Abstract
Background:
To determine the normal dimensions of spleen by ultrasonography in our environment exposed to endemic tropical infection and infestation.
Materials and Methods:
A prospective study of normal spleen ultrasound-based measurements in 200 Nigerian adults at the University of Benin Teaching Hospital Benin, Nigeria.
Results:
There were 91 males and 109 females; their age ranged between 20 and 60 years. For the males the mean age was 32.4 years (± 9.2 SD), mean height was 175.0 cm (±7.3 SD), mean weight was 72.5 kg (±10.1 SD), and mean body mass index was 23.6 (±2.8 SD) and the females the mean age was 29.7 years (±9.0 SD) mean height was 164.6 cm (±5.8 SD), mean weight 64.1 kg (±12.9 SD), and mean BMI was 24.9 (±1.4 SD). For the males the mean splenic length, width, depth, and volume were 11.1 cm (±0.9 SD), 4.4 cm (±0.5 SD), 7.8 cm (±0.6 SD), and 202.7 cm3 (±49.4 SD), respectively. For the females the corresponding values of splenic length, width, depth, and volume were 10.1 cm (±0.7 SD), 4.0 cm (±0.4 SD), 7.1 cm (±0.5 SD), and 153.7 cm3 (±33.2 SD), respectively.
Conclusion:
Comparison between mean splenic dimension parameters for males and females (from unpaired t-test determination) showed a statistically significant difference (P<0.001 for splenic length, width, depth, and volume). There was also statistically significant increasing value correlation between subjects’ weight and height (in favor of height) when compared to spleen length, width, depth, and volume. The other parameters show no significant correlation in both female and male. In particular there was also no statistically significant correlation of splenic measurements with age in either sex. This is similar to what was noted in other centers.
Keywords: Dimension, exposure, spleen, tropical environment, ultrasound
INTRODUCTION
The spleen is the largest organ in the reticuloendothelial system. A number of disorders are accompanied by altered spleen size, including infective, infestation, infiltrative, immunologic, and malignant conditions. In the environment of this study, our subjects are known to have been exposed to different topical infection and infestation, such as tuberculosis, malaria parasites, and poor sanitary conditions. The authors undertook this study, in order to determine whether, this peculiar tropical condition, could have any significant effect on the normal dimension of the spleen.
The spleen is an intraperitoneal organ located posterolaterally in the left hypochondrium between the fundus of the stomach and the left hemidiaphragm. In the supine position, the long axis of the spleen is in line with the tenth rib, but in the upright position it is more vertical. Its extreme or superior angle lies approximately 4 cm from the tenth thoracic spine and its lateral border at the mid-axillary line in the ninth intercostal space. The shape of the spleen is characteristically tetrahedral, but may be modified when enlarged. The splenic hilum is the only portion that is not covered by peritoneum, but here peritoneal reflections carry the main splenic vessels, the splenic arteries and veins.
The spleen though not firmly anchored in the body, is attached to the stomach by the gastrolineal ligament and to the dorsal body wall by the lienorenal ligament. The phrenicocolic ligament which is not attached directly to the spleen supports its inferior end. These attachments allow the spleen to enlarge as much as ten times and to shift to ectopic locations. A connective tissue capsule covers the spleen and projects fibres (trabeculae) into its pulp. The peritoneum covers the capsule.
During life, the spleen is a soft, purple colored organ which is considerably larger than in most cadavers.1 Normally, the spleen does not extend inferior to the left costal margin; hence a normal spleen is seldom palpable through the anterolateral abdominal wall.1 It varies in size and shape but it is usually 12 cm long, 5 cm thickness, and 7 cm wide.1 As a result of increased appreciation of the spleens function in the body's defense against disease, splenectomy is no longer performed as hastily as in the past.2
As a clinical guideline, the distance that the spleen extends below the left costal margin is often used to monitor spleen size, but clinical examination of splenic size is notoriously inaccurate.3 In a study evaluating spleen size in patients with sarcoidosis, splenomegaly was present in 57% of the patients (using sonographic criteria to evaluate size) but only clinically palpable in 8%.4 The range in reported sensitivity of assessing splenomegaly by palpation of the abdomen varies from 50% at best to the more realistic estimate of 17%.5 In addition, mild splenomegaly may be difficult to identify by clinical examination. Hosey et al. in a study of a healthy collegiate athletic population found that spleen size was larger in men and white athletes than in women and black athletes.
Prior to the development of sonography, size, particularly the measurement of radiographic spleen length was obtained from plain radiography. A potential cause of inaccuracy and drawback of this method was that plain radiographs give a composite shadow which may not be entirely the spleen.6
It has been found that splenic size is better evaluated by ultrasonography, computed tomography or magnetic resonance imaging than by plain radiography.6
Several studies have sought to develop standards for splenic size, utilizing a variety of imaging techniques such as computed tomography, scintigraphy, magnetic resonance imaging, and sonography.7–18 Volumetric measurements are most accurately obtained on computed tomography or magnetic resonance imaging.6,14 Nevertheless routine computed tomography for the diagnosis and serial follow-up of patients for suspected splenic enlargement is difficult to justify in view of the radiation exposure (especially in a pediatric or adolescent population) and the expense in our environment. The use of magnetic resonance imaging is similarly hampered by expense and limited availability in many areas of the world, particularly in developing countries.
Ultrasonography affords a useful noninvasive role in evaluating the spleen and used for best advantage, it can demonstrate the existence and composition of splenic masses, disruption of splenic texture or outline, progressive changes in masses and the size of the spleen. The aims of this study are to assess and document the splenic sizes in asymptomatic adults in a Nigerian population (Benin environment) and thereby serve as a baseline for comparison in cases of splenomegaly using transabdominal sonography. This study will also serve as a guide in management and follow up of such cases. The finding will be compared to what is obtained elsewhere, bearing in mind the peculiarity of our environment.
MATERIALS AND METHODS
This study comprised of prospective consecutive ultrasonic assessment of splenic sizes in 200 asymptomatic adult subjects (91 males and 109 females) who came to the Department of Radiology of the University of Benin Teaching Hospital Benin City for routine chest radiographs as part of their medical tests pre employment and preadmission into tertiary institutions. Benin City, a metropolitan community in Nigeria, West Africa is inhabited by diverse Nigerian tribes and the residents are exposed to tropical endemic diseases such as malaria, tuberculosis and water bone parasites. The period of the study was between 14th of March and 22nd of June 2005.
The center for the evaluation was the department of Radiology, University of Benin Teaching Hospital, Benin City and a Sonoace 1500 (Medison Corporation, South Korea 1998) ultrasound machine was used. The scan probe used was a curvilinear real time probe, with scan frequency of 3.5 mega Hertz (MHz).
Informed consent was obtained from the patients before being used for the study. The reason for the study, possible effects, and stages of examination was explained to the subjects as a group or individually.
The patients were reassured psychologically and made to relax before the investigation. Thereafter, the patient was asked to lie supine on the couch, with arms away from the chest wall; and instructed to take as shallow breaths as possible. Where necessary, scanning with the subject in deep inspiration was done to move the spleen from under the ribs.
Scanning using the 3.5 MHz ultrasound probe was done along the lower left costal margin from the ninth to eleventh ribs at the anterior, mid, and posterior axillary lines with the subject in the right lateral decubitus position and where necessary in the supine position. The intercostal spaces were used as scan window, for proper visualization of the entire spleen. All measurements were made on sections through the splenic hilum in order to create a constant reference point for repeating measurement according to the guidelines of the American Institute of Ultrasound in Medicine12 and as described by Lamb et al.19 Splenic length (the maximum distance between the dome of the spleen and the splenic tip) was done on the longitudinal section. Splenic width defined as the maximum distance between the medial and lateral borders of the spleen was measured on a plane perpendicular to the length.
Transverse scans were obtained with the transducer rotated through 90°. Splenic depth defined as the maximum antero-posterior dimension was measured on the transverse section.
Each dimension was rescanned and recorded three different times to the nearest millimeter and the median value obtained for accuracy of result. The volumes were calculated manually from the formula for a prolate ellipse.
Vital data obtained included age, sex, height, weight, occupation, and ethnicity.
The following subjects were excluded from the study:
Subjects with pathologies potentially involving the spleen.
Subjects with hemoglobinopathies.
Subjects with skin infections at the area of the spleen.
Subjects in whom the entire length of the spleen could not be properly documented and those with previous splenectomy.
Subjects with lymphoproliferative disorders such as lymphomas, leukemias, etc.
Subjects with focal lesions and nonuniform parenchyma.
Subjects who had fever either at the time of the scan or within at least four weeks prior to the scan
Gravid women.
Data analysis
The axial measurements of the spleen and the volume were compared with the sex, height, weight, and body mass index of the various subjects to determine if variations exist.
Data analysis was carried out using statistical package called InStat (GraphPad Inc, USA) and Statistical Package for Social Sciences (SPSS). Data comparison (statistical test of significance) was done with Chi-square test and Mann–Whitney test.
At 95% interval, two-tailed P-values less than or equal to 0.05 were considered to be statistically significant.
RESULTS
The age range of subjects in this study was between 20 and 60 years. Age group 25-29 had the highest number of male subjects made up of 28 males and age group 20-24 had the highest number of female subjects made up of 35 females.
For the males the mean age was 32.4 years (±9.2 SD), mean height was 175.0 cm (±7.3 SD), mean weight was 72.5 kg (±10.1 SD), and mean body mass index (BMI) was 23.6 (±2.8 SD). For the females the mean age was 29.7 years (±9.0 SD) mean height was 164.6 cm (±5.8 SD) mean weight 64.1 kg (±12.9 SD), and mean BMI was 24.9 (±1.4 SD).
Comparison between mean splenic dimension parameters for males and females (from unpaired t-test determination) showed a statistically significant difference (P<0.001 for splenic length, width, depth, and volume).
Table 1 shows height against mean splenic dimension of subjects.
Table 1.
Height group distribution of splenic measurements in males and females
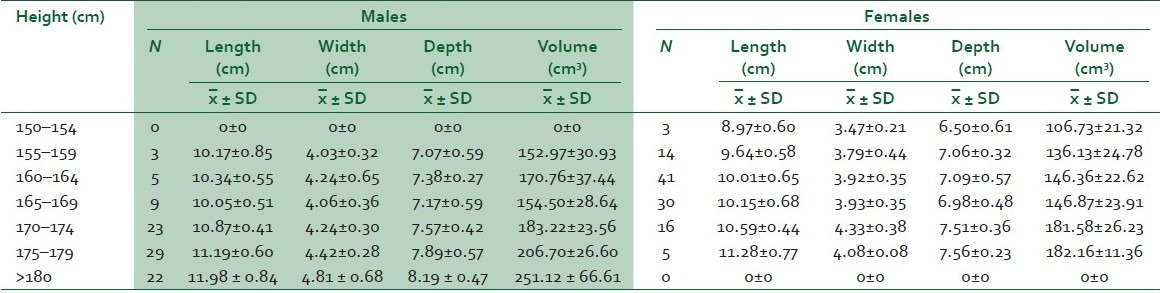
For the male subjects there was no statistically significant correlation between age of subjects and splenic length, width, or volume but there was significant correlation with splenic depth (r=0.234, P<0.01), which decreased with age.
There was also statistically significant correlation between subject weight and spleen length (r=0.405, P<0.001), width (r=-0.260, P<0.01), depth (r=0.394, P<0.001), and volume (r=0.402, P<0.001).
There was no statistically significant correlation between body mass index and mean splenic dimensions (r=–0.090, –0.119, 0.026, and –0.044 for spleen length, width, depth, and volume, respectively).
For the female subjects there was no statistically significant correlation between the age of the subjects and mean splenic dimensions (r=0.110, 0.204, 0.205, and 0.033 for spleen length, width, depth, and volume, respectively).
There was a statistically significant correlation between subject weight and mean splenic length (r=0.416, P<0.001), width (r=0.302, P<0.01), depth (r=0.221, P<0.01), but no statistically significant correlation with volume (r=0.173, P>0.05).
There was no statistically significant correlation between body mass index and mean splenic measurements (r=0.131, 0.131, 0.002, and 0.050 for splenic length, width, depth, and volume, respectively).
Regression coefficient of splenic length against age for males calculated with Pearson's product moment correlation was –0.172. With 89 degrees of freedom the critical r value is 0.205. There was thus no statistically significant correlation between splenic length and age for males.
For females, regression coefficient of splenic length against age calculated with Pearson's product moment correlation was 0.101. With 107 degrees of freedom the critical r value is 0.190. There was thus no statistically significant correlation between splenic length and age for females. There was also no statistically significant correlation of other splenic measurements with age in either sex.
Using Pearson's correlation coefficient (r) of splenic length against subject height for males, height correlated strongly with spleen length (r=0.784, P<0.001) [Figure 1].
Figure 1.
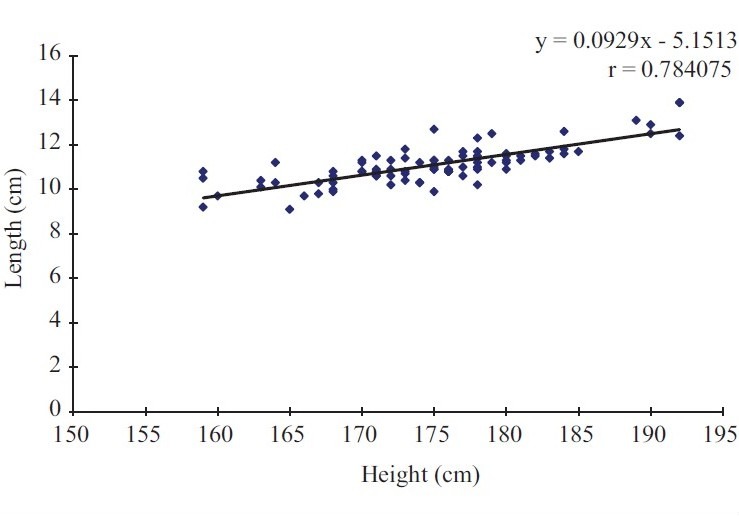
Scatter plot of spleen length (cm) against subject height (cm) for males
There was also statistically significant correlation between subject height and splenic width (r=0.595, P<0.001), depth (r=0.607, P<0.001), and volume (r=0.723, P<0.001). The strongest correlation was with splenic length, followed by the volume, depth, and slightly weaker correlation with splenic width.
The weight of the male subjects correlated with splenic length (r=0.400, P<0.001), depth (r=0.394, P<0.001) but showed a weaker correlation with width (r=0.260, P=0.013).
Using Pearson's correlation coefficient (r) of subject height with spleen length for females, height correlated with spleen length (r=0.545, P<0.001) [Figure 2].
Figure 2.

Scatter plot of spleen length (cm) against subject height (cm) for females
For the females, subject height correlated with splenic width (r=0.393, P<0.001), depth (r=0.278, P=0.003) and volume (r=0.290, P=0.002). The strongest correlation was with splenic length, followed by the width, the depth, and the volume.
Subject weight correlated with spleen length (r=0.438, P<0.001), width (r=0.302, P<0.01), but less with depth (r=0.221, P=0.021).
For all splenic measurements, there was a stronger correlation with subject height than with their weight for the males and females. In addition, for all splenic measurements, splenic length showed the strongest correlation with subject height than did the other splenic dimension parameters in both sexes.
There was a stronger correlation between subject height and respective splenic dimensions for the males than females.
There was also a stronger correlation between subject weight and splenic measurements for the males than females, except splenic width which correlated more strongly in females.
DISCUSSION
Ultrasound measurement of splenic length is standard practice, and it provides a very useful means of non-invasive examination of the spleen.6 Kluhs et al.20 in a study to investigate splenic weight determined sonographically and the weight of the spleen measured at autopsy or after splenectomy found that a significant correlation existed.
This study has shown the following mean dimensions of splenic sizes, for the males; the mean splenic length, width, depth, and volume were 11.1 cm (±0.9 SD), 4.4 cm (±0.5 SD), 7.8 cm (±0.6 SD), and 202.7 cm3 (±49.4 SD), respectively, and for the females the corresponding values of splenic length, width, depth and volume were 10.1 cm (±0.7 SD), 4.0 cm (±0.4 SD), 7.1 cm (±0.5 SD) and 153.7 cm3 (±33.2 SD), respectively. The height and weight of the subjects, for both male and female, respectively, increased linearly with the splenic dimensions. This was noted more prominently with the height of subjects. This was not different from other studies in different continents, where the peculiar endemicity in our environment does not exist. Loftus et al.21 in their study of a Chinese population suggested an upper limit of normal length of 12 cm. Some textbooks of ultrasound and other studies suggested an average splenic length of 12 cm, average width of 5 cm, and average depth (antero-posterior dimension) of 7 cm.12,22–25 These observations suggest that there is no significant racial bias of spleen size in this study as compared with Caucasians. This is similar to results obtained in other studies. Mustapha et al.16 in a study of an adult African population found mean spleen volumes that were smaller than data from Western sources and this could not be attributed to difference in body habitus. Okoye et al.25 found good correlation between subject height and splenic length. Spielman et al.3 also found a good correlation between subject height and spleen length in their study population which consisted of tall healthy athletes (r=0.4 for males and 0.3 for females). Splenic volumetric index has also been determined using ultrasound scanning by Pietri et al.26
Malaria, tuberculosis, and other water borne parasitic infections are a major public health concern in Nigeria. According to recent estimates, half of the Nigerian population has at least one episode of malaria annually, and majority of outpatient visits can be attributed to malaria.27 This is similar to exposure to tuberculosis and water borne parasitic infection. This by extrapolation may have exposed the subjects to immunological memory. The spleen is a reticuloendothelial organ involved in defense against infection and infestation and thus it is expected that the spleen may be slightly comparatively larger in exposed subjects than to what is obtained in nonexposed subjects. However, there was no statistically significant correlation of splenic measurements with age in either sex in our study.
From this study, malaria endemicity in Nigeria and other endemic infection and infestation appears not to have any significant effect on spleen sizes. The finding by Chauhan et al.28 suggested instead that spleen size was significantly decreased in people who had been affected by Plasmodium falciparium malaria.
Loftus et al.21 in a similar study in Hong Kong investigated the correlation between sonographic measurement of splenic length, volume and weight. Sonographic measurements before autopsy were obtained in 30 cadavers and these values were compared with the actual length, volume and weight of the spleen at autopsy. They found clear linear correlation between maximum sonographic length and actual length, volume and weight. The study showed that a single simple sonographic measurement gives clinically useful indication of true splenic size. Also in a study in Brazil, Rodrigues et al.22 examined 32 morphologically normal spleens from adult corpses and found a roughly linear correlation between actual spleen volume (y) and ultrasound spleen volume (x) (i.e. y=14.23 + 0.469x).
This study has been able correlate the baseline ultrasonic splenic dimensions in our environment to others elsewhere, and found no remarkable difference irrespective of our environmental peculiarities.
CONCLUSION
Normal splenic dimension was determined in our environment, with the known environmental peculiarity sited, but no significant difference was found in comparison with other studies sited without our kind of environmental peculiarity. This is suggesting immunological defence mechanism in our body does not have residual effect on splenic dimensions.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Moore KL. Clinically oriented anatomy. 3rd ed. USA: Williams and Wilkins; 1992. pp. 184–7. [Google Scholar]
- 2.Berman M. Spleen. In: Kawamura DM, editor. Diagnostic medical sonography: A guide to clinical practice. Abdomen and superficial structures. 2nd ed. Philadelphia: Lippincott-Raven; 1997. pp. 263–88. [Google Scholar]
- 3.Spielman AL, DeLong DM, Kliewer MA. Sonographic evaluation of spleen size in tall healthy athletes. AJR Am J Roentgenol. 2005;184:45–9. doi: 10.2214/ajr.184.1.01840045. [DOI] [PubMed] [Google Scholar]
- 4.Kataoka M, Nakata Y, Maeda T, Hosoya S, Nishizaki H, Ono Y, et al. Ultrasonographic analysis of splenomegaly in patients with sarcoidosis. Nihon Kyobu Shikkan Gakkai Zasshi. 1990;28:750–5. [PubMed] [Google Scholar]
- 5.Dommerby H, Stangerup M, Hancke S. Hepatosplenomegaly in infectious mononucleosis: Assessed by ultrasonic scanning. J Laryngol Otol. 1986;100:573–9. doi: 10.1017/s0022215100099680. [DOI] [PubMed] [Google Scholar]
- 6.Dick R. The liver and spleen. In: Sutton D, editor. Text book of Radiology and Imaging. London: Churchill Livingstone; 1998. pp. 981–1028. [Google Scholar]
- 7.Konus OL, Ozdemir A, Akkaya A, Erbas G, Celik H, Isik S. Normal liver, spleen and kidney dimensions in neonates, infants and children: Evaluation with sonography. AJR Am J Roentgenol. 1998;171:1693–8. doi: 10.2214/ajr.171.6.9843315. [DOI] [PubMed] [Google Scholar]
- 8.Friis H, Ndhlovu P, Mduluza T, Kaondera K, Franke D, Vennervald BJ, et al. Ultrasonographic organometry: Liver and spleen dimensions among children in Zimbabwe. Trop Med Int Health. 1996;1:183–90. doi: 10.1111/j.1365-3156.1996.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 9.Dittrich M, Milde S, Dinkel E, Baumann W, Weitzel D. Sonographic biometry of liver and spleen size in childhood. Pediatr Radiol. 1983;13:206–11. doi: 10.1007/BF00973157. [DOI] [PubMed] [Google Scholar]
- 10.Al-Imam O, Suleiman A, Khuleifat S. Ultrasound assessment of normal splenic length and spleen-to-kidney ratio in children. East Mediterr Health J. 2000;6:514–6. [PubMed] [Google Scholar]
- 11.Haddad-Zebouni S, Hindy R, Slaba S, Aoun N, Mourani C, Abi Ghanem S, et al. Ultrasonographic evaluation of the kidney, Liver and spleen size in children. Arch Pediatr. 1999;6:1266–70. doi: 10.1016/s0929-693x(00)88887-9. [DOI] [PubMed] [Google Scholar]
- 12.Frank K, Linhart P, Kortsik C, Wohlenberg H. Sonographic determination of spleen size: Normal dimensions in adults with a healthy spleen. Ultraschall Med. 1986;7:134–7. doi: 10.1055/s-2007-1011931. [DOI] [PubMed] [Google Scholar]
- 13.Loftus WK, Metreweli C. Ultrasound measurement of mild splenomegaly spleen/kidney ratio. Pediatr Radiol. 1998;28:98–100. doi: 10.1007/s002470050304. [DOI] [PubMed] [Google Scholar]
- 14.Breiman RS, Beck JW, Korobkin M, Glenny R, Akwari OE, Heaston DK, et al. Volume determinations using computed tomography. AJR Am J Roentgenol. 1982;138:329–33. doi: 10.2214/ajr.138.2.329. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg HK, Markowitz RI, Kolberg H, Park C, Hubbard A, Bellah RD. Normal splenic size in infants and children: Sonographic measurements. AJR Am J Roentgenol. 1991;157:119–21. doi: 10.2214/ajr.157.1.2048509. [DOI] [PubMed] [Google Scholar]
- 16.Mustapha Z, Tahir A, Tukur M, Bukar M, Lee WK. Sonographic determination of the normal spleen size in an adult African population. Eur J Radiol. 2010;75:e133–5. doi: 10.1016/j.ejrad.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Harris A, Kamishima T, Hao HY, Kato F, Omatsu T, Onodera Y, et al. Splenic volume on computed tomography utilizing automatically contouring software and its relationship with age, gender, and anthropometric parameters. Eur J Radiol. 2010;75:e97–101. doi: 10.1016/j.ejrad.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Kebede T, Adamassie D. Spleen length in childhood with ultrasound normal based on age at Tikur Anbessa Hospital. Ethiop Med J. 2009;47:49–53. [PubMed] [Google Scholar]
- 19.Lamb PM, Lund A, Kanagasabay RR, Martin A, Webb JA, Reznek RH. Spleen size: How well do linear ultrasound measurements correlate with three-dimensional CT volume assessments? Br J Radiol. 2002;75:573. doi: 10.1259/bjr.75.895.750573. [DOI] [PubMed] [Google Scholar]
- 20.Kluhs L, Teichgraber UK, Schneider U, Leudwig WD, Dorken B, Benter T. Accuracy of the sonographic determination of the splenic weight in comparison with the weight at autopsy. Article in German. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfah. 2003;175:532–5. doi: 10.1055/s-2003-38435. [DOI] [PubMed] [Google Scholar]
- 21.Loftus WK, Chow LT, Metreweli C. Sonographic measurement of splenic length: Correlation with measurement at autopsy. J Clin Ultrasound. 1999;27:71–4. doi: 10.1002/(sici)1097-0096(199902)27:2<71::aid-jcu4>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues AJ, Jr, Rodrigues CJ, Germano MA, Rasera Junior I, Cerri GG. Sonographic assessment of normal spleen volume. Clin Anat. 1995;8:252–5. doi: 10.1002/ca.980080403. [DOI] [PubMed] [Google Scholar]
- 23.Downey MT. Estimation of splenic weight from ultrasonographic measurements. Can Assoc Radiol J. 1992;43:273–7. [PubMed] [Google Scholar]
- 24.Loftus WK, Metreweli C. Normal splenic size in a Chinese population. J Ultrasound Med. 1997;16:345–7. [PubMed] [Google Scholar]
- 25.Okoye IJ, Agwu KK, Ochie K. Sonographic splenic sizes in normal adult Nigerian population. West Afr J Radio. 2005;12:37–43. [Google Scholar]
- 26.Pietri H, Boscaini M. Determination of a splenic volumetric index by ultrasonic scanning. J Ultrasound Med. 1984;3:19–23. doi: 10.7863/jum.1984.3.1.19. [DOI] [PubMed] [Google Scholar]
- 27.Federal ministry of Health (FMOH) Nigeria Demographic and Health Survey. 2003;10:1–8. [Google Scholar]
- 28.Chauhan R, Kapoor V, Vohra PA, Jhala PJ, Upadhyaya AK, Pathak KJ. The ‘small spleen’ in malaria. J Assoc Physicians India. 1996;7:483–5. [PubMed] [Google Scholar]


