Abstract
Progesterone secretion by the steroidogenic cells of the corpus luteum (CL) is essential for reproduction. Progesterone synthesis is under the control of LH, but the exact mechanism of this regulation is unknown. It is established that LH stimulates the LH receptor/choriogonadotropin receptor, a G-protein coupled receptor, to increase cAMP and activate cAMP-dependent protein kinase A (PKA). In the present study, we tested the hypothesis that cAMP/PKA-dependent regulation of the Wnt pathway components glycogen synthase kinase (GSK)-3β and β-catenin contributes to LH-dependent steroidogenesis in luteal cells. We observed that LH via a cAMP/PKA-dependent mechanism stimulated the phosphorylation of GSK3β at N-terminal Ser9 causing its inactivation and resulted in the accumulation of β-catenin. Overexpression of N-terminal truncated β-catenin (Δ90 β-catenin), which lacks the phosphorylation sites responsible for its destruction, significantly augmented LH-stimulated progesterone secretion. In contrast, overexpression of a constitutively active mutant of GSK3β (GSK-S9A) reduced β-catenin levels and inhibited LH-stimulated steroidogenesis. Chromatin immunoprecipitation assays demonstrated the association of β-catenin with the proximal promoter of the StAR gene, a gene that expresses the steroidogenic acute regulatory protein, which is a cholesterol transport protein that controls a rate-limiting step in steroidogenesis. Collectively these data suggest that cAMP/PKA regulation of GSK3β/β-catenin signaling may contribute to the acute increase in progesterone production in response to LH.
Luteinizing hormone stimulates cAMP/PKA regulation of GSK3β/β-catenin signaling that contributes to progesterone secretion.
The corpus luteum is a transient endocrine gland produced from the remnants of an ovulated follicle (1,2). The primary function of the corpus luteum is the secretion of progesterone. For pregnancy to progress normally, the steroidogenic cells of the corpus luteum must receive the appropriate signals to maintain the production of progesterone and extend the luteal life span (1,2,3,4,5). Insufficient progesterone secretion accounts for a significant proportion of early pregnancy loss in humans and domestic farm animals (6,7). Despite the vital nature of steroidogenesis, the mechanisms controlling progesterone synthesis in the corpus luteum are not completely understood.
LH, a pituitary gonadotropin, is possibly the most important regulator of corpus luteum function (1,8,9,10). This hormone induces ovulation and the differentiation of cells of the follicle into progesterone-secreting luteal cells (1,2). The LH/choriogonadotropin receptor (LHCGR) is a glycoprotein hormone receptor in the family of rhodopsin-like G protein-coupled receptors (11). Activation of the LHCGR leads to an increase in cAMP via Gs stimulation of adenylyl cyclase (12). The subsequent increase in cAMP/cAMP-dependent protein kinase (PKA) signaling coincides with an increase in progesterone synthesis. The PKA signaling pathway appears to be a predominant pathway for increasing progesterone in the corpus luteum, although other intracellular signals have been proposed that contribute to progesterone synthesis (5,13).
The rate-limiting step in steroid production in the corpus luteum is believed to be the movement of cholesterol from the cytosol to the inner mitochondrial membrane, a process mediated by the steroidogenic acute regulatory protein (STARD1, commonly referred to as StAR) (14). In patients with congenital lipoid adrenal hyperplasia, both adrenal and gonadal steroid synthesis is impaired due to a mutation in the StAR gene and StAR null mice have an identical phenotype (1,15,16,17). Although StAR protein expression is stimulated through multiple signaling pathways in various species (18), the cAMP/PKA pathway appears to play a major role in the acute steroidogenic response to tropic hormones such as LH (18,19).
Members of the Wnt pathway [Wnt ligands, Frizzled receptors, glycogen synthase kinase (GSK)-3, and β-catenin] are necessary for embryonic development and play a role in normal ovarian function (20,21,22). For example, aberrations in Wnt signaling results in a number of developmental defects such as pseudohermaphrodism, sex reversal, or delayed gonadal development (2,4,23). Mice that lack the Fzd4 receptor are infertile due to the lack of corpus luteum formation and progesterone secretion (21). Furthermore, misregulation of Wnt signaling in the ovary can result in granulosa cell tumors (24) due to elevated levels of β-catenin and up-regulation of genes regulating cell growth (25). Although β-catenin plays a role in cell adhesion through its association with α-catenin, E-cadherin, and the actin cytoskeleton, it is a key transcriptional coactivator in the canonical Wnt signaling pathway. In the absence of a Wnt signal, a large destruction complex (containing GSK3β, axin, adenomatous polyposis coli, casein kinase I, and other proteins) binds the free cytoplasmic β-catenin and promotes phosphorylation of serine/threonine residues (Ser33, Ser37, Thr41, Ser45) in its NH2 terminus, which targets β-catenin for degradation via the ubiquitin/proteasome pathway (26,27,28,29). The binding of a Wnt ligand to its cell surface receptor results in the disruption of the destruction complex, allowing β-catenin to accumulate in the cytoplasm and translocate to the nucleus in which it binds to various transcription factors and other transcriptional coregulators to regulate gene expression (26,27,28,29). Recent studies implicate β-catenin as an activating cofactor for the hormone-dependent gene expression in steroidogenic tissues; β-catenin regulates the inhibin gene in the adrenal (30) and aromatase gene (CYP19A1) present in the granulosa cells of developing ovarian follicles (31).
Understanding the mechanisms involved in the regulation of progesterone synthesis could lead to treatments to enhance corpus luteum function and prevent premature corpus luteum regression and early pregnancy loss. This understanding may also contribute to development of new ways to control fertility and possibly lead to novel cancer treatments. In the present study, we tested the hypothesis that cAMP/PKA-dependent regulation of GSK3β/β-catenin signaling contributes to LH-dependent steroidogenesis. Our data demonstrate a novel link between the cAMP/PKA pathway and the Wnt pathway at the level of GSK3β/β-catenin signaling. Our data indicate that the ability of LH to interrupt GSK3β activity contributes to increased levels of β-catenin, StAR expression, and progesterone synthesis.
Materials and Methods
Reagents
Mouse monoclonal anti-GSK3β and β-catenin antibodies were from BD Transduction Laboratories (San Diego, CA). Rabbit polyclonal antiphosphorylated GSK3β (Ser9) and phospho-Ser33/37/Thr41-β-catenin antibodies were from Cell Signaling Technology (Beverly, MA). Protease and phosphatase inhibitor cocktails, BSA (fraction V), monoclonal anti-β-actin, and horseradish peroxidase-conjugated antirabbit and antimouse secondary antibodies were purchased from Sigma Aldrich (St. Louis, MO). Bovine LH was purchased from Tucker Endocrine Research (Tucker, GA). Immobilon-P polyvinylidene diflouride (0.45 μm pore size) was provided by Millipore (Bedford, MA). Inhibitors H89, GF109203X, PD98059, LY294002, wortmannin, and rapamycin were obtained from BIOMOL (Plymouth Meeting, PA) or Calbiochem (La Jolla, CA). 8-Bromo-cAMP and forskolin were purchased from Sigma (St. Louis, MO). 8-(4-Chlorophenylthio)-2′-O-methyladenosine-cAMP, a specific activator of the exchange protein activated by cAMP (EPAC); and Rp-adenosine-3′,5′-cyclic monophosphorothioate (Rp-cAMPS), a specific competitive inhibitor of the activation of cAMP-dependent protein kinases by cAMP, were obtained from Biolog (Bremen, Germany). The hemagglutinin-tagged Ad.GSK3βS9A was obtained from Kenneth Walsh (Boston University School of Medicine) (32). The GSK3β Ser9 residue was mutated to an alanine rendering the GSK3β product insensitive to phosphorylation and inactivation, thus making it constitutively active. The Ad.Δ90 β-catenin was previously described (31). The first 90 N-terminal residues were deleted, resulting in the removal of the GSK3β phosphorylation sites resulting in a degradation-resistant, active form of β-catenin.
Isolation and culture of bovine luteal cells
Bovine ovaries were from a local slaughterhouse. The corpora lutea were from cows during early pregnancy and were processed as described previously (33). The corpus luteum was dissociated with type II collagenase (103 U/ml; Atlantic Biologicals, Lawrenceville, GA) in basal medium (Cambrex, Walkersville, MD) supplemented with 0.1% BSA, 100 U/ml penicillin G-sodium, 100 μg/ml streptomycin sulfate, and 10 μg/ml gentamicin sulfate. Trypan blue exclusion was used to determine cell viability. The primary cell preparations had a viability of greater than 90% and contained a mixture of large and small luteal cells at a ratio of one large cell to eight or nine small cells. The steroidogenic cells were then plated (∼1 × 105 cells/cm2) in basal medium with 5% fetal bovine serum (Invitrogen, Carlsbad, CA) for 24 h in 5% CO2 at 37 C in a humidified incubator. Cultures were serum starved overnight in basal medium. Before treatments, the cells were placed in fresh serum-free media and were allowed to equilibrate for 3 h. Treatments are described in the legends to the figures.
Preparation of cell extracts
After treatment, cells were washed with ice-cold PBS and were then lysed with lysis buffer [150 mm NaCl, 20 mm Tris-HCl (pH 7.4), 1 mm EGTA, 1 mm EDTA, 1% Triton X-100, protease inhibitor cocktail, and phosphatase inhibitor cocktails]. Cells were scraped from the plates and transferred to microcentrifuge tubes or, in the case of cells suspensions, transferred directly to microcentrifuge tubes. Cell lysates were then sonicated at 4 C with two 3-sec pulses. The lysates were then centrifuged for 15 min at 14,000 × g at 4 C. Using the Bradford reagent (Bio-Rad, Hercules, CA), protein concentrations of the supernatant were obtained. Samples, with the exception of immunoprecipitation and chromatin immunoprecipitation assay (ChIP) samples, were stored at −80 C until use.
Western blot analysis
Proteins (40 μg/lane) were separated by 10 or 15% SDS-PAGE gels and were transferred to polyvinylidene diflouride membranes for immunoblotting as previously described (34). Membranes were developed using Western Lightning ECL (PerkinElmer Life Sciences Inc., Waltham, MA) and were visualized on x-ray film or on a Image Station 440 (Kodak Digital Sciences, Rochester, NY). Images were quantified using Kodak Digital Sciences software.
cAMP and progesterone analysis
For cAMP analysis media were taken from cultures after 15 min and cAMP was measured using a cAMP kit obtained from Assay Designs, Inc. (Ann Arbor, MI). Conditioned media collected for progesterone analysis were stored frozen at −80 C. Progesterone determinations were performed using a progesterone RIA kit (Diagnostic Systems Laboratories, Inc., Webster, TX) according to the manufacturer’s instructions.
Protein kinase assays
PKA activity was determined in cell extracts prepared as described above. Protein extracts (1–1.5 mg/ml) were assayed in the presence or absence of exogenous cAMP (10 μm) using a modification of previously described methods (35) using a reaction mixture consisting of 130 μm PKA heptapeptide substrate (LRRASLG; Peninsula, Belmont, CA) in a buffer containing 20 mm Tris-HCl (pH 7.5), 100 μm 3-isobutyl-1-methylxanthine, 20 mm magnesium-acetate, and 200 μm [γ-32P]ATP. Protein samples (20 μl) were added to 50 μl of the reaction mix described above and incubated for 15 min at 30 C. The reaction was halted by spotting the assay mix (60 μl) onto P-81 phosphocellulose papers (Whatman, Hillsboro, OR). Papers were then washed five times for 5 min in 75 mm phosphoric acid and washed once in ethanol. Dried papers were counted in nonaqueous scintillation fluid and enzyme activity was expressed as picomoles per minute per milligram. GSK3 activity was measured following the protocol described in detail by McManus et al. (36). Briefly, GSK3 was immunoprecipitated from 200 μg cell lysate, washed extensively, and incubated with or without the phosphoprotein phosphatase (PP)2-A1 (25 mU/ml) before adding the glycogen synthase peptide as substrate. The assay (50 μl) contained washed protein G-sepharose immunoprecipitate, 50 mm Tris/HCl (pH 7.5), 0.1 mm EGTA, 0.1% (vol) 2-mercaptoethanol, 10 mm magnesium acetate, 0.1 mm [γ32P]ATP (∼200 cpm/pmol) and phospho-glycogen synthase peptide (YRRAAVPPSPSLSRHSSPHQpSEDEEE, 20 μm) and was carried out for 30 min at 30 C, with continuous agitation to keep the immunoprecipitate in suspension. Assays were completed as described above, and GSK3 activity was expressed as a reactivation ratio (i.e. GSK3 activity measured without PP2A1 treatment divided by GSK3 activity after PP2A1 treatment).
Adenovirus transduction
After plating (1 × 106 cells/ml per 10 cm2), the media on the cells were changed to serum-free media and cells were treated with Ad.GFP, Ad.GSK3βS9A (1 × 107/ml viral particles), or Ad.Δ90 β-catenin (1 × 109/ml viral particles). One hour after addition of virus, the remaining media were supplemented with 10% bringing the final serum concentration to 5%. The cells were then incubated overnight at 37 C in a humidified incubator with 5% CO2. Media were changed and the cells were allowed to equilibrate for 2 h before treatment.
RT-PCR
Luteal cells were plated in 60-mm dishes. After equilibration and treatment for 2 h with LH, RNA was isolated exactly as outlined in the Absolutely RNA purification kit (Stratagene, La Jolla, CA). After reverse transcription, the cDNA underwent PCR using bovine StAR primers (GenBank accession no. BC110213; forward, CCCTCTCTACAGCGACCAAG; reverse, ATCCCTTGAGGTCAATGCTG) at 55 C annealing temperature and 30 cycles.
ChIP assay
Luteal cells (7 × 106 cells) were plated in 10-cm2 culture dishes. After equilibration and treatment for 30 min, the cells were washed twice with ice-cold PBS and then cross-linked with 1% formaldehyde at room temperature for 10 min. Cells were rinsed twice with ice-cold PBS, scraped from plates in collection buffer [100 mm Tris-HCl (pH 9.0), 10 mm dithiothreitol], and incubated at 30 C for 15 min. Cells were then pelleted by centrifuging at 1500 × g for 5 min at room temperature. The cells were resuspended in 1 ml ice-cold PBS and then centrifuged at 2000 × g for 5 min at 4 C. The PBS was removed and the cells were washed sequentially with 1 ml of buffer 1 [0.25% Triton X-100, 10 mm EDTA, 0.5 mm EGTA, 10 mm HEPES (pH 6.5)] and buffer 2 [200 mm NaCl, 1 mm EDTA, 0.5 mm EGTA, 10 mm HEPES (pH 6.5)]. After removal of buffer 2, the cells were resuspended in 0.3 ml of lysis buffer [1% sodium dodecyl sulfate (SDS), 10 mm EDTA, 50 mm Tris-HCL (pH 8.1), protease inhibitor cocktail]. Samples were then sonicated three times with 10-sec pulses at the maximum setting (model W-225; Heat Systems-Ultrasonics, Inc., Farmingdale, NY).
Samples and the sonicator probe were placed on ice for a minimum of 3 min between sonications to prevent overheating of the samples. After sonication, the samples were centrifuged at 14,000 × g at 4 C for 15 min. Then 0.2 ml of sample was removed and diluted in 0.3 ml of supernatant buffer [1% Triton X-100, 2 mm EDTA, 150 mm NaCl, 20 mm Tris-HCl (pH 8.1)], bringing samples to a final volume of 0.5 ml. Salmon sperm DNA (2 μg), 45 μl of a 50% slurry [in Tris/EDTA (TE) buffer] of protein A sepharose beads, and 20 μl of normal mouse serum were added to the samples. Samples were then immunocleared by tumbling the mixture for at least 2 h at 4 C. Immunocleared samples were moved to a fresh microcentrifuge tube and primary antibody was added (1:100), and the samples were tumbled overnight at 4 C.
The next day, 45 μl of a 50% slurry (in TE buffer) of protein A sepharose beads and salmon sperm DNA (2 μg) were added to the immunoprecipitation reaction, and the samples were then tumbled an additional 2 h at 4 C. The supernatant was then removed and stored at −80 C and the beads were washed sequentially with 0.5 ml of Buffer 3 [0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl (pH 8.1), 150 mm NaCl], Buffer 4 [0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl (pH 8.1), 500 mm NaCl], and buffer 5 [0.25 m LiCl, 1% Nonidet P-40, 1% Na-deoxycholate, 1 mm EDTA, 10 mm Tris-HCl (pH 8.1)], tumbling each wash for 10 min at 4 C. The samples were then washed three times with TE buffer. Immunoprecipitated proteins were then removed from the beads by extracting three times with 100 μl of extraction buffer (1% SDS, 0.1 m NaHCO3) and pooling the extractions. The eluates and inputs (the remainder of the samples that have not gone through the immunoprecipitation procedure) were placed in a 65 C water bath overnight to reverse the cross-links (37). DNA was then purified from the samples using the Quick Spin kit (QIAGEN, Valencia, CA). For PCR, in a 50 μl reaction volume, 1 μl of template from a 30-μl DNA extraction was used with a 56 C annealing temperature and 35 cycles and StAR primers (GenBank accession no. Y17260; forward, CAAATGGCCAAAGACTCCTG; reverse, GGCCAGATGATGTGTTTCTG). After the first 35 cycles were complete, 5 μl of samples were added to a new PCR for a further 25 cycles.
Statistical analysis
All experiments were performed at least twice using different cell preparations with qualitatively similar results. The data are presented as the means ± se of the averages from multiple experiments. Data shown from two experiments are shown as the average response and the individual values obtained in each experiment. The differences in means were analyzed by ANOVA followed by t test for paired comparisons or multiple range testing.
Results
LH stimulates the phosphorylation of GSK3β by a cAMP/PKA-dependent mechanism
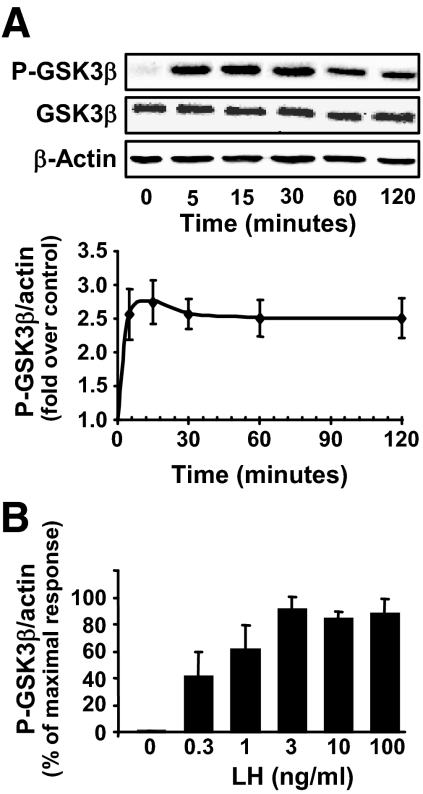
In contrast to many enzymes in protein kinase signaling cascades, the phosphorylation of GSK3β at Ser9 does not result in GSK3β activation; rather it deactivates the enzyme. Initial experiments determined the temporal response to saturating levels of LH (100 ng/ml). LH stimulated a rapid (within minutes) and sustained elevation in the Ser9 phosphorylation of GSK3β (Fig. 1A). LH did not alter total cellular GSK3β in incubations lasting up to 24 h. Experiments to determine the least effective and optimal concentrations of LH for inducing GSK3β phosphorylation revealed that as little as 0.3 ng/ml of LH stimulated GSK3β phosphorylation and LH concentrations of 3 ng/ml or greater were maximally effective (Fig. 1B). The LH-induced increase in GSK3β phosphorylation on Ser9 residues was correlated with a reduction (25 ± 7%, mean ± sem, n = 3) in GSK3 protein kinase activity.
Figure 1.
LH and cAMP stimulate the phosphorylation of GSK3β on ser9 (P-GSK3β) in bovine luteal cells. Primary cultures of steroidogenic luteal cells were treated for various periods of time with LH (0–100 ng/ml). Western blot analysis was performed to determine the levels of GSK3β phosphorylated on ser9 and total GSK3β. β-Actin served as a loading control. A, Time course (0–120 min) of the response to LH (100 ng/ml). A representative Western blot is shown and the graph represents means ± sem from four separate experiments (n = 4). B, Concentration response to LH at 15 min, means ± sem, n = 4.
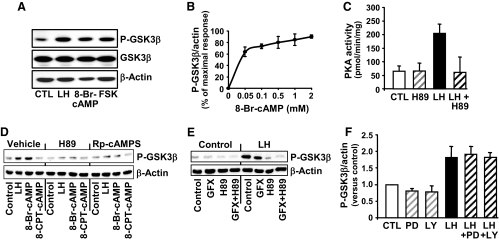
Experiments were performed to demonstrate the intracellular mediators that contributed to the response to LH. Compared with controls, treatment with LH for 15 min increased cAMP (5.6 ± 2.6 vs. 238 ± 18 pmol/ml, mean ± sem, n = 3). Similar to the response after treatment with LH, treatment with forskolin, an adenylyl cyclase activator, and 8-bromoadenosine-cAMP (8-Br-cAMP), a nonhydrolyzable cAMP analog, increased the phosphorylation of GSK3β (Fig. 2A). As little as 0.05 mm 8-Br-cAMP increased phosphorylation of GSK3β (Fig. 2B). Treatment with LH increased PKA activity (Fig. 2C), which was abrogated by pretreatment with H89, a PKA inhibitor (Fig. 2C). To determine whether GSK3β phosphorylation was mediated by PKA, luteal cells were pretreated with the PKA inhibitors H89 or Rp-cAMPS and then treated for 15 min with LH, 8-Br-cAMP, or 8-(4-chlorophenylthio) cAMP (8-CPT-cAMP), a cAMP analog that selectively activates EPAC and not PKA (38). We observed that LH and 8-Br-cAMP, but not 8-CPT-cAMP increased GSK3β phosphorylation (Fig. 2D). Incubation with the PKA inhibitors H89 and Rp-cAMPS resulted in inhibition of LH- and 8-Br-cAMP-dependent stimulation of GSK3β phosphorylation (Fig. 2D). We also examined the possibility that other protein kinases were responsible for phosphorylating GSK3β in response to LH (39,40,41,42). Because PKA and protein kinase C (PKC) have similar substrate phosphorylation motifs, we tested the ability of the PKC inhibitor GF109203X to block the effects LH on GSK3β phosphorylation (Fig. 2E). Relatively high concentrations of GF109203X (10 μm) only slightly inhibited LH-stimulated phosphorylation of GSK3β, whereas the PKA inhibitor H89 completely inhibited the response to LH. In other experiments, pretreatment of luteal cells with the MAPK kinase (MEK)-1/ERK inhibitor PD98059 (50 μm) or the phosphatidylinositol 3-kinase inhibitor LY294002 (20 μm) did not reduce the stimulatory effect of LH on GSK3β Ser9 phosphorylation (Fig. 2F). Similarly, pretreatment with the MEK1 inhibitor UO126 (20 μm) or the mammalian target of rapamycin inhibitor rapamycin (20 nm) did not alter the response to LH (data not shown).
Figure 2.
The stimulatory response of LH on GSK3β phosphorylation (P-GSK3β) is mediated by cAMP/protein kinase (A). Primary cultures of steroidogenic luteal cells were treated as described below. Western blot analysis was performed to determine the levels of total GSK3β and GSK3β phosphorylated on ser9. β-Actin served as a loading control. A, Response to 15 min treatment with control media (CTL), LH (100 ng/ml), 8-Br-cAMP (1 mm), or forskolin (FSK; 10 μm). B, Concentration response to 15 min of treatment with 0.05–2 mm 8-Br-cAMP, means ± sem, n = 4. C, The stimulatory effect of LH on PKA activity is inhibited by the PKA inhibitor H89. Luteal cells were pretreated for 60 min with H89 (20 μm) before treatment for 15 min with LH (100 ng/ml). Protein kinase activity represents means ± sem from three separate experiments. D, PKA inhibitors prevent the stimulatory effects of LH and 8-Br-cAMP on GSK3β phosphorylation. Luteal cells were pretreated for 60 min with the PKA inhibitors H89 (20 μm) or Rp-cAMPS (0.5 μm) and then treated with LH (100 ng/ml), 8-Br-cAMP (1 mm) or 8-(4-chlorophenylthio)-2′-O-methyladenosine-3′, 5′-cAMP (8-CPT-cAMP, 100 μm), a selective activator of EPAC but not PKA. E, Luteal cells were pretreated with the PKC inhibitor GF109203X (GFX; 10 μm), H89, or a combination of inhibitors for 60 min before treatment with LH (100/ml) for 15 min. F, Luteal cells were pretreated for 1 h with the MEK1/2 inhibitor PD98059 (PD; 50 μm) or the phosphatidylinositol 3-kinase inhibitor LY294002 (LY; 20 μm) before treatment with LH for 15 min. Results are means ± sem from three separate experiments.
Activation of GSK3β inhibits LH-stimulated progesterone synthesis
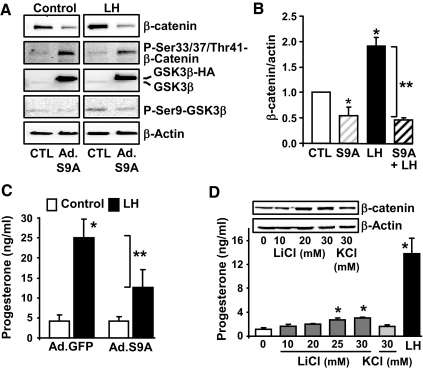
Experiments were performed to determine whether alterations in GSK3β activity would affect cellular β-catenin and progesterone secretion. Elevations GSK3β activity would be expected to increase phosphorylation and degradation of its target protein β-catenin, an important transcription coregulatory factor. Luteal cells were infected with an adenovirus that allowed the expression of a constitutively active mutant of GSK3β (Ad.GSK3βS9A), which cannot be phosphorylated due to a Ser9-to-Ala9 substitution (32). As predicted, treatment with Ad.GSK3βS9A resulted in increased phosphorylation of the N-terminal β-catenin phosphorylation sites (Ser33/37/Thr41) and reductions in cellular β-catenin (Fig. 3A). Treatment with LH increased GSK3β phosphorylation, total β-catenin (Fig. 3B), and progesterone secretion (Fig. 3C). However, adenoviral-induced expression of GSK3βS9A was capable of overriding the stimulatory effect of LH on β-catenin levels (Fig. 3, A and B) and progesterone secretion (Fig. 3C). In contrast, treatment with LiCl, a GSK3β inhibitor (43), but not KCl, resulted in modest but significant elevations in progesterone secretion (Fig. 3D), which were accompanied by increased levels of β-catenin.
Figure 3.
Modulation of GSK3β activity in luteal cells alters LH-stimulated progesterone secretion. A–C, Luteal cells were infected with an adenovirus-expressing GFP (Ad.GFP) or a hemagglutinin-tagged GSK3β mutant with a ser9 (S9) to alanine mutation (Ad.GSKS9A), which is constitutively active. After 24 h, cells were incubated in the presence or absence of LH (100 ng/ml) for 6 h. A, A representative Western blot panel shows levels of β-catenin, phospho-β-catenin (Ser33/37/Thr41), GSK3β, and phospho (P)-ser9-GSK3β with β-actin as a loading control. B, Levels of total β-catenin protein normalized to actin. *, P < 0.05, control (CTL) vs. Ad.GSKS9A; *, P < 0.05, CTL vs. LH; *, P < 0.05, Ad.GSKS9A vs. LH; **, P < 0.05, LH vs. Ad.GSKS9A+LH. C, Progesterone secretion was measured in aliquots of the media. Results are means ± sem from four separate experiments. **, P < 0.05, Ad.GFP+LH vs. Ad.GSKS9A+LH; *, P < 0.05, Ad.GFP vs. Ad.GFP+LH. D, Effects of LiCl, a GSK3β inhibitor, on progesterone secretion. Luteal cells were treated for 6 h with LiCl (10–30 mm), KCl (30 mm), or LH (100 ng/ml). Shown are representative Western blots of β-catenin and β-actin as a loading control. Progesterone secretion was measured in aliquots of the media. Results are means ± sem from four separate experiments. *, P < 0.05, vs. control.
β-Catenin augments LH-induced progesterone synthesis
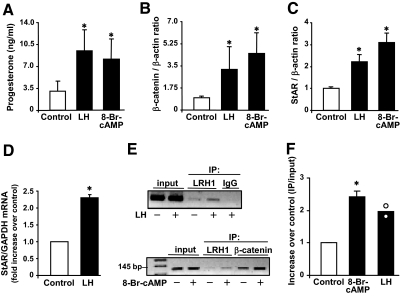
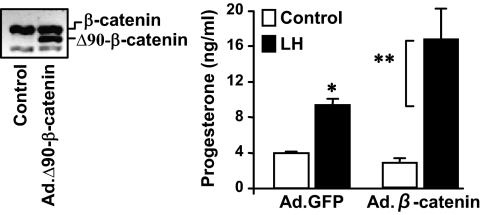
Experiments were performed to evaluate the effects of LH on GSK3β and β-catenin under conditions optimal for progesterone secretion. Treatment with LH or 8-Br-cAMP significantly increased (P < 0.05, n = 4) progesterone (Fig. 4A) and β-catenin (Fig. 4B). Using identical conditions, LH and 8-Br-cAMP increased levels of StAR protein (P < 0.05, n = 4), a rate-limiting cholesterol transport protein necessary for progesterone synthesis (Fig. 4C). In keeping with previous reports (44,45), we observed an increase in StAR mRNA (P < 0.05, n = 3) within 2 h of LH treatment (Fig. 4D). To determine whether β-catenin may interact with the StAR promoter, ChIP assays were performed using antibodies to β-catenin. After treatment with LH or 8-Br-cAMP, ChIP analysis revealed that β-catenin was associated with the proximal portion of the StAR promoter (Fig. 4, E and F). This region of the StAR promoter possesses consensus binding regions for the nuclear receptors steroidogenic factor 1 (NR5A1) and liver receptor homolog 1 (NR5A2) (also commonly known as steroidogenic factor 1 (SF1) and liver receptor homolog-1 (LRH1), respectively), which are known to contribute to StAR expression. ChIP analysis also revealed that, in luteal cells, LRH-1 associates with the StAR promoter after stimulation with either 8-Br-cAMP or LH (Fig. 4E). To determine whether increasing β-catenin contributes to progesterone secretion, luteal cells were infected with an adenovirus expressing a truncated β-catenin (Ad.Δ90β-catenin) protein that lacks 90 N-terminal amino acids (Fig. 5). The N-terminal truncation mutant lacks the residues comprising the GSK3β phosphorylation sites (31), which impairs N-terminal phosphorylation and degradation of β-catenin. Compared with luteal cells infected with the control GFP adenovirus, luteal cells infected with Ad.Δ90β-catenin showed a significant augmentation (∼2-fold, P < 0.05, n = 3) in the ability of LH to stimulate progesterone secretion (Fig. 5). Treatment with Ad.Δ90β-catenin in the absence of LH did not elevate progesterone secretion.
Figure 4.
LH increases β-catenin and StAR in luteal cells. Primary cultures of steroidogenic luteal cells were treated as described below. A–C, Luteal cells were treated with control media or LH (100 ng/ml) or 8 Br-cAMP (1 mm) for 6 h. Results are means ± sem, n = 4 separate experiments. A, Progesterone secretion in response to LH and 8-Br-cAMP. B, β-Catenin protein expressed as a ratio of β-catenin to β-actin. C, StAR levels expressed as a ratio of StAR to β-actin. D, Luteal cells were treated with control media or LH (100 ng/ml) for 2 h. StAR mRNA levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Results are means ± sem, n = 3. E, Luteal cells were treated with LH (100 ng/ml) or 8-Br-cAMP (1 mm) for 30 min followed by immunoprecipitation (IP) using antibodies to β-catenin or LRH1 or IgG and subsequent ChIP assay showing either LRH1 or β-catenin binding to the proximal StAR promoter. F, Luteal cells were treated with LH (100 ng/ml) or 8-Br-cAMP (1 mm) for 30 min followed by IP of β-catenin and ChIP assay showing β-catenin binding to the proximal StAR promoter. Results are expressed as a ratio of the signal observed in the IP to that of the input. Shown are means ± sem, n = 3 for treatment with 8-Br-cAMP and the averages (bars) and individual values (white circle) from two experiments with LH. *, P < 0.05 vs. control.
Figure 5.
β-Catenin enhances LH-stimulated progesterone secretion. Luteal cells were infected the adenoviruses Ad.GFP or Ad.Δ90β-catenin for 24 h before treatment with control media or LH (100 ng/ml) for 6 h. A representative Western blot is shown illustrating overexpression of Ad.Δ90β-catenin in luteal cells. Progesterone secretion was determined from aliquots of conditioned media. Data are means ± sem from three separate experiments. *, P < 0.05, Ad.GFP+LH vs. Ad.GFP+control; **, P < 0.05, Ad.GFP+LH vs. Ad.β-catenin+LH.
Discussion
The present results provide novel evidence for the convergence of cAMP/PKA and GSK3β/β-catenin signaling pathways in the regulation of progesterone synthesis. In the canonical Wnt signaling pathway (26,27,29), the binding of a Wnt ligand to its receptor causes the disruption of the β-catenin destruction complex, resulting in a reduction in GSK3-mediated phosphorylation of β-catenin and a subsequent increase in the content of β-catenin. Studies in other systems indicate that GSK3 activity can also be reduced by phosphorylation of the N-terminal Ser9 residue in GSK3β (46,47,48). The evidence presented in this study demonstrates that cAMP/PKA mediates the action of LH on the phosphorylation of GSK3β in luteal cells. First, the acute effects of LH on the phosphorylation of GSK3β were in keeping with the known rapid actions of LH on activation of the LHCGR and the increase in cAMP and stimulation of PKA activity. Importantly, the actions of LH were apparent at physiological concentrations of LH. Second, the actions of LH were mimicked by cAMP analogs that activate PKA but not by analogs like 8-CPT-cAMP, which selectively activate EPACs (38). Third, based on a pharmacological approach, PKA was identified as the LH inducible protein kinase responsible for stimulating GSK3β phosphorylation in luteal cells. In this regard, the PKA inhibitors H89 and Rp-cAMPS blocked the LH-stimulated phosphorylation of GSK3β, whereas treatment with inhibitors of other signaling pathways (phosphatidylinositol 3-kinase/AKT signaling, mammalian target of rapamycin/p70S6kinase, MEK1/ERK, PKC) that may contribute to GSK3β phosphorylation (39,40,41,42) had little or no effect on LH-stimulated phosphorylation Ser9 on GSK3β. Taken together, the present studies establish GSK3β as a downstream target of LH-induced cAMP/PKA signaling in bovine luteal cells.
Of physiological relevance are the observations that modulating the activity of GSK3β affected progesterone production in luteal cells. The present studies clearly show that LH-increased β-catenin in luteal cells and stimulated progesterone secretion by luteal cells, both of which were significantly reduced on treatment with a constitutively active mutant of GSK3β (Ad.GSKS9A). These results underscore the importance of GSK3β/β-catenin signaling in the acute steroidogenic response to LH. Support for this idea was provided by the findings that LH increased levels of β-catenin and that overexpression of β-catenin enhanced the stimulatory effect of LH on progesterone synthesis. Moreover, ChIP analysis demonstrated that β-catenin was associated with the proximal promoter of the StAR promoter in response to treatment with LH or 8-Br-cAMP. These data suggest that the increase in β-catenin was linked to the increase in progesterone production, possibly via its actions as a coactivator for the StAR gene. Based on studies in other systems, it seems likely that the increases in β-catenin protein may interact with transcription factors such as SF1 or LRH1, which have been shown to stimulate the transcription of genes known to promote steroidogenesis in the gonad (15,17,31). A recent report indicates that SF1 and LRH1 are associated with the proximal StAR promoter in midluteal phase bovine corpora lutea when the tissue is highly steroidogenic (49). In other systems, β-catenin has been shown to interact with SF1 to promote aromatase expression in granulosa cells (31) and LRH1 to promote cyclin mediated cell proliferation in pancreatic LTPA cells (50). Because a large number of transcription factors and cotranscription factors may be targeted for degradation or nuclear export when phosphorylated by GSK3β (51), additional studies are required to identify other factors affected by LH- and PKA-dependent inhibition of GSK3β activity in luteal cells.
Whereas the present findings strongly implicate the GSK3/β-catenin pathway in luteal cell progesterone synthesis, direct modulation of Wnt signaling and β-catenin levels do not appear to be sufficient for maximal steroidogenesis. Overexpression of a stabilized mutant of β-catenin missing the N-terminal phosphorylation sites was not sufficient to increase progesterone synthesis. Likewise, treatment with GSK3β inhibitors resulted in only modest increases in progesterone synthesis. Based on these results, we propose that the LH-stimulated GSK3β/β-catenin pathway forms an integral component of the pathway leading to progesterone synthesis, but other PKA-dependent actions of LH are also required to achieve optimal steroidogenesis (Fig. 6). For example, increases in StAR protein may not contribute to steroidogenesis in the absence of PKA-dependent phosphorylation of StAR in Leydig cells (52). Furthermore, PKA can also regulate the phosphorylation and activity of other transcription factors or coactivators, including β-catenin. Recent reports indicate that PKA regulates the stability and/or activity of β-catenin by phosphorylation on Ser552 and Ser675 in COS-7 and L cells (29,53,54).
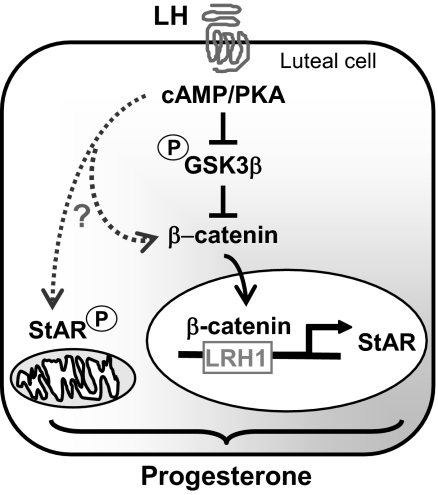
Figure 6.
Convergence of cAMP/PKA and GSK3/β-catenin signals contributes to progesterone synthesis in luteal cells. Our data provide evidence that LH via a cAMP/PKA mechanism phosphorylates and inhibits GSK3β, leading to the accumulation of β-catenin and the interaction of β-catenin with the StAR promoter, events that play a role in the stimulation of progesterone synthesis. LH, presumably acting via cAMP/PKA, exerts control (?) over other events such as the phosphorylation of StAR, β-catenin, or other cellular mediators to provide optimal stimulation of progesterone synthesis.
Taken together, the results of these experiments demonstrate a novel intersection of LH stimulated PKA signaling and GSK3/β-catenin signaling in steroidogenic luteal cells. Activation of PKA by LH and its subsequent phosphorylation and inactivation of GSK3β provide a new link in the intracellular mechanisms used by LH in the regulation of corpus luteum function. Future studies are required to further elucidate the roles that GSK3β and β-catenin play in the response to LH in the ovary.
Footnotes
This work was supported by grants from the Department of Veterans Affairs Biomedical Laboratory Research and Development Merit Review Research Program (to J.S.D.); the National Research Initiative Competitive Grant 2006-35203-17249 (to J.S.D.) from the U.S. Department of Agriculture Cooperative State Research, Education and Extension Service; the Olson Center for Women’s Health at the University of Nebraska Medical Center (to J.S.D.); and a University of Nebraska Medical Center Research Assistantship to (to L.R.).
Disclosure Summary: The authors have nothing to declare.
First Published Online October 9, 2009
Abbreviations: 8-Br-cAMP, 8-Bromoadenosine-cAMP; ChIP, chromatin immunoprecipitation assay; 8-CPT-cAMP, 8-(4-chlorophenylthio; cAMP; EPAC, exchange protein activated by cAMP; GSK, glycogen synthase kinase; LHCGR, LH/choriogonadotropin receptor; LRH1, liver receptor homolog-1; MEK, MAPK kinase; PKA, protein kinase A; PKC, protein kinase C; PP, phosphoprotein phosphatase; Rp-cAMPS, Rp-adenosine-3′,5′-cyclic monophosphorothioate; SDS, sodium dodecyl sulfate; SF1, steroidogenic factor 1; StAR, steroidogenic acute regulatory protein; TE, Tris/EDTA.
References
- Davis JS, Rueda BR 2002 The corpus luteum: an ovarian structure with maternal instincts and suicidal tendencies. Front Biosci 7:d1949–d1978 [DOI] [PubMed] [Google Scholar]
- Stouffer RL 2003 Progesterone as a mediator of gonadotrophin action in the corpus luteum: beyond steroidogenesis. Hum Reprod Update 9:99–117 [DOI] [PubMed] [Google Scholar]
- Narko K, Saukkonen K, Ketola I, Bützow R, Heikinheimo M, Ristimäki A 2001 Regulated expression of prostaglandin E(2) receptors EP2 and EP4 in human ovarian granulosa-luteal cells. J Clin Endocrinol Metab 86:1765–1768 [DOI] [PubMed] [Google Scholar]
- Regan JW 2003 EP2 and EP4 prostanoid receptor signaling. Life Sci 74:143–153 [DOI] [PubMed] [Google Scholar]
- Niswender GD 2002 Molecular control of luteal secretion of progesterone. Reproduction 123:333–339 [DOI] [PubMed] [Google Scholar]
- Allen WR 2001 Luteal deficiency and embryo mortality in the mare. Reprod Domest Anim 36:121–131 [PubMed] [Google Scholar]
- Hinney B, Henze C, Kuhn W, Wuttke W 1996 The corpus luteum insufficiency: a multifactorial disease. J Clin Endocrinol Metab 81:565–570 [DOI] [PubMed] [Google Scholar]
- Weems CW, Reynolds LP, Huie JM, Hoyer GL, Behrman HR 1985 Effects of prostaglandin E1 or E2 (PGE1; PGE2) on luteal function and binding of luteinizing hormone in nonpregnant ewes. Prostaglandins 29:161–173 [DOI] [PubMed] [Google Scholar]
- Chasalow FI, Pharriss BB 1972 Luteinizing hormone stimulation of ovarian prostaglandin biosynthesis. Prostaglandins 1:107–117 [DOI] [PubMed] [Google Scholar]
- Hizaki H, Segi E, Sugimoto Y, Hirose M, Saji T, Ushikubi F, Matsuoka T, Noda Y, Tanaka T, Yoshida N, Narumiya S, Ichikawa A 1999 Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP(2). Proc Natl Acad Sci USA 96:10501–10506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Themmen APN, Huhtaniemi IT 2000 Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev 21:551–583 [DOI] [PubMed] [Google Scholar]
- Vassart G, Pardo L, Costagliola S 2004 A molecular dissection of the glycoprotein hormone receptors. Trends Biochem Sci 29:119–126 [DOI] [PubMed] [Google Scholar]
- Benyo DF, Zeleznik AJ 1997 Cyclic adenosine monophosphate signaling in the primate corpus luteum: maintenance of protein kinase A activity throughout the luteal phase of the menstrual cycle. Endocrinology 138:3452–3458 [DOI] [PubMed] [Google Scholar]
- Arakane F, Kallen CB, Watari H, Foster JA, Sepuri NB, Pain D, Stayrook SE, Lewis M, Gerton GL, Strauss 3rd JF 1998 The mechanism of action of steroidogenic acute regulatory protein (StAR). StAR acts on the outside of mitochondria to stimulate steroidogenesis. J Biol Chem 273:16339–16345 [DOI] [PubMed] [Google Scholar]
- Manna PR, Eubank DW, Lalli E, Sassone-Corsi P, Stocco DM 2003 Transcriptional regulation of the mouse steroidogenic acute regulatory protein gene by the cAMP response-element binding protein and steroidogenic factor 1. J Mol Endocrinol 30:381–397 [DOI] [PubMed] [Google Scholar]
- Caron KM, Soo SC, Wetsel WC, Stocco DM, Clark BJ, Parker KL 1997 Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci USA 94:11540–11545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna PR, Dyson MT, Eubank DW, Clark BJ, Lalli E, Sassone-Corsi P, Zeleznik AJ, Stocco DM 2002 Regulation of steroidogenesis and the steroidogenic acute regulatory protein by a member of the cAMP response-element binding protein family. Mol Endocrinol 16:184–199 [DOI] [PubMed] [Google Scholar]
- Stocco DM, Wang X, Jo Y, Manna PR 2005 Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol 19:2647–2659 [DOI] [PubMed] [Google Scholar]
- Balasubramanian K, Lavoie HA, Garmey JC, Stocco DM, Veldhuis JD 1997 Regulation of porcine granulosa cell steroidogenic acute regulatory protein (StAR) by insulin-like growth factor I: synergism with follicle-stimulating hormone or protein kinase A agonist. Endocrinology 138:433–439 [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H 2005 Wnt signalling in stem cells and cancer. Nature 434:843–850 [DOI] [PubMed] [Google Scholar]
- Hsieh M, Boerboom D, Shimada M, Lo Y, Parlow AF, Luhmann UF, Berger W, Richards JS 2005 Mice null Frizzled4 (Fzd4−/−) are infertile and exhibit impaired corpora lutea formation and function. Biol Reprod 73:1135–1146 [DOI] [PubMed] [Google Scholar]
- Boerboom D, Paquet M, Hsieh M, Liu J, Jamin SP, Behringer RR, Sirois J, Taketo MM, Richards JS 2005 Misregulated Wnt/β-catenin signaling leads to ovarian granulosa cell tumor development. Cancer Res 65:9206–9215 [DOI] [PubMed] [Google Scholar]
- Goldberg VJ, Ramwell PW 1975 Role of rostaglandins in reproduction. Physiol Rev 55:325–351 [DOI] [PubMed] [Google Scholar]
- Laguë MN, Paquet M, Fan HY, Kaartinen MJ, Chu S, Jamin SP, Behringer RR, Fuller PJ, Mitchell A, Doré M, Huneault LM, Richards JS, Boerboom D 2008 Synergistic effects of Pten loss and WNT/CTNNB1 signaling pathway activation in ovarian granulosa cell tumor development and progression. Carcinogenesis 29:2062–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS 2005 Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-β-catenin signaling axis. Science 310:1504–1510 [DOI] [PubMed] [Google Scholar]
- Mulholland DJ, Cheng H, Reid K, Rennie PS, Nelson CC 2002 The androgen receptor can promote β-catenin nuclear translocation independently of adenomatous polyposis coli. J Biol Chem 277:17933–17943 [DOI] [PubMed] [Google Scholar]
- Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, Sun Z 2002 Linking β-catenin to androgen-signaling pathway. J Biol Chem 277:11336–11344 [DOI] [PubMed] [Google Scholar]
- Helmbrecht K, Kispert A, von Wasielewski R, Brabant G 2001 Identification of a Wnt/β-catenin signaling pathway in human thyroid cells. Endocrinology 142:5261–5266 [DOI] [PubMed] [Google Scholar]
- Hino S, Tanji C, Nakayama KI, Kikuchi A 2005 Phosphorylation of β-catenin by cyclic AMP-dependent protein kinase stabilizes β-catenin through inhibition of its ubiquitination. Mol Cell Biol 25:9063–9072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gummow BM, Winnay JN, Hammer GD 2003 Convergence of Wnt signaling and steroidogenic factor-1 (SF-1) on transcription of the rat inhibin α gene. J Biol Chem 278:26572–26579 [DOI] [PubMed] [Google Scholar]
- Parakh TN, Hernandez JA, Grammer JC, Weck J, Hunzicker-Dunn M, Zeleznik AJ, Nilson JH 2006 Follicle-stimulating hormone/cAMP regulation of aromatase gene expression requires β-catenin. Proc Natl Acad Sci USA 103:12435–12440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurk C, Maatz H, Rocnik E, Bialik A, Force T, Walsh K 2005 Glycogen-synthase kinase3β/β-catenin axis promotes angiogenesis through activation of vascular endothelial growth factor signaling in endothelial cells. Circ Res 96:308–318 [DOI] [PubMed] [Google Scholar]
- Chakravorty A, Joslyn MI, Davis JS 1993 Characterization of insulin and insulin-like growth factor-I actions in the bovine luteal cell: regulation of receptor tyrosine kinase activity, phosphatidylinositol-3-kinase, and deoxyribonucleic acid synthesis. Endocrinology 133:1331–1340 [DOI] [PubMed] [Google Scholar]
- Arvisais EW, Romanelli A, Hou X, Davis JS 2006 AKT-independent phosphorylation of TSC2 and activation of mTOR and ribosomal protein S6 kinase signaling by prostaglandin F2α. J Biol Chem 281:26904–26913 [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Forgèt MA, Sisson JH 2003 Ethanol stimulates ciliary beating by dual cyclic nucleotide kinase activation in bovine bronchial epithelial cells. Am J Pathol 163:1157–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR 2005 Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J 24:1571–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M 2000 Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843–852 [DOI] [PubMed] [Google Scholar]
- Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Døskeland SO, Blank JL, Bos JL 2002 A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 4:901–906 [DOI] [PubMed] [Google Scholar]
- Welsh GI, Foulstone EJ, Young SW, Tavare JM, Proud CG 1994 Wortmannin inhibits the effects of insulin and serum on the activities of glycogen synthase kinase-3 and mitogen-activated protein kinase. Biochem J 303(Pt 1):15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS, Johnson GV 2004 The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci 29:95–102 [DOI] [PubMed] [Google Scholar]
- Patel S, Doble B, Woodgett JR 2004 Glycogen synthase kinase-3 in insulin and Wnt signalling: a double-edged sword? Biochem Soc Trans 32:803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Vandenheede JR, Cohen P 1994 The mechanism by which epidermal growth factor inhibits glycogen synthase kinase 3 in A431 cells. Biochem J 303(Pt 1):27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryves WJ, Harwood AJ 2001 Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem Biophys Res Commun 280:720–725 [DOI] [PubMed] [Google Scholar]
- Clark BJ, Soo SC, Caron KM, Ikeda Y, Parker KL, Stocco DM 1995 Hormonal and developmental regulation of the steroidogenic acute regulatory protein. Mol Endocrinol 9:1346–1355 [DOI] [PubMed] [Google Scholar]
- Juengel JL, Meberg BM, Turzillo AM, Nett TM, Niswender GD 1995 Hormonal regulation of messenger ribonucleic acid encoding steroidogenic acute regulatory protein in ovine corpora lutea. Endocrinology 136:5423–5429 [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA 1995 Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785–789 [DOI] [PubMed] [Google Scholar]
- Dai F, Yu L, He H, Chen Y, Yu J, Yang Y, Xu Y, Ling W, Zhao S 2002 Human serum and glucocorticoid-inducible kinase-like kinase (SGKL) phosphorylates glycogen syntheses kinase 3β (GSK-3β) at serine-9 through direct interaction. Biochem Biophys Res Commun 293:1191–1196 [DOI] [PubMed] [Google Scholar]
- Sakoda H, Gotoh Y, Katagiri H, Kurokawa M, Ono H, Onishi Y, Anai M, Ogihara T, Fujishiro M, Fukushima Y, Abe M, Shojima N, Kikuchi M, Oka Y, Hirai H, Asano T 2003 Differing roles of Akt and serum- and glucocorticoid-regulated kinase in glucose metabolism, DNA synthesis, and oncogenic activity. J Biol Chem 278:25802–25807 [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Komiyama J, Viger RS, Okuda K 2009 The expression of the nuclear receptors NR5A1 and NR5A2 and transcription factor GATA6 correlates with steroidogenic gene expression in the bovine corpus luteum. Mol Reprod Dev 76:873–880 [DOI] [PubMed] [Google Scholar]
- Botrugno OA, Fayard E, Annicotte JS, Haby C, Brennan T, Wendling O, Tanaka T, Kodama T, Thomas W, Auwerx J, Schoonjans K 2004 Synergy between LRH-1 and β-catenin induces G1 cyclin-mediated cell proliferation. Mol Cell 15:499–509 [DOI] [PubMed] [Google Scholar]
- Grimes CA, Jope RS 2001 The multifaceted roles of glycogen synthase kinase 3β in cellular signaling. Prog Neurobiol 65:391–426 [DOI] [PubMed] [Google Scholar]
- Arakane F, King SR, Du Y, Kallen CB, Walsh LP, Watari H, Stocco DM, Strauss 3rd JF 1997 Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J Biol Chem 272:32656–32662 [DOI] [PubMed] [Google Scholar]
- Taurin S, Sandbo N, Yau DM, Sethakorn N, Dulin NO 2008 Phosphorylation of β-catenin by PKA promotes ATP-induced proliferation of vascular smooth muscle cells. Am J Physiol Cell Physiol 294:C1169–C1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO 2006 Phosphorylation of β-catenin by cyclic AMP-dependent protein kinase. J Biol Chem 281:9971–9976 [DOI] [PubMed] [Google Scholar]