Abstract
The human monocarboxylate transporter 8 (hMCT8) protein mediates transport of thyroid hormone across the plasma membrane. Association of hMCT8 mutations with severe psychomotor retardation and disturbed thyroid hormone levels has established its physiological relevance, but little is still known about the basic properties of hMCT8. In this study we present evidence that hMCT8 does not form heterodimers with the ancillary proteins basigin, embigin, or neuroplastin, unlike other MCTs. In contrast, it is suggested that MCT8 exists as monomer and homodimer in transiently and stably transfected cells. Apparently hMCT8 forms stable dimers because the complex is resistant to denaturing conditions and dithiothreitol. Cotransfection of wild-type hMCT8 with a mutant lacking amino acids 267–360 resulted in formation of homo-and heterodimers of the variants, indicating that transmembrane domains 4–6 are not involved in the dimerization process. Furthermore, we explored the structural and functional role of the 10 Cys residues in hMCT8. All possible Cys>Ala mutants did not behave differently from wild-type hMCT8 in protein expression, cross-linking experiments with HgCl2 and transport function. Our findings indicate that individual Cys residues are not important for the function of hMCT8 or suggest that hMCT8 has other yet-undiscovered functions in which cysteines play an essential role.
Monocarboxyate transporter 8 (MCT8) is an important thyroid hormone transporter. This study presents basic characteristics of the MCT8 structure.
Thyroid hormone (TH), the common name for the prohomone T4 and the active hormone T3, is essential for normal metabolism and development of many tissues. Because TH metabolism and action takes place intracellularly, transport of TH is required across the plasma membrane. This process takes place via TH transporters (1,2). The majority of the currently known TH transporters accepts a wide variety of compounds and demonstrate a relatively low apparent affinity toward TH. So far, only organic anion-transporting polypeptide-1C1 (3,4,5), monocarboxylate transporter (MCT)-8 (6), and MCT10 (7) are reported to have high specificity for TH. Recently the biological importance of MCT8 became apparent when loss-of-function mutations in MCT8 were associated with severe psychomotor retardation and disturbed TH levels, also known as Allan-Herndon-Dudley syndrome (8).
Evidence has accumulated that MCT1–4, which transport monocarboxylates such as pyruvate and lactate, require ancillary proteins for normal cellular function. Apparently depending on the cell type, MCT1 interacts with basigin (BSG; CD147) or the homologous protein embigin (EMB; GP70), MCT3 and MCT4 only with BSG and MCT2 only with EMB (9,10,11,12). The association of these MCTs with their partners is required for translocation to the plasma membrane and transport activity (11).
Western blotting of lysates of cells transfected with human (h)-MCT8 revealed, in addition to a band of 61 kDa, in accordance with the calculated molecular mass of hMCT8, a band suggesting a molecular mass approximating 260 kDa (13). It is currently unknown whether this complex represents the association of human (h) MCT8 with an ancillary protein. The relevance of this question is that mutations in possible protein partners of hMCT8 may also result in neurological abnormalities similar to Allan-Herndon-Dudley syndrome. In addition, basic properties of the hMCT8 protein are mostly unclear. In this paper we investigate the possible dimerization and the effects of Cys mutants on the characteristics of the hMCT8 protein.
Materials and Methods
Animals
The 5A11/Bsg-knockout mouse strain was generated using a neomycin gene-insertion strategy, as described previously (14). Care and handling of these animals was in accordance with the guidelines established by the University of Pennsylvania Institutional Animal Care and Use Committee.
Materials
[3′-125I]T3 was obtained from GE Healthcare (Little Chalfont, UK); nonradioactive iodothyronines from Henning (Berlin, Germany); 4,4′-diisothiocyanatostilbene-2,2′-disulfonate (DIDS) and HgCl2 from Sigma (St. Louis, MO); bis(sulfosuccinimidyl) suberate (BS3) from Pierce (Etten-Leur, The Netherlands); and FuGENE6 transfection reagent from Roche Diagnostics (Almere, The Netherlands).
Cell culture
COS1 and JEG3 cells were cultured in six-well culture dishes with DMEM/F12 medium supplemented with 9% heat-inactivated fetal bovine serum and 100 nm sodium selenite. Depending on the experiment, cells were transfected with 500 ng pcDNA3.hMCT8 [wild type (wt) or mutated; from our MCT8 cDNA library (15)] alone or in combination with 500 ng pCMV-SPORT6.hBSG, pcDNA3.hEMB, rat neuroplastin (rNP)-55, or rNP65 in pOPRSVICat. The full-coding sequence of human MCT3 cDNA was amplified from human retinal pigment epithelium and cloned into the pTargeT vector (Promega, Madison, WI). COS1 cells were transfected with 1 μg hMCT3 or hMCT8 using FuGENE6.
Flp-293-hMCT8 cells, HEK293-derived cells (Invitrogen) stably transfected with hMCT8, were grown in DMEM/F12 medium supplemented with 9% heat-inactivated fetal bovine serum, 100 nm sodium selenite, and 100 μg/ml hygromycin.
RT-PCR
Total RNA was isolated from 1 × 106 cells using the High Pure RNA isolation kit (Roche Diagnostics) according to the manufacturer’s guidelines. cDNA was synthesized using 0.5 μg RNA and TaqMan reverse transcriptase reagent (Roche Diagnostics) in a total volume of 50 μl. The oligonucleotide primers of human glyceraldehyde-3-phosphate dehydrogenase, BSG, EMB, neuroplastin (NP)-55, and NP65 were designed across introns (see supplemental Table S1 published on The Endocrine Society’s Journals Online web site at http:// endo. endojournals.org).
TH uptake experiments
TH uptake studies were performed as described previously (13). Briefly, after 48 h transfection, COS1 and JEG3 cells were washed with incubation medium (Dulbecco’s PBS containing 0.1% d-glucose and 0.1% BSA). Uptake of T3 was tested by incubation of the cells for 5 min at 37 C with 1 nm (2 × 105 cpm) [125I]T3 in 1.5 ml incubation medium. After incubation, cells were briefly washed with the medium, lysed with 0.1 m NaOH, and counted in a γ-counter.
Western blotting
SDS-PAGE was performed as reported recently, except for different concentrations (0–250 mm) of dithiothreitol (DTT) and 7.5% acrylamide gels (13,16). The purified polyclonal antibody 1306, which has been raised against the C-terminal part of hMCT8, was used for Western blotting.
Immunofluorescence
Transfected COS7 cells and frozen sections of mouse brain were labeled with MCT3 and MCT8 antibodies as previously described (17). The MCT8 antibody was produced in rabbits immunized with the carboxyl terminal cytoplasmic tail of MCT8 by Dr. Ian Simpson (College of Medicine, Pennsylvania State University, Hershey, PA). Images were obtained on a laser scanning confocal microscope (LSM510; Zeiss Microscope Imaging, Thornwood, NY) with a ×63 oil objective.
Cross-linking experiments
Cross-linking of proteins on intact cells was carried out using the membrane-impermeable reagents DIDS (20 μm), BS3 (1–5 mm), or HgCl2 (1 μm to 1 mm). Subsequently, cells were harvested in 100 mm phosphate (pH 7.2), 2 mm EDTA (PE) buffer, and washed twice with cold PBS.
Cloning and side-directed mutagenesis
The cloning of wt hMCT8 has been described recently (13). The hMCT8 Cys>Ala mutants C184A, C231A, C244A, C281A, C283A, C436A, C481A, C491A, C497A, and C546A were generated by side-directed mutagenesis using the QuickChange kit (Stratagene, Amsterdam, The Netherlands). The presence of the desired mutations was confirmed by DNA sequencing.
Affinity-labeling with BrAc[125I]T3
Affinity labeling was carried out as described elsewhere (13). At 80% confluency, COS1 cells in six-well plates were cotransfected with pcDNA3.hMCT8 and pcDNA3.rD1. After 48 h, the cells were washed with DMEM/F12 and preincubated with DMEM/F12 at 37 C. Subsequently BrAc[125I]T3 (2 × 105 cpm/well) was added and cells were incubated for 4 h at 37 C. Cells were washed with PBS and harvested in 200 μl phosphatidylethanolamine buffer. After protein correction (Bradford assay), SDS-PAGE loading buffer (four times) was added with a final concentration of 10 mm DTT. Samples were analyzed by SDS-PAGE (10% gels; Pierce). Radioactivity was measured by phosphor imaging (Typhoon 9200; GE Healthcare, Piscataway, NJ).
Results
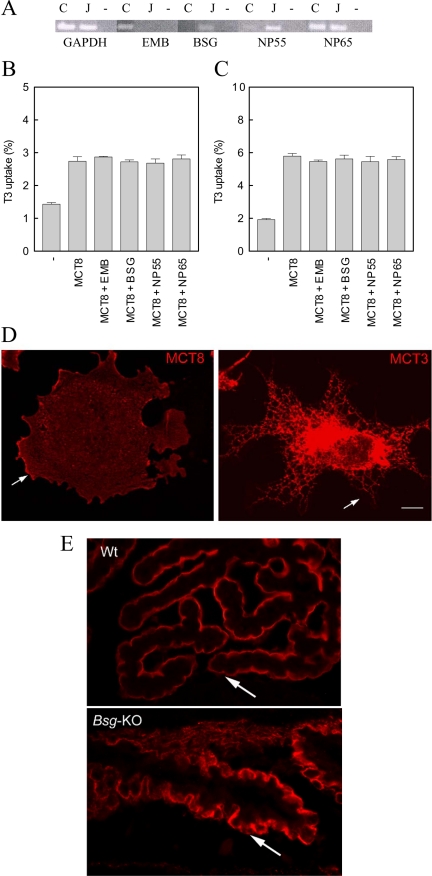
Western blotting of lysates of COS1 and JEG3 cells transfected with hMCT8 both demonstrated, in addition to the 61-kDa monomer, a high molecular mass band, possibly representing the association of hMCT8 with an ancillary protein (13). Therefore, we measured mRNA expression of the ancillary proteins BSG, EMB, NP55, and NP65 in these cell lines. COS1 cells showed high expression of EMB and NP65 mRNA, low expression of NP55 mRNA, and no expression of BSG mRNA. JEG3 cells showed marked expression of BSG, NP55, and NP65 mRNA, but EMB mRNA was undectectable (Fig. 1A).
Figure 1.
Panel A, RT-PCR (35 cycles) of EMB, BSG, NP55, and NP65 mRNA levels in COS1 (C) and JEG3 (J) cells. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 27 cycles) was used as control. Uptake after 5 min incubation with 1 nm [3′-125I]T3 in COS1 cells (panel B) and JEG3 cells (panel C) cotransfected (ratio 1:1) with hMCT8 and hBSG, hEMB, rNp55, and rNp65. Panel D, Immunofluorescence localization of MCT3 and MCT8 in COS1 cells transfected with hMCT8 or hMCT3. Arrows indicate the plasma membrane. Bar, 10 μm. Panel E, Immunofluorescence localization of Mct8 in the choroid plexus in wt and Bsg knockout mice. Arrows indicate the apical plasma membrane of the choroid plexus epithelium.
Western blotting of lysates of cells cotransfected with hMCT8 and each of the investigated ancillary proteins did not show an increased intensity of the hMCT8-related bands (data not shown). Because ancillary proteins do not necessarily induce protein synthesis but rather stimulate protein translocation and functional activity, we tested T3 uptake in cotransfection studies. Cotransfection of hMCT8 and hEMB, hBSG, rNp55 or rNp65 in COS1 (Fig. 1B) and JEG3 (Fig. 1C) cells did not result in increased T3 uptake compared with hMCT8 alone.
COS1 cells transfected with hMCT8 cDNA were labeled with MCT8 antibody. The immunofluorescence staining pattern observed is consistent with cell membrane labeling indicating that the protein was trafficked to the cell surface (Fig. 1D). In contrast, in cells transfected with MCT3 cDNA, the MCT3 protein, which is known to require BSG for trafficking to the cell surface, is retained in the endoplasmic reticulum (Fig. 1D).
To rule out that (other) endogenous ancillary proteins are sufficient for proper function of hMCT8, we attempted to knock down their endogenous expression. However, different knockdown strategies using RNA interference were unsuccessful (data not shown). Therefore, we performed immunohistochemistry in tissues of Bsg−/− animals.
In the choroid plexus, in which no Emb and Np have been reported (our unpublished data and (18), a clear Mct8 signal was observed at the apical plasma membrane in tissue from both wild-type and Bsg−/− mice (Fig. 1E). Also in several other tissues, a clear plasma membrane localization of Mct8 was observed, in contrast with Mct1 and Mct3, which showed a cytoplasmic staining in the Bsg-knockout mice (17). Taken together, these data suggest that MCT8 does not require these ancillary proteins for normal function.
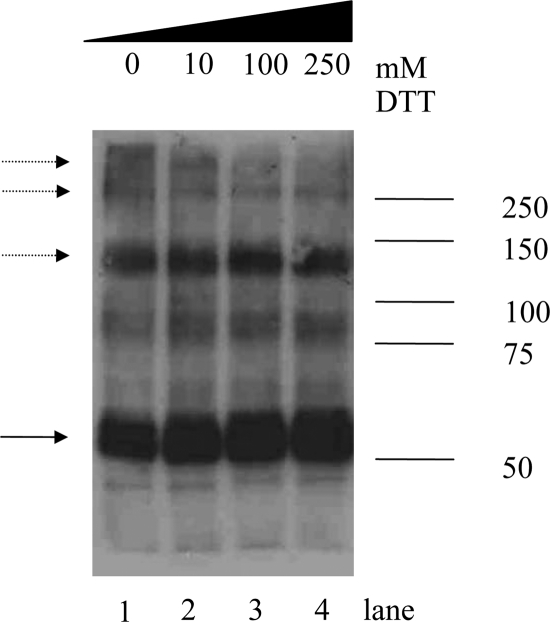
Previously Western blotting was performed after separation of the hMCT8-transfected cell lysate on a 12% SDS-PAGE gel (13). To explore the possibility of homodimerization for hMCT8, COS1 cells were transfected and analyzed on 7.5% SDS-PAGE gels and subsequent Western blotting. This revealed three major bands at 60, 120, and 250 kDa, suggesting the existence of hMCT8 as monomer, dimer, and tetramer (Fig. 2, lane 2). Faint higher-molecular-mass bands are visible, possibly representing multimeric hMCT8 complexes. In addition, there are multiple faint bands between 75 and 100 kDa, the nature of which remains unknown. The same results were obtained after transfection of JEG3 cells with hMCT8 (data not shown).
Figure 2.
Expression of hMCT8 in COS1 cells. Lysates of cells transfected with hMCT8 were separated by SDS-PAGE (7.5%) and analyzed by Western blotting. Expression of hMCT8 protein is shown with increasing DTT concentrations (0–250 mm). The blot was probed with hMCT8-specific antibody 1306. The solid arrow indicates hMCT8 monomer; the dashed arrows point to hMCT8 dimers.
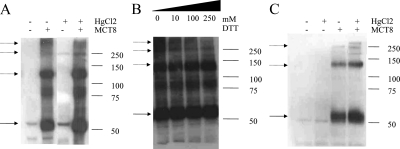
Because the bands at 120 and 250 kDa are resistant to denaturing conditions and even 10 mm DTT, indicating strong protein-protein interactions, we performed SDS-PAGE after treatment with increasing DTT concentrations. Figure 2 shows that with increasing concentrations of DTT the 250-kDa band and the higher-molecular-mass bands disappear, suggesting that disulfide bonds are involved in the oligomerization of hMCT8. In addition, exposure of intact COS1 cells transfected with hMCT8 to HgCl2, which is a known Cys cross-linker, suggests an increased intensity of the higher-molecular-mass bands (Fig. 3A). This cross-linking is a reversible process because the cross-linked oligomeric hMCT8 species decreased with DTT treatment (Fig. 3B). Flp293-hMCT8 cells also demonstrated a 250-kDa band after HgCl2 treatment, consistent with the presence of tetrameric hMCT8 (Fig. 3C). The cross-linkers DIDS, which is known to cross-link MCT1 and BSG, and BS3 did not have any effect on the hMCT8 protein pattern (data not shown).
Figure 3.
Cross-linking of hMCT8 with HgCl2 (10 μm). A, The effect of HgCl2 on the expression of hMCT8 in transiently transfected COS1 cells. B, The effect of increasing concentrations of DTT (0–250 mm) on cross-linked hMCT8 in COS1 cells. C, Expression of lysates from Flp293-hMCT8, HEK293 cells stably transfected with hMCT8, with and without HgCl2 treatment. The solid arrow indicates hMCT8 monomer; the dashed arrows point to hMCT8 dimers.
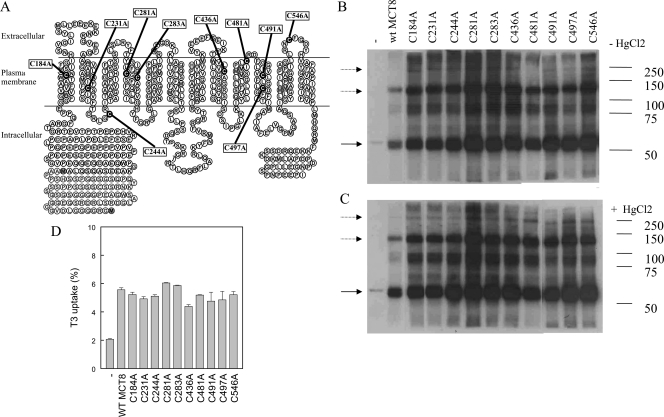
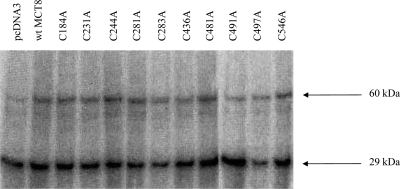
The increased oligomerization of hMCT8 with HgCl2 treatment and its reversion with DTT treatment suggested the involvement of Cys residues in this process. hMCT8 contains 10 Cys residues, which may participate in disulfide bond formation (Fig. 4A). Therefore, we investigated the effects of all possible Cys to Ala mutations. Western blots demonstrated no changes in protein patterns of any of the Cys mutants compared with wt hMCT8 (Fig. 4B). In addition, we did not detect differences between mutants and wt hMCT8 after HgCl2 treatment (Fig. 4C). To determine whether the Cys>Ala substitutions result in an altered MCT8 function, transport of T3 was measured. No differences in T3 uptake were observed between the various Cys mutants and wt hMCT8 (Fig. 4D).
Figure 4.
Expression of cysteine-to-alanine hMCT8 mutants in COS1 cells. A, A putative model of hMCT8. Cys residues are depicted in black circles and Cys>Ala mutants are depicted in boxes. Western blotting of mutant hMCT8 without (B) and with (C) 10 μm HgCl2 treatment. The solid arrow indicates hMCT8 monomer; the dashed arrows point to hMCT8 dimers. D, Uptake of [3′-125I]T3 (5 min) in COS1 cells transiently transfected with cysteine-to-alanine mutants compared with wt hMCT8.
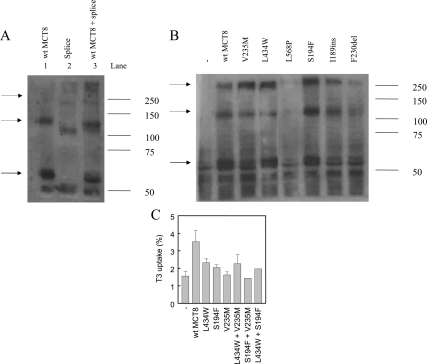
The oligomerization of hMCT8 was further investigated using cDNA coding for a hMCT8 splice variant identified in one of our patients that results in the deletion of amino acids 267–360 (15). The splice site mutant results in a monomeric hMCT8 protein of about 51 kDa, corresponding to the 94 amino acid loss (Fig. 5A, lane 2). The bands of 110 and 240 kDa represent oligomeric mutant hMCT8. When wt hMCT8 and the splice site mutant were cotransfected, an intermediately migrating band of about 115 kDa appeared, demonstrating heterodimerization between wt hMCT8 and the splice site mutant (Fig. 5A, lane 3). This confirms that the hMCT8 protein forms homodimers in vitro.
Figure 5.
Expression of mutant hMCT8 in JEG3 cells, corresponding to mutations in MCT8 patients. A, Expression of hMCT8 (lane 1), a splice site mutant, which lacks amino acids 267–360 (lane 2), and cotransfection of wt and splice site mutant hMCT8 (lane 3). B, Expression of different hMCT8 mutants. The solid arrow indicates wt hMCT8 monomer; the dashed arrows point to wt hMCT8 dimers and oligomers. C, Uptake of 1 nm [3′-125I]T3 (5 min) in JEG3 cells (co)transfected with a (combination of) hMCT8 mutants.
Intriguingly, whereas the monomeric expression of the splice site mutant was decreased, the oligomeric bands seem more intense compared with wt hMCT8. Therefore, we performed Western blotting of a series of hMCT8 mutants to investigate the expression of the oligomeric bands. Most MCT8 mutants (V235M, S194F, I189ins), which show monomeric protein expression, have a relatively increased intensity of oligomeric bands compared with wt hMCT8 (Fig. 5B).
To investigate whether oligomerization could be functional for transporter activity, we wondered whether cotransfection of two mutant hMCT8 plasmids could (partially) rescue transport activity. We used the L434W and S194F mutants, which show some residual activity, and the V235M mutant, which has no residual function (19). Cotransfection of a combination of the mutants did not result in a clear recovery of function (Fig. 5C).
Recent results suggested that hMCT8 undergoes affinity labeling by BrAc[125I]T3 (13). Because Cys is a likely target for affinity labels such as BrAcT3, the Cys>Ala mutants enabled us to explore whether Cys residues are responsible for affinity labeling of hMCT8. BrAc[125I]T3 incubation of intact COS1 cells cotransfected with hMCT8 (wt or Cys mutants) and rD1 resulted in a clear labeling of a 29-kDa and an approximately 60-kDa protein, corresponding to the molecular mass of rD1 and hMCT8, respectively (Fig. 6). However, no differences in intensity of these bands was noticed between the Cys mutants and wt hMCT8.
Figure 6.
Affinity labeling of cells transfected with wt and cysteine-to-alanine hMCT8 mutants. COS1 cells were cotransfected with wt hMCT8 or cysteine-to-alanine mutants and rD1. COS1 cells cotransfected with pcDNA3 and rD1 were used as control. Intact cells were incubated for 4 h at 37 C with BrAc[125I]T3.
Discussion
In the present study, we explored dimerization of the TH transporter hMCT8. The well-studied MCT family members MCT1–4 all require ancillary proteins for their expression and normal function (9,10,11,12,20). BSG is the most common ancillary protein because it is capable of making functional complexes with MCT1, MCT3, and MCT4. However, depending on the cell, MCT1 may also associate with EMB, which is the requisite ancillary protein for MCT2. In contrast with EMB, BSG is widely expressed and believed to be the most important ancillary protein in adult life.
Our data obtained using COS1 as well as JEG3 cells demonstrate that neither coexpression of BSG or EMB, which are differentially expressed in the cell types studied, nor the homologous NP55 or NP65 isoforms result in increased hMCT8 expression or activity. The observation that hMCT8, in contrast with MCT3, is localized at the plasma membrane in transfected COS1 cells suggests that the lack of effect of exogenous ancillary proteins is not due to sufficient levels of endogenous ancillary proteins in our MCT8 overexpression system.
This is supported by similar coexpression studies that demonstrate that MCT1 is properly trafficked only if it is associated with BSG (9). Notably, these experiments were done in COS cells, indicating that endogenous ancillary proteins are insufficient for effective trafficking of MCT1 in an overexpression model.
Furthermore, our results are supported by the observation that in Bsg knockout mice Mct8 is normally expressed at the plasma membrane in the choroid plexus in which Bsg is the main ancillary protein. In contrast, Mct1, Mct3, and Mct4 are not trafficked to the plasma membrane in many tissues in these animals.
Taken together, our results suggest that the studied ancillary proteins are not required for proper function of hMCT8. This is not surprising because the amino acid homology between hMCT1, hMCT2, hMCT3, and hMCT4 is 38–58%, whereas hMCT1–4 show only 21–25% amino acid identity with hMCT8. However, this does not preclude a role for other as-yet-unknown regulatory proteins in the proper cellular control of hMCT8.
In previous experiments using 12% SDS-PAGE gels, only 61- and 260-kDa bands were observed when Western blots were made of hMCT8-expressing cell lysates (13). In the present study, we investigated the hMCT8 protein pattern using 7.5% SDS-PAGE gels because lower-percentage acrylamide gels separate large proteins better. Our present findings clearly show hMCT8-related protein bands of 60, 120, and 250 kDa and even higher molecular mass bands. Because hMCT8 has no predicted phosphorylation or glycosylation sites, the present data strongly suggest that hMCT8 forms homodimers and tetramers.
Cotransfection of wt hMCT8 (predicted molecular mass 59.5 kDa) with a splice site mutant (predicted molecular mass 49 kDa) revealed heterodimerization, thereby confirming the existence of hMCT8 as a homodimer. This is in agreement with a study in which an alternative approach was used to demonstrate homodimerization of wt hMCT8 and heterodimerization of wt hMCT8 with the A224V hMCT8 mutant (21). The high-molecular-mass bands on Western blots suggest the presence of tetramers and even multimers of a higher order, possibly octamers. The existence of these higher-order multimers may be aggresomes, which is a general cellular response occurring when the capacity of the degrading pathway is exceeded by the production of aggregation-prone misfolded proteins (22). However, the observation that not only overexpressing cells but also stably transfected cells show hMCT8 oligomerization indicate that these oligomers represent relevant quaternary structures of hMCT8.
The hMCT8 dimer and tetramer are DTT reducible and appear further stabilized by the Cys cross-linker HgCl2, suggesting that disulfides as well as free cysteines are involved in this process. Thus, we hypothesized that Cys residues are important for hMCT8 dimerization. Indeed, Cys residues are involved in the dimerization of other 12-transmembrane domain (TMD) transporter proteins, which are also DTT reducible and HgCl2 reactive (23,24,25). However, we did not observe differences in dimerization between any of the possible Cys>Ala mutants and wt hMCT8, which argues against the involvement of Cys residues in the dimerization process. This is strengthened by the fact that high concentrations of DTT affected only higher-order multimers. Our data suggest that although Cys residues may be necessary for the formation of higher-order multimers, other amino acids may play a more prominent role in the formation of hMCT8 dimer and tetramer. However, it should be stressed that we have mutated only individual Cys residues, and therefore, it is not excluded that the function of one Cys residue may be taken over after mutation by another Cys residue. It should also be mentioned that Cys residues may be involved in intermolecular protein binding through not only disulfide formation but also complexation with metals such as Zn2+. Indeed, it has been demonstrated that the dopamine transporter exists as a Zn2+-containing protein, which stabilizes the dopamine-dopamine transporter interaction (26). Because the amino group-targeted cross-linkers BS3 and DIDS did not stabilize hMCT8 dimers, Lys residues do not seem to be present at suitable distances in the MCT8 dimerization domain(s).
Apparently the loss of residues 267–360 in the splice site mutant does not hamper dimerization. Prediction programs suggest that the regions of the splice site mutant corresponding to TMDs 1, 2, and 7–12 in wt hMCT8 have not been changed. In contrast, the 94 amino acid loss result in the deletion of TMDs 4–6. Additionally, the amino acids that form TMD 3 in wt hMCT8 do not traverse the plasma membrane in the splice mutant. If this is correct, it means that the region comprising TMDs 3–6 is not necessary for dimerization.
BrAc[125I]T3 is a well-known affinity label for a number of proteins interacting with TH, i.e. D1 and protein disulfide isomerase (27). Recently we demonstrated that hMCT8 facilitates uptake of BrAc[125I]T3 (7). In addition, a clear labeling of a 55-kDa protein was observed, which we assumed represented BrAcT3 labeling of hMCT8 itself. We reasoned that BrAc[125I]T3 was targeted to a reactive amino acid such as cysteine in the T3 binding site of hMCT8.
However, in none of the cell lysates transfected with the Cys>Ala mutants BrAc[125I]T3 labeling of the 60 kDa protein changed, which is now well explained if the 60 kDa-labeled protein is not hMCT8. Indeed, preliminary results indicate that protein disulfide isomerase is labeled by BrAc[125I]T3 and not hMCT8 (data not shown). Apparently the transport of BrAc[125I]T3 by the Cys mutants was not hampered because rD1 was equally labeled in cells cotransfected with the various mutants compared with wt hMCT8.
Because hMCT8 is a plasma membrane protein and no decreased function or protein expression of the Cys mutants were seen, there is no doubt that all these mutants are properly trafficked to the plasma membrane.
Taken together, the Cys mutants did not behave differentially compared with wt hMCT8 in any of experiments we carried out. This seems remarkable because Cys residues usually have important biological functions because the reactive thiol group is among others involved in forming disulfide bridges, catalytic sites, and posttranslational modifications. Our findings may indicate that Cys residues are not important at all for the function of hMCT8 or suggest that hMCT8 has other yet-undiscovered functions in which Cys residues play an essential role. Again, it should be stressed that our results were obtained with individual Cys>Ala mutants. To exclude redundancy in function, the study of a single mutant in which all Cys residues are mutated is needed.
Although it is clear that hMCT8 forms homodimers, the role of hMCT8 dimerization remains tentative. Therefore, further studies are required to establish the function of hMCT8 dimerization.
Supplementary Material
Footnotes
This work was supported by The Netherlands Organization for Scientific Research Grant 9120.6093 from The Netherlands Organization for Scientific Research (to W.E.V. and E.C.H.F.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online September 24, 2009
Abbreviations: BS3, Bis(sulfosuccinimidyl)suberate; BSG, basigin; DIDS, 4,4′-diisothiocyanatostilbene-2,2′-disulfonate; DTT, dithiothreitol; EMB, embigin; h, human; MCT, monocarboxylate transporter; NP, neuroplastin; rNP, rat neuroplastin; TH, thyroid hormone; TMD, transmembrane domain; wt, wild type.
References
- Hennemann G, Docter R, Friesema EC, de Jong M, Krenning EP, Visser TJ 2001 Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocr Rev 22:451–476 [DOI] [PubMed] [Google Scholar]
- Visser WE, Friesema EC, Jansen J, Visser TJ 2008 Thyroid hormone transport in and out of cells. Trends Endocrinol Metab 19:50–56 [DOI] [PubMed] [Google Scholar]
- Pizzagalli F, Hagenbuch B, Stieger B, Klenk U, Folkers G, Meier PJ 2002 Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter. Mol Endocrinol 16:2283–2296 [DOI] [PubMed] [Google Scholar]
- Sugiyama D, Kusuhara H, Taniguchi H, Ishikawa S, Nozaki Y, Aburatani H, Sugiyama Y 2003 Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood-brain barrier: high affinity transporter for thyroxine. J Biol Chem 278:43489–43495 [DOI] [PubMed] [Google Scholar]
- Tohyama K, Kusuhara H, Sugiyama Y 2004 Involvement of multispecific organic anion transporter, Oatp14 (Slc21a14), in the transport of thyroxine across the blood-brain barrier. Endocrinology 145:4384–4391 [DOI] [PubMed] [Google Scholar]
- Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ 2003 Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem 278:40128–40135 [DOI] [PubMed] [Google Scholar]
- Friesema EC, Jansen J, Jachtenberg JW, Visser WE, Kester MH, Visser TJ 2008 Effective cellular uptake and efflux of thyroid hormone by human monocarboxylate transporter 10 (MCT10). Mol Endocrinol 22:1357–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesema EC, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MH, Kuiper GG, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ 2004 Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364:1435–1437 [DOI] [PubMed] [Google Scholar]
- Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP 2000 CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J 19:3896–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp NJ, Wang D, Yoon H, Hjelmeland LM 2003 Polarized expression of monocarboxylate transporters in human retinal pigment epithelium and ARPE-19 cells. Invest Ophthalmol Vis Sci 44:1716–1721 [DOI] [PubMed] [Google Scholar]
- Wilson MC, Meredith D, Fox JE, Manoharan C, Davies AJ, Halestrap AP 2005 Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is EMBIGIN (gp70). J Biol Chem 280:27213–27221 [DOI] [PubMed] [Google Scholar]
- Manoharan C, Wilson MC, Sessions RB, Halestrap AP 2006 The role of charged residues in the transmembrane helices of monocarboxylate transporter 1 and its ancillary protein basigin in determining plasma membrane expression and catalytic activity. Mol Membr Biol 23:486–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesema EC, Kuiper GG, Jansen J, Visser TJ, Kester MH 2006 Thyroid hormone transport by the human monocarboxylate transporter 8 and its rate-limiting role in intracellular metabolism. Mol Endocrinol 20:2761–2772 [DOI] [PubMed] [Google Scholar]
- Igakura T, Kadomatsu K, Kaname T, Muramatsu H, Fan QW, Miyauchi T, Toyama Y, Kuno N, Yuasa S, Takahashi M, Senda T, Taguchi O, Yamamura K, Arimura K, Muramatsu T 1998 A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis. Dev Biol 194:152–165 [DOI] [PubMed] [Google Scholar]
- Friesema EC, Jansen J, Heuer H, Trajkovic M, Bauer K, Visser TJ 2006 Mechanisms of disease: psychomotor retardation and high T3 levels caused by mutations in monocarboxylate transporter 8. Nat Clin Pract Endocrinol Metab 2:512–523 [DOI] [PubMed] [Google Scholar]
- Jansen J, Friesema EC, Kester MH, Milici C, Reeser M, Grüters A, Barrett TG, Mancilla EE, Svensson J, Wemeau JL, Busi da Silva Canalli MH, Lundgren J, McEntagart ME, Hopper N, Arts WF, Visser TJ 2007 Functional analysis of monocarboxylate transporter 8 mutations identified in patients with X-linked psychomotor retardation and elevated serum triiodothyronine. J Clin Endocrinol Metab 92:2378–2381 [DOI] [PubMed] [Google Scholar]
- Gallagher SM, Castorino JJ, Philp NJ 2009 Interaction of monocarboxylate transporter 4 with β1-integrin and its role in cell migration. Am J Physiol Cell Physiol 296:C414–C421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein HG, Smalla KH, Bogerts B, Gordon-Weeks PR, Beesley PW, Gundelfinger ED, Kreutz MR 2007 The immunolocalization of the synaptic glycoprotein neuroplastin differs substantially between the human and the rodent brain. Brain Res 1134:107–112 [DOI] [PubMed] [Google Scholar]
- Jansen J, Friesema EC, Kester MH, Schwartz CE, Visser TJ 2008 Genotype-phenotype relationship in patients with mutations in thyroid hormone transporter MCT8. Endocrinology 149:2184–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole RC, Halestrap AP 1997 Interaction of the erythrocyte lactate transporter (monocarboxylate transporter 1) with an integral 70-kDa membrane glycoprotein of the immunoglobulin superfamily. J Biol Chem 272:14624–14628 [DOI] [PubMed] [Google Scholar]
- Biebermann H, Ambrugger P, Tarnow P, von Moers A, Schweizer U, Grueters A 2005 Extended clinical phenotype, endocrine investigations and functional studies of a loss-of-function mutation A150V in the thyroid hormone specific transporter MCT8. Eur J Endocrinol 153:359–366 [DOI] [PubMed] [Google Scholar]
- Johnston JA, Ward CL, Kopito RR 1998 Aggresomes: a cellular response to misfolded proteins. J Cell Biol 143:1883–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastrup H, Karlin A, Javitch JA 2001 Symmetrical dimer of the human dopamine transporter revealed by cross-linking Cys-306 at the extracellular end of the sixth transmembrane segment. Proc Natl Acad Sci USA 98:10055–10060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastrup H, Sen N, Javitch JA 2003 The human dopamine transporter forms a tetramer in the plasma membrane: cross-linking of a cysteine in the fourth transmembrane segment is sensitive to cocaine analogs. J Biol Chem 278:45045–45048 [DOI] [PubMed] [Google Scholar]
- Henriksen U, Fog JU, Litman T, Gether U 2005 Identification of intra- and intermolecular disulfide bridges in the multidrug resistance transporter ABCG2. J Biol Chem 280:36926–36934 [DOI] [PubMed] [Google Scholar]
- Bjorklund NL, Volz TJ, Schenk JO 2007 Differential effects of Zn2+ on the kinetics and cocaine inhibition of dopamine transport by the human and rat dopamine transporters. Eur J Pharmacol 565:17–25 [DOI] [PubMed] [Google Scholar]
- Schoenmakers CH, Pigmans IG, Hawkins HC, Freedman RB, Visser TJ 1989 Rat liver type I iodothyronine deiodinase is not identical with protein disulfide isomerase. Biochem Biophys Res Commun 162:857–868 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.