme-Verlag; 2002. [Google Scholar]
- 122.Reidenberg MM. Abuse liability of l-deprenyl: examination of the clinical and preclinical pharmacological data. Clinic Pharmacol Therap. 1994;56:1–796. [Google Scholar]
- 123.Remick R, Froese C, Keller F. Common side effects associated with monoamine oxidase inhibitors. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13:497–504. doi: 10.1016/0278-5846(89)90137-1. [DOI] [PubMed] [Google Scholar]
- 124.Reynolds GP, Riederer P, Sandler M, Jellinger K, Seemann D. Amphetamine and 2-phenylethylamine in post-mortem Parkinsonian brain after (-) deprenyl administration. J Neural Transm. 1978;43:271–277. doi: 10.1007/BF01246964. [DOI] [PubMed] [Google Scholar]
- 125.Richard IH, Kurlan R, Tanner C, Factor S, Hubble J, Suchowersky O, Waters C. Serotonin syndrome and the combined use of deprenyl and antidepressant in Parkinson's disease. Neurology. 1997;48:1070–1077. doi: 10.1212/wnl.48.4.1070. [DOI] [PubMed] [Google Scholar]
- 126.Riederer P. An interdisciplinary approach to the Understanding of normal Behaviour and neuropsychiatric Disorders in particular. In: Ban TA, Healy D, Shorter E, editors. Reflections on twentieth-century Psychopharmacology. Vol. 4. Budapest, Hungary: Animula Publishing House; 2004. pp. 319–327. [Google Scholar]
- 127.Riederer P, Burger R. Ist Schokolade ein psychopharmakon? Die rolle von β-Phenylethylamin als psychostimulus. PPT Heft. 2009;1:26–31. [Google Scholar]
- 128.Riederer P, Danielczyk W, Grünblatt E. Monoamine oxidase-B inhibition in Alzheimer's diease. Neurotoxicology. 2004;25:271–277. doi: 10.1016/S0161-813X(03)00106-2. [DOI] [PubMed] [Google Scholar]
- 129.Riederer P, Konradi C, Schay V, et al. Localization of MAO-A and MAO-B in human brain: a step in understanding the therapeutic action of -Deprenyl. Adv Neurol. 1987;45:111–118. [PubMed] [Google Scholar]
- 130.Riederer P, Lachenmayer L. Selegiline's neuroprotective capacity revisited. J Neural Transm. 2003;110:1273–1278. doi: 10.1007/s00702-003-0083-x. [DOI] [PubMed] [Google Scholar]
- 131.Riederer P, Lachenmayer L, Laux G. Clinicalapplications of MAO-inhibitors. Curr Med Chem. 2004;11:2033–2043. doi: 10.2174/0929867043364775. [DOI] [PubMed] [Google Scholar]
- 132.Riederer P, Youdim MBH. Monoamine oxidase activity and monamine metabolism in brains of parkinsonian patients treated with l-deprenyl. J Neurochem. 1986;46:1359–1365. doi: 10.1111/j.1471-4159.1986.tb01747.x. [DOI] [PubMed] [Google Scholar]
- 133.Riederer P, Youdim MBH, Birkmayer W, Jellinger K. Monoamine oxidase activity during (--)deprenyl therapy: human brain post-mortem studies. Adv Biochem Psychopharmacol. 1978;19:377–382. [PubMed] [Google Scholar]
- 134.Riederer P, Youdim MBH, Rausch WD, Birkmayer W, Jellinger K, Seemann D. On the mode of action of L-deprenyl in the human central nervous system. J Neural Transm. 1978;43:217–226. doi: 10.1007/BF01246958. [DOI] [PubMed] [Google Scholar]
- 135.Robinson DS, Amsterdam JD. The selegiline transdermal systemin major depressive disorder: a systematic review of safety and tolerability. J Affect Disord. 2008;105:15–23. doi: 10.1016/j.jad.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 136.Robinson DS, Gilmor ML, Yang Y, Moonsammy G, Azzaro AJ, Oren DA, Campbell BJ. Treatment effects of selegiline transdermal system on symptoms of major depressive disorder: a meta-analysis of short-term, placebo-controlled, efficacy trials. Psychopharmacol Bull. 2007;40:15–28. [PubMed] [Google Scholar]
- 137.Sampaio C, Ferreira JJ. ADAGIO trial hints that rasagiline slows disease progression. Nat Rev Neurol. 2010;6:126–128. doi: 10.1038/nrneurol.2010.2. [DOI] [PubMed] [Google Scholar]
- 138.Sano I. Biochemistry of the extrapyramidal system. Shinkei Kenkyu no Shimpo. Adv Neurol Sci. 1960;5:42. [PubMed] [Google Scholar]
- 139.Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha tocopherol, or both as treatment for Alzheimer disease. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 140.Schapira A, Albrecht S, Barone P, Comella C, Hsu H, Massey D, et al. the PROUD Study Group. Immediate vs. delayed-start pramipexole in early Parkinson's disease: the PROUD study; Poster 1.278 on the 13th WFN World Congress on Parkinson's Disease and related disorders; 13-15 December; Miami (VS). 2009. [Google Scholar]
- 141.Schwarzschild MA. Rasagiline in Parkinson's Disease. Letter to the editor. N Engl J Med. 2010;362:658. [PubMed] [Google Scholar]
- 142.Shoulson I, Oakes D, Fahn S, Lang A, Langston JW, Kieburtz K, Rudolph A the Parkinson Study Group. Impact of sustained deprenyl (selegiline) in levodopa-treated Parkinson's disease: a randomized placebo-controlled extension of the deprenyl and tocopherol antioxidative therapy in parkinsonism trial. Ann Neurol. 2002;51:604–612. doi: 10.1002/ana.10191. [DOI] [PubMed] [Google Scholar]
- 143.Siderowf A, Stern M. Clinical trials with rasagiline. Neurology. 2006;66:S80–S88. doi: 10.1212/wnl.66.10_suppl_4.s80. [DOI] [PubMed] [Google Scholar]
- 144.Sterling J, Herzig Y, Goren T, Finkelstein N, Lerner D, Goldenberg W, Miskolczi I, Molnar S, Rantal F, Tamas T, Toth G, Zagyva A, Zekany A, Finberg J, Lavian G, Gross A, Friedman R, Razin M, Huang W, Krais B, Chorev M, Youdim MB, Weinstock M. Novel dual inhibitors of AChE and MAO derived from hydroxy aminoindan and phenethlamine as potential treatment for Alzheimer's disease. J Med Chem. 2002;45:5260–5279. doi: 10.1021/jm020120c. [DOI] [PubMed] [Google Scholar]
- 145.Steur EN, Ballering LA. Moclobemide and selegeline in the treatment of depression in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1997;63:547. doi: 10.1136/jnnp.63.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Szelenyi I, editor. Inhibitors of monoamine oxidase B. Pharmacology and Clinical Use in Neurodegenerative Disorders. Basel/Switzerland: Birkhäuser Verlag; 1993. [Google Scholar]
- 147.Tariot PN, Cohen RM, Sunderland T, Newhouse PA, Yount D, Mellow AM, Weingartner H, Mueller EA, Murphy DL. L-deprenyl in Alzheimer's disease. Preliminary evidence for behavioral change with monoamine oxidase B inhibition. Arch Gen Psychiatry. 1987;44:427–433. doi: 10.1001/archpsyc.1987.01800170041007. [DOI] [PubMed] [Google Scholar]
- 148.Thebault JJ, Guillaume M, Levy R. Tolerability, safety, pharmacodynamics, and pharmacokinetics of rasagiline: a potent, selective, and irreversible monoamine oxidase type B inhibitor. Pharmacotherapy. 2004;24:1295–1305. doi: 10.1592/phco.24.14.1295.43156. [DOI] [PubMed] [Google Scholar]
- 149.Thomas CE, Huber EW, Ohlweiler DF. Hydroxyl and peroxyl radical trapping by the monoamine oxidase-B inhibitors deprenly, MDL 72,974A: implications for protection of biological substrates. Free Radic Biol Med. 1997;22:733–737. doi: 10.1016/s0891-5849(96)00402-9. [DOI] [PubMed] [Google Scholar]
- 150.Varga E, Tringer L. Clinical trial of a new typepromptly acting psychoenergic agent (phenylisopropyl methyl-porpinyl-HC1, E-250) Acta Med Acad Shi Hung. 1967;23:189–295. [PubMed] [Google Scholar]
- 151.Veazey C, Aki SO, Cook KF, Lai EC, Kunik ME. Prevalence and treatment of depression in Parkinson's disease. J Neuropsychiatry Clin Neurosci. 2005;17:310–323. doi: 10.1176/jnp.17.3.310. [DOI] [PubMed] [Google Scholar]
- 152.Versiani M, Mardi A, Figueira I, et al. Tolerability ofmoclobemide, a new reversible inhibitor of monoamine oxidase-A, compared with other antidepressants and placebo. Acta Psychiatr Scand Suppl. 1990;360:24–28. doi: 10.1111/j.1600-0447.1990.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 153.Weinreb O, Amit T, Youdim MBH. Rasagiline; a monoamine oxidase B inhibitor and neuroprotective anti-parkinson drug. Progress in Neurobiology. 2010;92:330–344. doi: 10.1016/j.pneurobio.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 154.Wermuth L, Bech P. Depression in Parkinson's disease. A review. Acta Neurol Scand. 2006;114:360–380. doi: 10.1111/j.1600-0404.2006.00718.x. [DOI] [PubMed] [Google Scholar]
- 155.Yasar S, Justinova Z, Lee SH, Stefanski R, Goldberg SR, Tanda G. Metabolic transformation plays a primary role in the psychostimulant-like discriminiative-stimulus effects of selegiline [(R)-(-)deprenyl] J Pharmacol Exp Ther. 2006;317:387–394. doi: 10.1124/jpet.105.096263. [DOI] [PubMed] [Google Scholar]
- 156.Yasar S, Winger G, Nickel B, Schulze G, Goldberg SR. Preclinical Evaluation of l-Deprenyl: Lack of Amphetamine-Like Abuse Potential. In: Szelanyi I, editor. Inhibitors of monoamine oxidase B. Pharmacology and Clinical Use in Neurodege-nerative Disorders. Basel/Switzerland: Birkhäuser Verlag; 1993. pp. 216–233. [Google Scholar]
- 157.Yogev-Falach M, Amit T, Bar-Am O, Weinstock M, Youdim MBH. The involvement of mitorgen-activated protein (MAP) kinase in the regulation of amyloid precursor protein processing by novel cholinesterase inhibitors derived from rasagiline. FASEB J. 2002;16:1674–1676. doi: 10.1096/fj.02-0198fje. [DOI] [PubMed] [Google Scholar]
- 158.Yogev-Falach M, Bar-Am O, Amit T, Weinstock M, Youdim MBH. A multifunctional, neuroprotective drug, ladostigil (TV3326), regulates holo-APP translocation and processing. FASEB J. 2006;20:2177–2179. doi: 10.1096/fj.05-4910fje. [DOI] [PubMed] [Google Scholar]
- 159.Youdim MB. My love with monoamine oxidase, iron and Parkinson's disease. J Neural Transm Suppl. 2006;71:V–IX. [PubMed] [Google Scholar]
- 160.Youdim MBH. Rasagiline in Parkinson's Disease. Letter to the editor. N Engl J Med. 2010;362:657–658. doi: 10.1056/NEJMc0910491. [DOI] [PubMed] [Google Scholar]
- 161.Youdim MB, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- 162.Youdim MBH, Tipton KF. Rat striatal monoamine oxidase-B inhibiton by l-deprenyl and rasagiline: its relationship to 2-phenylethylamine-induced stereotypy and Parkinson's disease. Parkinsonism Relat Disord. 2002;8:247–253. doi: 10.1016/s1353-8020(01)00011-6. [DOI] [PubMed] [Google Scholar]
- 163.Zornberg GL, Bodkin JA, Cohen BM. Severe adverse interaction between pethidine and selegiline. Lancet. 1991;337:246. doi: 10.1016/0140-6736(91)92219-r. [DOI] [PubMed] [Google Scholar]