Abstract
Background:
To estimate the efficacy of daily administration of 25 mg mifepristone for the treatment of uterine leiomyoma.
Materials and Methods:
A total of 30 women were to receive 25 mg mifepristone daily for a period of 6 months. Abdominal ultrasonography was performed before treatment, at 3 months and after 6 months, to evaluate the leiomyoma size and uterine volume. Endometrial biopsy was done after the treatment. Efficacy was estimated by the reduction in leiomyoma size, uterine volume, and improvement in quality of life.
Results:
After 180 days of treatment, there was a 47% decrease in the leiomyoma volume and a 53% decrease in the uterine volume. Symptomatic improvement was noted. Twenty-three of 30 women (75.7%) became amenorrheic after the treatment. Endometrial biopsy after treatment revealed simple hyperplasia in two of 30 women.
Conclusion:
25 mg mifepristone produces reduction in leiomyoma size and uterine volume and produces symptomatic improvement in women with fibroids.
Keywords: Fibroid, leiomyoma, mifepristone
INTRODUCTION
Uterine leiomyomata are the most common benign tumors in women and nearly half of all women aged 35 to 49 years have uterine leiomyomata.1 They present with severe bleeding per vaginam leading to iron deficiency anemia.2 Approximately 7% of leiomyomata report moderate to severe pain abdomen.3 Leiomyoma uteri is the most common indication for hysterectomy which accounts for a large number admissions to hospitals. Availability of a safe and effective nonsurgical treatment of symptomatic leiomyomata would be of considerable clinical and public health importance.4
Mifepristone, synthesized from its precursor norethindrone, competitively binds and inhibits progesterone receptors.5 Our study was designed to see the effects of mifepristone on leiomyomata, the regression in size of uterus, the improvement of symptoms including the correction of anemia, cessation of menses, and the side effects of mifepristone.
MATERIALS AND METHODS
This study was a prospective clinical trial conducted in the Gynaecology clinic of Medical College, Kolkata, between July and December, 2009 for a period of 6 months. The study included 30 women of reproductive and perimenopausal age group (20-42 years) of single as well as multiple parity. These women had varied presentation, most commonly being menorrhagia and abdominal pain. None of them were desirous of pregnancy and had agreed to use nonhormonal methods of contraception. Monthly log of all episodes of vaginal bleeding during the course of treatment was noted. A baseline ultrasonography, if it showed a uterine volume on TAS >160 ml or at least one leiomyoma of 2.5 cm, was included in the study. A repeat USG after 6 months was done. The side effects of the drug were also assessed.
Women desirous to have children or users of hormonal contraceptives or other long-acting hormones/Gonadotropin releasing hormone (GnRH) analogues up to 6 months prior to the onset of the study were excluded from the study. Also, pre-existing hepatic, renal, thyroid disorders and abnormal vaginal bleeding were the other exclusion criteria.
The study was approved by the Ethical and Scientific committee, MCH, Kolkata. All the women gave a written consent. The subjects were given 25 mg of a mifepristone preparation (it comes as 200 mg tablet, so 1/8th of the tablet was given daily) for 6 months. Besides mifepristone, the subjects were allowed to take analgesics; the type and amount consumed were recorded in the monthly logs. Menstrual blood loss was assessed by pictorial charts.
A monthly blood loss index was calculated from menstrual history by assigning values 1–4 to each day of spotting, indicating light, moderate, and heavy flow, respectively, and then summing the results. The uterine fibroid symptom Quality of life scale 1-100,6 with higher scores indicating better quality of life, was used to measure perceived impact of leiomyomata on day-to-day activities. Each of these questionnaire was administered at baseline and then monthly. Monthly assessments of the presence and intensity of likely leiomyoma symptoms (including pelvic pain, pelvic pressure, urinary frequency, low back pain, and pain during intercourse) were done. The drug side effects (including nausea, vomiting, diarrhea, headache, hot flushes, and weight gain) were assessed.
Uterine volume and leiomyoma size and number were assessed by abdominal USG at baseline 3 months and 6 months. The uterus was measured in three planes and total volume was calculated. Monthly hemoglobin levels and pregnancy test were done. Liver function test (LFT) was done at baseline, 3rd and 6th months. All participants underwent endometrial biopsy at baseline and 6-month follow-up.
RESULTS
A total of 30 women met all the eligibility criteria and consented during the allotted tenure. None of the subjects dropped out during the course of the study.
Their ages ranged between 20 and 45 years, and the highest number of subjects belonged to the age group of 30 to 35 years [Figure 1].
Figure 1.
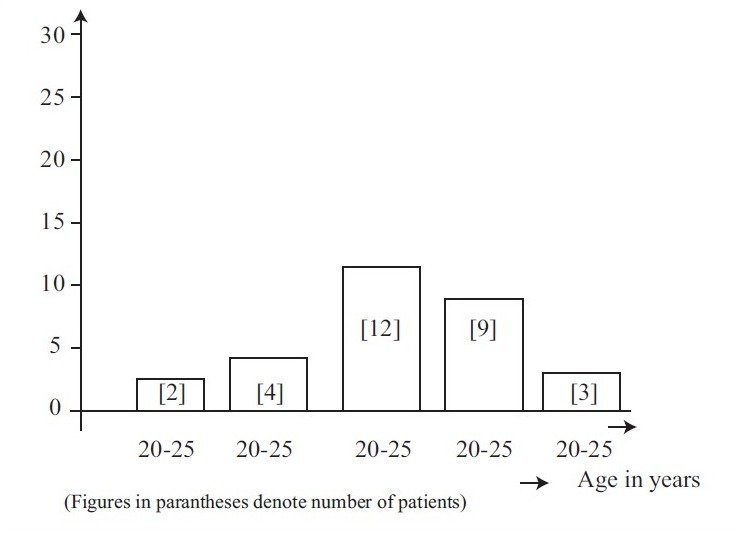
Age distribution
There was an inverse relationship between parity and occurrence of fibroid. The higher the parity, the lesser the incidence of fibroid. In the present study, 16 of 30 women were nulliparous.
Pelvic pain and pelvic discomfort was the commonest presentation in 30 patients, 13 complained of dysmenorrhea, vague dragging lower abdominal pain and 11 of 30 women presented with menorrhagia.
In our study, the size of fibroid varied from being not palpable per abdomen up to 20 weeks uterine size.
Treatment with mifepristone resulted in marked reduction of bleeding. By the sixth month, 23 of 30 (75.5%) women became amenorrheic. Anemia (defined as Hb levels below 12.0° g/dl) were present in 24 of 30 (80%) women at baseline and after six months of treatment, two of 30 (6.6%) remained anemic [Figure 2].
Figure 2.
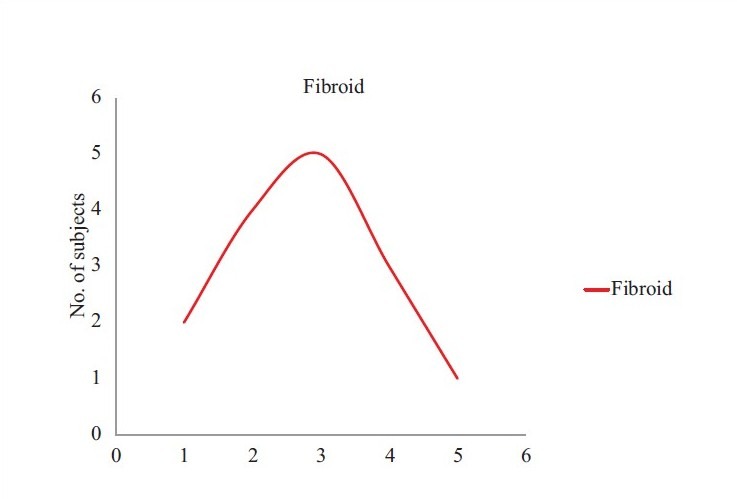
Graphical representation of increase in haemoglobin percentage after treatment
The subjects reported decrease in pain [Figure 3]. Uterine volume decreased an average of 160 ml among the subjects by the end of 6 months [Figure 4]. Symptoms of pelvic pain, urinary frequency, low back pain, and dyspareunia also showed improvements. The primary outcome was mean change in leiomyoma-specific overall quality of life scale, 1-100, with higher scores indicating better quality of life.
Figure 3.
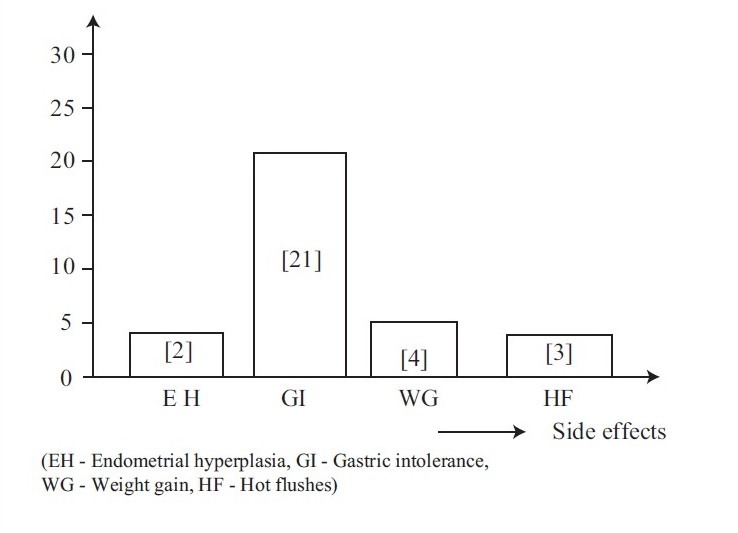
Graphical representation of side effects
Figure 4.
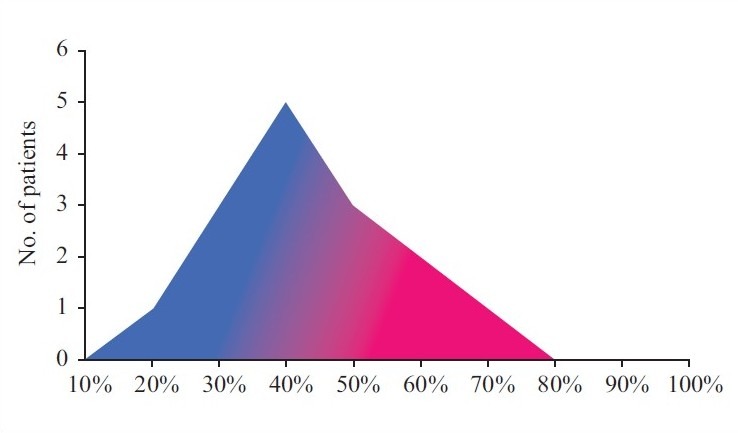
Graphical representation of decrease in mean uterine volume
DISCUSSION
Leiomyomata contain both estrogen and progesterone receptors7 and they are more in number in fibroids when compared with the myometrium.8 Since leiomyoma has ovarian steroid dependency, we reasoned out that mifepristone may have an inhibitory effect on growth of leiomyomata.
The effects of mifepristone on uterine leiomyomata have been studied worldwide in several clinical trials; mifepristone can be compared with GnRH agonists in terms of efficiency.9 The dosage of mifepristone used in multiple studies ranged from 5 to 50 mg and were either used solely to manage leiomyomata or used prior to surgery10 to decrease the size. It still remains to be established as to what the optional dosage should be to be effective for treatment of leiomyomata. In a systematic review of six studies, it was found that mifepristone decreased the size of fibroid considerably and decreased the symptoms namely menorrhagia, dysmenorrhea, and pelvic pressure.11
In our study, we observed that treatment with mifepristone 25 mg daily for 6 months substantially decreased bleeding, uterine volume, and size of fibroid in symptomatic cases and improved the quality of life for these women. The drug was well tolerated by the women as evidenced by no dropouts. There were no major side effects too. The effects of mifepristone in decreasing bleeding and size of fibroids are consistent with previous studies of the drug.12 Previous trials suggested that maintenance of symptomatic improvement and reduction in leiomyoma volume was seen up to one year.13
Further studies are required to determine whether benefits of the drug given for 6 months will be sustained if the drug is continued and so, it is yet to be determined for how long and whether any serious side effects emerge or not by its prolonged use.
In conclusion, treatment of women with symptomatic leiomyoma using low-dose mifepristone for 6 months results in decrease in leiomyoma size and bleeding; improves the quality of life for these women. It requires larger sample size to comment about the long-term effects of mifepristone as to how long the benefits will be sustained or whether the drug is safe for a long time administration.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Day BD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–7. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 2.Wegeinke G, Bavid DD, Hertz Picciotto I, Harlow SD, Steege JF, Hill MC, et al. Self reported heavy bleeding associated with uterine leiomyomata. Obstet Gynecol. 2003;101:431–7. doi: 10.1016/s0029-7844(02)03121-6. [DOI] [PubMed] [Google Scholar]
- 3.Lippman SA, Warner M, Samuels S, Olive D, Vercellini P, Eskenazi B. Uterine fibroids and gynecologic pain symptoms in a population based study. Fertil Steril. 2003;80:1488–94. doi: 10.1016/s0015-0282(03)02207-6. [DOI] [PubMed] [Google Scholar]
- 4.Becker ER, Spalding J, Duchane J, Horowitz IR. Inpatient surgical treatment patterns for patients with uterine fibroids in the United States, 1998-2002. J Nat Med Assoc. 2005;97:1336–42. [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy AA, Castellano P. RU486: Pharmacology and potential use in the treatment of endometriosis and leiomyomata uteri. Curr Opin Obstet Gynecol. 1994;6:269–78. [PubMed] [Google Scholar]
- 6.Fiscella K, Eisinger SH, Meldrum S, Feng C, Fisher SG, Guzick DS. Effect of mifepristone for symptomatic leiomyomata on quality of life and uterine size: A randomized controlled trial. Obstet Gynecol. 2006;108:1381–7. doi: 10.1097/01.AOG.0000243776.23391.7b. [DOI] [PubMed] [Google Scholar]
- 7.Wilson EA, Yang F, Rees ED. Estriadol and progesterone binding in uterine leiomyomata and in normal uterine tissue. Obstet Gynecol. 1980;55:20–4. [PubMed] [Google Scholar]
- 8.Soules MR, McCasty KS. Leiomyomatas: Steroid Receptor content. Am J Obstet Gynecol. 1982;143:6–11. doi: 10.1016/0002-9378(82)90676-7. [DOI] [PubMed] [Google Scholar]
- 9.Malartic C, Morel O, Allerman G, Tulpin L, Desfeux P, Barranger E. Role of mifepristone for the treatment of uterine Fibroid. Gynecol Obstet Fertil. 2008;36:668–74. doi: 10.1016/j.gyobfe.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Engman M, Granberg S, Williams AR, Meng CX, Lalitkumar PG, Gemzell-Danielsson K. Mifepristore for treatment of uterine leiomyoma. A prospective randomized placebo controlled trial. Hum Reprod. 2009;24:1870–9. doi: 10.1093/humrep/dep100. [DOI] [PubMed] [Google Scholar]
- 11.Steinauerer J, Pritts EA, Jackson R, Jacoby AF. Systematic Review of mifepristone for the treatment of uterine Leiomyomata. Obstet Gynecol. 2004;103:1331–6. doi: 10.1097/01.AOG.0000127622.63269.8b. [DOI] [PubMed] [Google Scholar]
- 12.Murphy AA, Kettel LM, Morale AJ, Roberts NJ, Yen SS. Regression of uterine leiomyomata in response to the antiprogesterone RU486. J Clin Endocrinol Metab. 1993;76:313–7. doi: 10.1210/jcem.76.2.8432797. [DOI] [PubMed] [Google Scholar]
- 13.Eisenger SH, Bonfiglio T, Fiscella K, Meldrum S, Guzick DS. Twelve month safety and efficiency of low dose Mifepristone for uterine myomas. J Minim Invasive Gynecol. 2005;12:227–33. doi: 10.1016/j.jmig.2005.01.022. [DOI] [PubMed] [Google Scholar]


