Abstract
We analyzed the effect of short-term water deficits at different periods of sunflower (Helianthus annuus L.) leaf development on the spatial and temporal patterns of tissue expansion and epidermal cell division. Six water-deficit periods were imposed with similar and constant values of soil water content, predawn leaf water potential and [ABA] in the xylem sap, and with negligible reduction of the rate of photosynthesis. Water deficit did not affect the duration of expansion and division. Regardless of their timing, deficits reduced relative expansion rate by 36% and relative cell division rate by 39% (cells blocked at the G0-G1 phase) in all positions within the leaf. However, reductions in final leaf area and cell number in a given zone of the leaf largely differed with the timing of deficit, with a maximum effect for earliest deficits. Individual cell area was only affected during the periods when division slowed down. These behaviors could be simulated in all leaf zones and for all timings by assuming that water deficit affects relative cell division rate and relative expansion rate independently, and that leaf development in each zone follows a stable three-phase pattern in which duration of each phase is stable if expressed in thermal time (C. Granier and F. Tardieu [1998b] Plant Cell Environ 21: 695–703).
The responses of tissue expansion and cell division to environmental stresses have been well documented in organs that grow in one dimension and in steady state, such as roots or monocot leaves during linear elongation. Material elements flow through the elongation zone but spatial distributions of RER and cell division rate do not change with time (Gandar and Hall, 1988; Silk, 1992). This steady state remains unchanged if temperature is fluctuating, provided that durations and rates are expressed in thermal instead of clock time (Ben Haj Salah and Tardieu, 1995). In this case, environmental events that occur at different timings of organ development cause similar consequences on growth processes, because the structure of the expanding zone does not change with time. The spatial distributions of RER and RDR are influenced by environmental factors such as light (Muller et al. [1998] on maize roots), water deficit (Durand et al. [1995] on tall fescue leaves and Ben Haj Salah and Tardieu [1997] on maize leaves, and Sacks et al. [1997] on maize roots), and salinity (Bernstein et al. [1993] on sorghum leaves). In all cases, RER is mainly affected in zones distal to the meristem, whereas proximal zones are less affected. Expansion processes are usually more affected than division processes, resulting in a smaller cell length in stressed plants.
The consequences of environmental stresses are more complex for dicot leaves, which grow in two dimensions and in which no steady state can be defined (Wolf et al., 1986). The effect of short-term water deficits on both leaf area and cell number depends on the timing of the deficit (Lecoeur et al., 1995). A given water deficit affects differently the zones located near the base and near the tip of the leaf, in terms of cell expansion and of mitotic index (Heckenberger et al., 1998). A short-term water deficit affects expansion rate during the deficit, but also during a long period after plants have been rewatered (Lecoeur et al., 1995). We have attempted to use a recent model of leaf development (Granier and Tardieu, 1998a, 1998b) to interpret these behaviors.
Sunflower (Helianthus annuus L.) leaf development consists of a three-phase process with the transitions occurring with a tip-to-base gradient within the leaf (Granier and Tardieu, 1998a). During the first period increases in area and cell number in the leaf zone are both exponential, i.e. RER and RDR are constant. This period has a constant duration in a given zone of the leaf if expressed in thermal time (Granier and Tardieu, 1998b). In nonstressing conditions, RER and RDR measured during this period are common to all leaf zones and to different plant leaves if expressed in thermal time (Granier and Tardieu, 1998b). A second period follows with a decline in RDR but with a maintained RER. During the third period RER declines. This three-phase development is observed in each leaf zone, but periods with exponential expansion and with exponential division are shorter near the leaf tip than near the leaf base.
We have analyzed the way in which this simple pattern was modified by reproducible short-term water deficits experienced at different phases of leaf development to interpret the complex responses to water deficit in dicot leaves. A water deficit at a given time was experienced at different phases of development in different leaf zones, e.g. the leaf tip can be in the third phase while the leaf base is still in the first phase during the stress. Water deficits of similar intensities were also applied at different leaf ages to test whether differences in the effects due to the gradient of development within a leaf were conserved for several timings of stress.
MATERIALS AND METHODS
Plant Culture and Growth Conditions
Sunflower (Helianthus annuus L., hybrid Albena) plants were grown in a greenhouse in Montpellier (southern France) during four growing periods: April 1995, July 1995, September 1995, and July 1996. Seeds were sown in 60 columns (0.14 m in diameter, 0.65 m in height) containing a 1:1 mixture (v/v) of a loamy soil and an organic compost. Each column was filled with 5.25 ± 0.25 kg of dry soil. Experiments were carried out with natural light except in April 1995, in which additional light was provided by a bank of sodium lamp. Light was measured continuously using a PPFD sensor (LI-190SB, Li-Cor, Lincoln, NE). Air temperature and RH were measured every 20 s (model HMP35A, Vaisala Oy, Helsinki, Finland). Leaf temperature was measured with a copper-constantan thermocouple (0.4 mm in diameter) located in the apical meristem before leaf emergence, and appressed under the lamina after the leaf appeared. Temperature, PPFD, and RH were averaged and stored every 600 s in a data logger (LTD-CR10 wiring panel, Campbell Scientific, Shepshed, Leicestershire, UK). Environmental conditions for the four growing periods are presented in Table I.
Table I.
Environmental conditions in the greenhouse during the four growing periods
| Experiment | Air Temperature Day/Night | Cumulative PPFD | Duration of Expansion with VPD > 3 kPa | Photoperiod |
|---|---|---|---|---|
| °C | mol m−2 d−1 | % of total | h | |
| April 1995 | 22.0 /15.9 | 18.1 | 0.0 | 14.5a |
| July 1995 | 29.0 /22.5 | 26.6 | 11.3 | 15.0 |
| September 1995 | 25.9 /21.3 | 19.7 | 0.2 | 13.5 |
| July 1996 | 26.5 /22.4 | 26.7 | 1.4 | 15.0 |
Means were calculated over the period of expansion of leaf 8. Duration of periods with high air vapor pressure deficit (VPD) was calculated relative to total duration of expansion.
Additional light was given in the greenhouse during the expansion of the leaf.
Columns were fully irrigated, covered with foil, and allowed to drain freely for 24 h. Soil water content at that time was consistently 0.40 ± 0.07 g g−1 in all experiments, and was taken as the estimate of soil water-retention capacity. The lower limit of ASW was determined in a preliminary experiment (T. Simonneau, unpublished data), which related soil water content to predawn leaf water potential. It was characterized as soil water content when predawn leaf water potential was −1.5 MPa and was 0.14 ± 0.02 g g−1. Maximum ASW is defined as the difference between soil water contents at water-retention capacity and lower limit of available water multiplied by the weight of dry soil in columns. Five columns per treatment were weighted before each watering, one to three times per day, depending on evaporative demand. This allowed us to calculate the volume of nutrient solution (modified one-tenth-strength Hoagland solution supplemented with minor nutrients) required to maintain soil water content at a constant value.
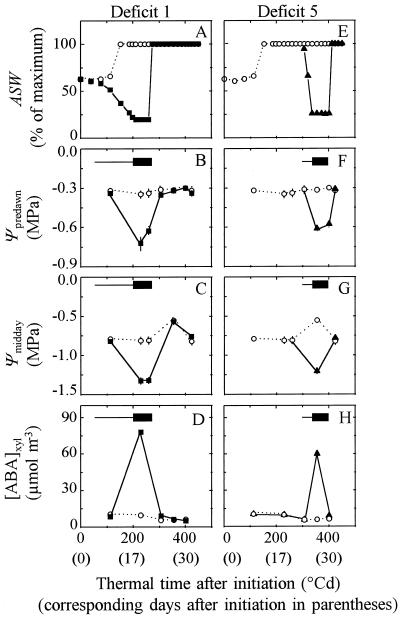
In the control treatment, ASW was maintained at 70% of the maximum value until the emergence of leaf 8 and later at 100%. The relatively low ASW imposed to control plants at the beginning of the experiment allowed young plants with a low transpiration rate to rapidly deplete the soil at the beginning of the drought treatment. In the water-deficit treatment watering was stopped until ASW reached 23% of the maximum and was managed afterward to maintain it at a constant level for 4 to 5 d (Fig. 1, A and E). Columns were then rewatered and soil was maintained at maximum ASW until the end of the experiment. Similar water deficits were experienced at six different periods of development of leaf 8 (Table II). Deficit periods were numbered from 1 to 6, from the earliest to the latest, respectively. Deficits 1 to 4 were imposed at the same time that the watering rate of control plants was increased such that ASW reached the maximum value (Fig. 1A). Cessation of irrigation occurred later in deficits 5 and 6, when the transpiration rate was large enough to deplete soil water in 2 or 3 d (Fig. 1E). Deficits 1 and 5 were experienced during April 1995, deficits 2 and 6 during July 1996, deficit 3 during July 1995, and deficit 4 during September 1995.
Figure 1.
Change with time in ASW (in % of maximum) (A and E), in predawn leaf water potential (Ψpredawn) (B and F), in midday leaf water potential (Ψmidday) (C and G), and in the [ABA] in the xylem ([ABA]xyl) (D and H) during two soil water deficits (deficits 1 and 5, April 1995). ○, Control plants; ▪, early deficit 1; and ▴, late deficit 5. Horizontal, thin bars, Periods with declining soil water content; horizontal, thick bars, periods during which ASW was maintained at 23% of maximum.
Table II.
Characteristics of water deficits during all experiments
| Deficit | Cessation of Irrigation | Constant Deficit | Rewatering | Ψpredawn | [ABA] | Reduction in Leaf Area | Reduction in Cell No. |
|---|---|---|---|---|---|---|---|
| °Cd (d) | MPa | μmol m−3 | % of control | ||||
| 1 | 53 (4) | 200 (15) | 268 (19) | −0.68 | 78 | 41 | 40 |
| 2 | 143 (10) | 193 (12) | 286 (16) | −0.68 | – | 44 | 45 |
| 3 | 178 (9) | 211 (10) | 305 (14) | −0.65 | 80 | 30 | 25 |
| 4 | 243 (13) | 288 (15) | 336 (18) | −0.62 | – | 34 | 20 |
| 5 | 299 (21) | 337 (24) | 395 (27) | −0.60 | 60 | 18 | 0 |
| 6 | 323 (15) | 351 (17) | 449 (21) | −0.68 | 83 | 10 | 0 |
Cessation of watering, beginning of the period with constant water deficit, and rewatering are positioned in degree days (°Cd) and in days (in parentheses) after the initiation of leaf 8. Predawn leaf water potential (Ψpredawn) and [ABA] are presented for each deficit period. Reductions in final leaf area and final cell number per leaf are presented during the same experiment.
Leaf water potential was measured before dawn and at noon. At least five mature leaves per treatment were excised and placed in a pressure chamber for measurement. The amount of 100 mm3 of xylem sap from the leaves used for predawn measurements was collected at a pressure of about 0.35 MPa above the balancing pressure. The extracted sap was stored at −80°C for subsequent ABA analysis by radioimmunoassay (Quarrie et al., 1988). During July 1995, stomatal conductance and photosynthesis of the youngest, fully expanded leaf was measured at noon using a ventilated, closed chamber with a manually controlled null-balance system (volume, 106 mm3; contact area, 7600 mm2; model LI-6200, Li-Cor).
Growth Measurements
A leaf was considered initiated when its primordium was visible (about 40 μm long) on the apical meristem with a stereomicroscope (model wild F8Z, Leica) at a magnification of ×80. The areas of three leaves on position 8 on the stem were measured every 2nd d from initiation to emergence of the leaf by dissecting the apex under the microscope, excising the studied leaf, and measuring its area with an image analyzer (model V 4.10, Bioscan-Optimas, Edmonds, WA). When the leaf was 25 mm long, it was marked with India ink by a stamp, which drew a regular grid of 70 points. Five leaves were photographed with a video camera every day at noon and the area was determined with the image analyzer. Cell area in the upper epidermis of three leaves was measured every 2nd d from 5 d after leaf initiation until end of expansion. A transparent negative film was obtained with a varnish spread on the leaf. Films were placed under a microscope (Leica-Leitz DM RB, Wetzlar, Germany) coupled to the image analyzer. The areas of 50 epidermal cells were measured in 3 to 8 (depending on leaf length) transects perpendicular to the midrib and labeled by their distance to the point of petiole insertion. Spatial analysis of tissue expansion and cell division were carried out using triangulation of the grid drawn on the lamina (Granier and Tardieu, 1998a). Areas of several triangles were pooled in four 2.5-mm-wide zones perpendicular to the midrib of the leaf: base, MB, MT, and tip zone. When the leaf was 25 mm long, the midpoints of each zone were located at 23.75, 18.75, 11.25, and 1.25 mm from the leaf tip, respectively.
Rates and durations were expressed in time or in thermal time (Granier and Tardieu, 1998b). Thermal time was calculated by daily integration of leaf temperature minus a base temperature of 4.8°C (common x intercept of the relationships of all of the variables with leaf temperature). RER of triangle i on day j (RERi,j) was calculated as the local slope (at time j) of the relationship between the logarithm of the area of triangle i (Ai,j) and thermal time:
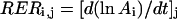 |
1 |
taking into account Ai,j on days j − 1, j, and j + 1 by linear regression.
RER of the whole leaf was calculated in the same way, but considering leaf area instead of zone area. RDR in zone i at time j (RDRi,j) was calculated as the local slope of the relationship between the logarithm of cell number (Ni,j) and thermal time:
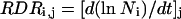 |
2 |
taking into account Ni on days j − 1, j, and j + 1 in the same way as in Equation 1. RDR in the whole leaf was calculated in the same way, but considering cell number per leaf instead of cell number per zone. cdt was calculated from RDR in a zone or in the leaf:
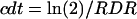 |
3 |
Expansion and epidermal cell division in a zone were considered to begin on the day when the leaf was initiated on the apex. They were considered to end on the day when RER or RDR reached 0 in the considered zone. Duration of the period with exponential increase in zone area or in cell number per zone was calculated from leaf initiation to the day when RER or RDR decreased below 15% of its mean value, averaged from initiation in the zone.
Reductions in final area, final cell number, and final cell area in a zone or in the leaf were calculated as the difference between the values in control and droughted plants divided by the value in control plants. Reductions in RER and RDR were calculated in the same way and were averaged over the water-deficit period.
Flow Cytometry and Calculation of the Durations of Cell-Cycle Phases
During July 1996, 10 leaves in position 8 on the stem were collected at 6 am and were dissected into three areas corresponding to the zone B, MB, and MT. The epidermal tissue of each zone was detached with a scalpel and chopped with a razor blade in a plastic Petri dish containing 2 cm3 of extraction buffer (Dolezel et al., 1989). The suspension obtained was passed through a 50-μm nylon filter and nuclei were stained with 100 mm3 of propidium iodine (1% in water). Fluorescence intensity of 10,000 nuclei, linked to DNA content, was measured with a FACSCAN-argon laser flow cytometer (488 nm, 15 mW, Becton Dickinson). Proportions of nuclei with 2c and 4c were interpreted as the proportions of nuclei in phases G0-G1 and G2-M of the cell cycle. Nuclei with intermediate amounts of DNA were considered in phase S (Granier and Tardieu, 1998a). The durations of each phase of the cell cycle were calculated as the product of the percentage of cells in this phase at time j, estimated by flow cytometry, by cdt (for arguments, see Granier and Tardieu, 1998a). The duration of phase S-G2-M (tS-G2-m, j) was therefore calculated as:
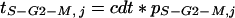 |
4 |
where pS-G2-M, j is the frequency of cells in phases S, G2, and M at time j. The duration of phase G0-G1 was calculated in the same way.
RESULTS
Characteristics of Water Deficits
Predawn leaf water potential of control plants remained between −0.25 and −0.32 MPa during the studied period, without appreciable difference between the periods when ASW was at either 70% or 100% of the maximum value (Fig. 1, B and F). Depending on evaporative demand, it took 2 to 11 d after cessation of irrigation for ASW to decline to 23% of the maximum value (Fig. 1, A and E; Table II). Predawn leaf water potential declined rapidly and reached a minimum value, which was maintained until rewatering, at values which ranged from −0.60 to −0.68 MPa in water-deficit periods 1 to 6 (Table II). Midday leaf water potential was consistently lower than predawn leaf water potential by 0.4 to 0.6 MPa in all treatments (Fig. 1, C and G). The [ABA] in the xylem sap reached 60 to 83 μmol m−3 during water deficit, and remained constant at about 10 μmol m−3 in control plants (Table II; Fig. 1, D and H). Stomatal conductance was reduced by 36% during water deficit (Table III), but photosynthesis was similar in all treatments (insignificant difference). The increase in leaf temperature due to partial stomatal closure was also very slight (mean difference, 0.7°C, insignificant difference). After rewatering, leaf water potential and [ABA] returned rapidly (less than 1 d) to the corresponding values in control treatment.
Table III.
Net photosynthesis, stomatal conductance, and leaf temperature measured during the period with constant deficit during deficit 6, July 1995
| Treatment | Net Photosynthesis | Stomatal Conductance | Leaf Temperature |
|---|---|---|---|
| mol m−2 s−1 | °C | ||
| Control | 27.26 ± 2.4 | 1.49 ± 0.1 | 30.2 ± 0.4 |
| Deficit 6 | 24.3 ± 4.2 | 0.95 ± 0.4 | 30.9 ± 0.7 |
Measurements were carried out on five plants per treatment at noon with high radiation (>1000 μmol m−2 s−1). Intervals of confidence are at P = 0.05.
Change with Time in Leaf Expansion Rate, as Affected by Water Deficit
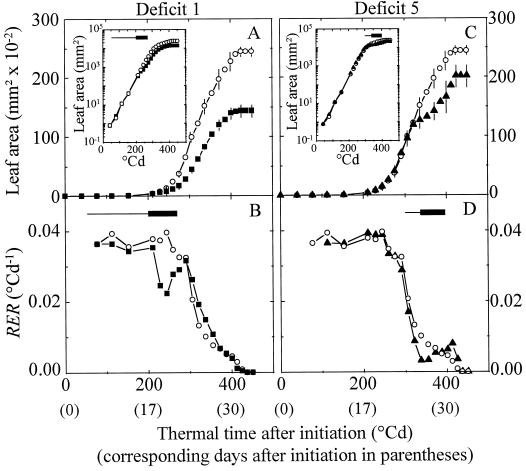
Leaf area of control plants followed an exponential increase (constant RER, Fig. 2, B and D) for 260°Cd after leaf initiation. A decline in RER occurred afterward, from 260°Cd to 432°Cd after leaf initiation. As presented earlier (Granier and Tardieu, 1998b), these durations differed between experiments if expressed in clock time (21–31 d from leaf initiation to end of expansion), but were common to all experiments if expressed in thermal time (432°Cd ± 12°Cd). None of the water deficits experienced by plants affected these durations. Absolute expansion rate was reduced during early deficit 1, but the maximum effect of deficit was observed after rewatering and lasted 10 d until end of expansion. In contrast, RER was affected during the water deficit only (Fig. 2B). It was reduced by 40% during deficit 1 (Fig. 2B), and by 40%, 30%, and 38%, respectively, by deficits 2 to 4 (not shown). When water deficit occurred later in leaf development (deficits 5 and 6), it reduced final leaf area to a lesser extent than early deficits of similar intensities (Fig. 2C; Table II). However, RER was reduced to the same extent as in early water deficit (30% and 40% of control, Fig. 2D).
Figure 2.
Change with time in leaf area (A and C) and in RER (B and D) of leaf 8 of control plants (○), of plants with early deficit 1 (▪), and of plants with late deficit 5 (▴) in April 1995. Positions of the periods of water deficits 1 and 5 are as in Figure 1. Insets, Change with time in leaf area with a log scale. For better legibility, interval of confidence at 0.05 are given every 2 d for leaf area (n = 5, A and C).
Local expansion rates in zone B and MT followed the same time courses as in the whole leaf (Fig. 3, A and D). RER was first constant and similar in all zones of control plants, then it decreased first at the leaf tip and progressively toward the base (not shown; Granier and Tardieu, 1998a), resulting in a larger final area in zone B than in MT. The respective positions of the periods with exponential expansion (constant RER) and with declining RER are presented in Figure 4A for each leaf zone. This timing was common to all experiments if expressed in thermal time (Granier and Tardieu, 1998b) and was affected by none of the water deficits. Early deficit 1 caused a reduction in absolute expansion rate during and after the deficit (Fig. 3A) in zone B, with a reduction in RER by 40% during the deficit only (not shown). It caused a smaller effect in MT, in which RER was already declining during the deficit, than in zone B, which was still in the exponential phase during the deficit. Deficit 5 caused a smaller effect than deficit 1 on zone B area and had virtually no effect on zone MT, in which RER was already close to 0 during deficit 5. These results suggest that reductions in RER were similar in all zones and for all timings of water deficit (mean value 36%), and that the effect of these deficits on final area progressively decreased as the deficit occurred later in the development of the considered zone.
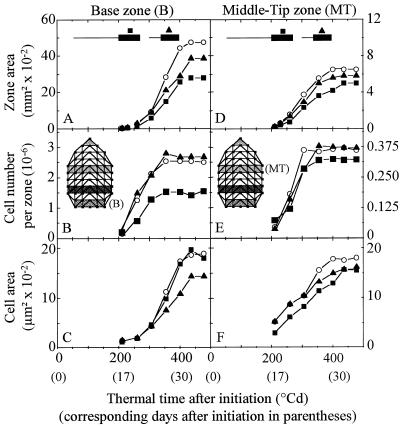
Figure 3.
Change with time in the area of zone B (A) and MT (D), in the cell number per zone B (B) and MT (E), in cell area in zone B (C) and MT (F) in leaves of control plants (○), and of plants in deficit 1 (▪) and of plants in deficit 5 (▴). For better legibility, interval of confidence at 0.05 are given at the end of expansion of the considered zone for zone area (n = 5, A and D) and cell area (n = 50, C and F).
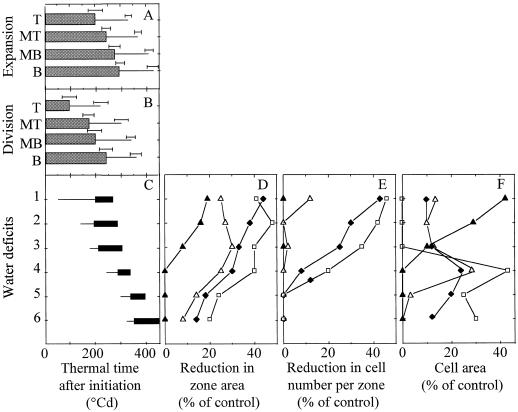
Figure 4.
Respective positions of periods with expansion (A) and cell division (B) in four zones drawn on the lamina, and positions of the six water-deficit periods (C). The reductions in the final area, final cell number, and final cell area in each zone are presented, respectively, in D, E, and F for each deficit period. They are calculated as the difference between values in control and droughted plants divided by the value in control plants. A, Thick bars, Periods with constant RER; thin bars, periods with declining RER. Horizontal error bars, Interval of confidence (P = 0.95) on durations, calculated over all experiments and treatments. B, Thick bars, Periods with constant RDR; thin bars, periods with declining RDR. Horizontal error bars, Interval of confidence (P = 0.95) on durations, calculated over all experiments and treatments. C, Thin bars, Periods with declining soil water content; thick bars, periods during which ASW was maintained at 23% of maximum. D, E, and F, Tip zone (T, ▴), MT (▵), MB (♦), and base of the leaf (B, □).
Change with Time in Cell Division Rate and Cell-Cycle Duration as Affected by Water Deficit
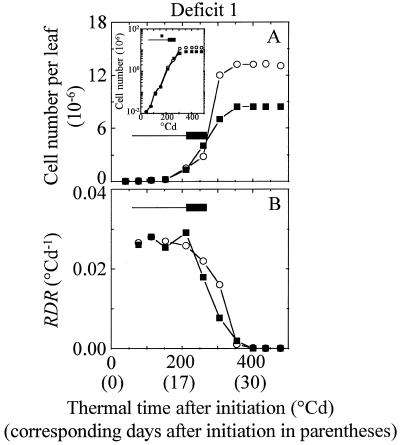
Cell number per leaf followed an exponential increase (constant RDR) for 202 ± 19°Cd after leaf initiation. RDR declined afterward until 330 ± 16°Cd (Fig. 5). Water deficit had no effect on these durations. This pattern was observed in each leaf zone, with earlier cessation of cell division in zones near the leaf tip (MT, Fig. 3E) than in the leaf base (Fig. 3B). The durations of periods with constant and with declining RDR were similar in all of the experiments if expressed in thermal time (Fig. 4B). Deficit 1 slowed the absolute increase in cell number during the deficit, but also after rewatering. In contrast, the reduction in RDR was observed during the deficit period only, and only appeared 50°Cd after the beginning of the period with constant water deficit. RDR was reduced by 39% during deficit 1 (Fig. 5B) and by 40%, 30%, and 38%, respectively, during deficits 2 to 4 (not shown). Reductions in RDR were similar in zone B, MB, and MT (not shown). Deficits 5 and 6, which occurred after cessation of division, had no effect on cell number.
Figure 5.
Change with time in cell number per leaf (A) and corresponding RDR (B) in the epidermis of leaves 8 of control plants (○) and of plants with early deficit 1 (▪). Position of the period of water deficit 1 is as in Figure 1. Inset, Change with time in leaf cell number with a log scale.
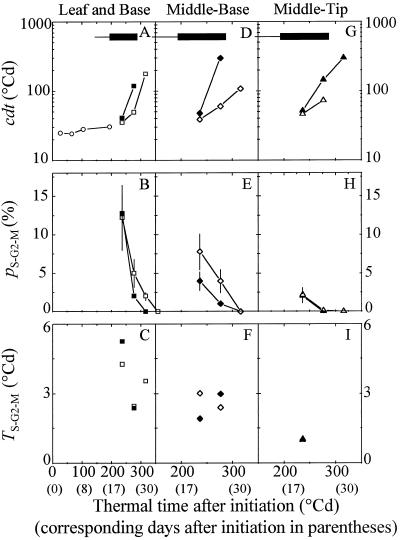
cdt remained stable (24°Cd in thermal time) until 200°Cd after leaf initiation in zone B of control plants, and increased rapidly afterward (Fig. 6A). This increase in cdt occurred earlier in MB and MT of well-watered plants (Fig. 6, A, D, and G). Water deficit had a relatively low effect on cdt calculated at 220°Cd but considerably affected it after 275° Cd. The proportion of nuclei in phase S-G2-M decreased in all of the zones and treatments as cdt was increasing (Fig. 6, B, E, and H). Water deficit did not affect the proportion of nuclei in phase S-G2-M at 220°Cd in zone B, consistent with the small difference in cdt at that time. The proportion of nuclei in phase S-G2-M was decreased by water deficit in MB, but differences could not be detected in MT, where measured proportions were already low at 220°Cd. Therefore, both in situ calculation of cdt and flow cytometry detected an appreciable effect of water deficit on the cell cycle, but this effect did not appear immediately after the onset of water deficit. The duration of phase S-G2-M remained in a narrow range in all of the leaf zones and regardless of watering treatments (1–4°Cd, i.e. 1.2–4.8 h at 25°C) because the decrease in the proportion of nuclei in phase S-G2-M was compensated by an increase in cell-cycle duration (Fig. 6, C, F, and I). This suggests that an increase in cell-cycle duration by water deficit was due to an increase in the duration of phase G0-G1.
Figure 6.
Change with time in cdt (A, D, and G), in percentage of nuclei in the S-G2-M phase of the cycle (B, E, and H), and in duration of the S-G2-M phase (tS-G2-M) (C, F, and I) in three zones drawn on the lamina experiment in July 1996. A, B, and C, Zone B (see Fig. 5). D, E, and F, MB. G, H, and I, MT. Open symbols, Control plants; closed symbols, water deficit 2. cdt in the whole leaf is given in A (○) during the period with exponential increase in leaf cell number. Horizontal thick or thin bars represent water-deficit periods as in Figure 1. Note that the x scale begins at 0°Cd in A, B, and C, and at 150°Cd in D, E, F, G, H, and I. Vertical bars, Interval of confidence at P = 0.05.
Change with Time in Cell Area as Affected by Water Deficit
Cell area was larger in MT than in zone B at 205°Cd after leaf initiation (Fig. 3, C and F). It increased slowly in zone B, in which RER and RDR were maximum, and increased rapidly in MT, in which RDR was already declining (Fig. 4, A and B). Cell expansion rate was reduced by early water deficit 1 in MT but not in zone B (Fig. 3, C and F). In contrast, it was more reduced in zone B than in MT by late deficit 5 (Fig. 3, C and F).
Effect of the Timing of Water Deficit on Final Leaf Area, Cell Number, and Cell Area
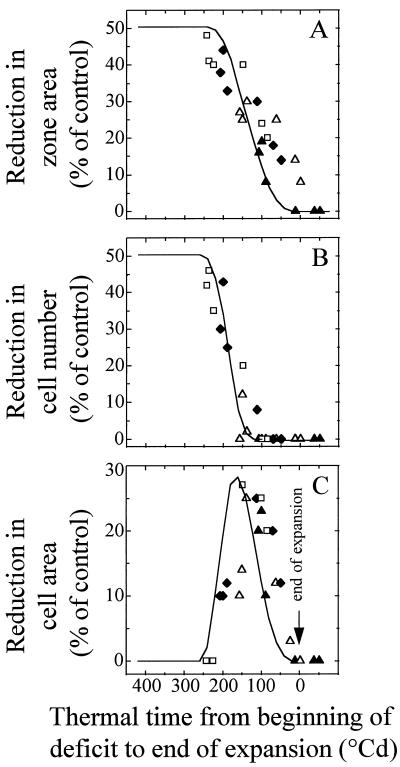
Figure 4 shows the effect of timings of water deficit, of expansion in each leaf zone, and of cell division in each leaf zone and are compared. In particular, the respective positions of the periods with exponential expansion and with exponential cell division are presented in Figure 4, A and B, for each leaf zone. The deficits of similar intensities but experienced at different stages of development of the considered leaf zone had contrasting effects on the final area of the zone (Fig. 4D). The overall tendency was that the effect on final zone area was larger with earlier water-deficit periods, and for a given water-deficit period, was larger in younger (base) versus older (tip) zones of the leaf. Water deficit had a very small effect (reductions in area smaller than 10%) when it occurred during the period with declining RER, although absolute expansion rate was highest during this period (Fig. 2, A and C). Reductions in final area of a leaf zone are presented in Figure 7A as a function of the duration of the period, which elapsed from the beginning of constant water deficit until cessation of expansion of the considered zone. A unique relationship applied to all of the experiments, all zones of the leaf, and all timings of water deficits. The effect of water deficits on final area in a zone decreased with the age of the considered zone.
Figure 7.
Effect of the timing of water deficit on zone area, cell number per zone and mean cell area, experimental points (symbols, as in Fig. 4), and simulated relationships (lines). A, Reduction in final area of individual zones of leaves subjected to water deficits 1 to 6, plotted against the duration (°Cd) of the period from the beginning of the deficit period to the end of expansion in the considered zone. B, Reduction in cell number in the same zones subjected to deficits 1 to 6, with the same x axis as in A. C, Reduction in cell area in the same zones subjected to deficits 1 to 6, with the same x axis as in A. Symbols are as in Figure 5, D, E, and F. Simulated curves in A and B were calculated independently of experimental points, by considering reductions in RER and RDR by 36% and 39%, respectively, during periods of water deficits lasting 75°Cd (see text and Fig. 8). Simulated curve in C was calculated from those in A and B, assuming that the effect of water deficit on RDR and RER are independent.
Water deficits of similar intensities also had different effects on the final cell number per zone, depending on their timings (Fig. 4E). In each zone maximum effects were observed with earliest deficits. This effect decreased afterward and was lower in zones near the tip than near the base of the leaf. Reductions in final cell number per zone are presented in Figure 7B with the same representation as in Figure 7A. As in the case of expansion, a unique relationship applied to all of the experiments, all zones of the leaf, and all timings of water deficits. It suggests that the effect of water deficits on final cell number in a zone decreased with the age of the zone. In contrast, the corresponding analysis applied to cell area provided a more complex pattern. Earliest water deficits (200°Cd–300°Cd before end of expansion of the considered zone) had a low effect on cell area (Figs. 4F and 7C). The maximum effect on cell area was observed for water deficits that occurred from 100°Cd to 200°Cd before end of expansion, i.e. when RER was still constant but RDR was already declining (Fig. 4F).
Simulation of Reductions in Final Area, Final Cell Number, and Final Cell Area as a Function of the Timing of Water Deficit
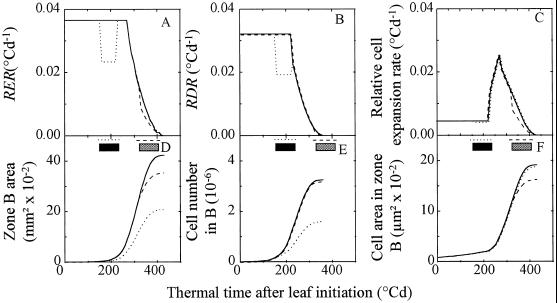
Experimental patterns of observed reductions in zone area, in cell number per zone, and in cell area were compared with tendencies simulated by using the three-phase development model described in the introduction. Expansion and cell division in zones of control leaves were simulated considering the time courses of RER (Fig. 8A) and of RDR (Fig. 8B), as presented in Granier and Tardieu (1998b). Effects of water deficits were simulated by uniformly reducing RER by 36% and RDR by 39% (mean reductions observed in experimental data) regardless of the considered zone and the timing of water deficit. Simulated changes with time in relative cell expansion rate were calculated at each time step and in all treatments as the difference between changes in RER and RDR (Fig. 8C). Changes with time in zone area (Fig. 8D) and cell number per zone (Fig. 8E) were then calculated with initial values of 0.2 mm2 and 2500 cells, respectively, for zone area and cell number per zone at leaf initiation. Examples of simulations corresponding to deficits 1 and 5 are presented in Figure 8. Consistent with experimental data, temporary reduction in RER (respectively in RDR, Fig. 8, A and B) resulted in a permanent reduction in absolute expansion rate (absolute cell division rate, respectively, Fig. 8, D and E). The early deficits, experienced when RER and RDR were maximum, had the greatest effect on final zone area and cell number but had no effect on final cell area, to the difference of the late deficit.
Figure 8.
Simulated time courses of RER (A), RDR (B), and relative cell expansion rate (C) in zone B of the leaf. Simulations of zone area (D), cell number (E), and cell area (F) in zone B in control plants and with two water deficits. Simulations of RER and RDR of control plants (—) are based on the model presented in Granier and Tardieu (1998b). Two water deficits lasting 75°Cd are simulated by imposing a reduction in RER by 36% and a reduction in RDR by 39% during the deficit. All other characteristics, presented in C to F, are deduced from time courses of RER and RDR presented in A and B (see text). ······, Early water deficit occurring while RER and RDR are constant. The position of this deficit is represented by the black, thick, horizontal line. ----, Late water deficit occurring during the period with declining RER and RDR. Its position is represented by the gray, thick, horizontal line.
The consequences of 22 similar deficit periods of 75°Cd (mean duration of constant water deficits, Table II) were simulated every 20°Cd from leaf initiation to end of expansion. Reductions in zone area, in cell number per zone, and in cell area were calculated for each simulated period of deficit, and are presented in Figure 7 (solid lines) as a function of the duration of the period elapsing from the beginning of water deficit to end of expansion of the considered zone. Regardless of the considered zone, deficits occurring more than 200°Cd before the end of expansion, i.e. when RER was maximum, uniformly reduced final zone area by 50%, consistent with experimental data corresponding to earliest water deficits. Later, the predicted effect of water deficit decreased, consistent with experimental data (although with a slight overestimation of the effect of earliest deficits and underestimation of that of latest deficits). Similarly, simulated deficits occurring more than 180°Cd before the end of expansion (period with maximum RDR) uniformly decreased final cell number by 50%. Effects of later deficit were smaller, consistent with experimental data. Simulated effects on cell area had a bell shape. Consistent with experimental data, simulations predicted a very small effect of early deficits, which occur while increases in cell number per zone and in zone area are both exponential. A maximum effect was predicted during the period when RER is still maximal but RDR is already declining. This effect decreased when deficit is experienced while RER is declining. The bell-shape tendency broadly fitted experimental data, although with a bias on the position of the maximum effect due to the error in prediction of the reduction in final zone area. The relatively simple processes presented in Figure 8, A and B, can therefore account for the diversity of effects of similar water deficits presented in Figure 4, D, E, and F, depending on timing of deficit and on the considered zone.
DISCUSSION
Water deficit of similar intensities but experienced at different stages of leaf development had markedly different effects on the final area, cell number, and cell area within a given zone of the leaf. However, basic processes of leaf development were affected essentially in the same way in all zones by all timings of water deficit.
Expansion, as estimated by RER, was affected to the same magnitude during all deficit periods and in all leaf zones, regardless of the actual value of RER in the corresponding leaf zone in well-watered plants. This suggests that mechanisms leading to a reduction in local expansion rate were conserved in all studied conditions, consistent with the fact that the [ABA] in the xylem sap was similar during all deficit periods. Considered deficits reduced RER, although they had virtually no effect on photosynthesis rate, consistent with earlier studies (Boyer, 1970; Saab et al., 1995). After rewatering and a return to near zero of the [ABA] in the xylem sap, RER rapidly returned to its value in the control treatment and slightly exceeded it, whereas absolute expansion rate was still lower than in control plants, as observed by Lecoeur et al. (1995). Duration of expansion was affected neither in the whole leaf nor in the individual zone.
Cell division was affected by water deficit, as both RDR and the proportion of cells in the S-G2-M phase were markedly reduced in droughted plants. RDR was reduced to a same extent in all experiments and in all leaf zones. If we assume that all cells in a given zone are dividing at similar rates (for arguments, see Granier and Tardieu, 1998a), the cdt can be considered as a correct estimate of the duration of cell cycle of epidermal cells (Green and Bauer, 1977; Beemster et al., 1996). Our calculations lead to the conclusion that increase in the cdt was due to a lengthening of phase G0-G1 without a detectable effect on the duration of phase S-G2-M. Such lengthening of phase G0-G1 has been reported in other situations: spatial gradient in RDR in meristems (Nougarède and Rondet, 1978) or in dicot leaves (Granier and Tardieu, 1998a), and effects of Suc starvation (Van't Hof, 1973) or of toxic metals on roots (Powell et al., 1986). A delay (about 50°Cd) was observed between the beginning of water deficit and the reductions in RDR, and in proportion of nuclei in the S-G2-M phase. It may be because the cell cycle was partially blocked at a key point in phase G0-G1. Therefore, it took some time for this blockage to result in a significant reduction in cell-cycle duration, because nuclei that had already crossed this point in droughted plants could finish their cycle.
Reductions in final zone area or in final cell number per leaf zone depended on the position of the period of water deficit relative to the timing of development in the studied zone. Both simulation and experiments showed that the final area of a zone was most affected when the water-deficit period occurred while RER was maximal. This effect decreased with time, together with RER. The same effect was observed for final cell number, but with an earlier decrease in the effect of water deficit because RDR began to decline earlier than RER (Granier and Tardieu, 1998a, 1998b). It is noteworthy that periods with maximum absolute increase in area or in cell number, which have been the object of most analyses (Rawson and Turner, 1982; Sadras et al., 1993; Palmer et al., 1996), were not those during which water deficit caused a maximum effect. The apparent ”after effect” of early deficits was due to a characteristic of exponential processes that expansion rate at each time is proportional to leaf area at that time. A temporary reduction in RER during water deficit resulted in a definitive reduction in absolute expansion rate because the area was smaller (Fig. 8). This negative after-effect decreased as the deficit period was closer to the end of expansion.
The effect of the timing of water deficit on reduction in area, cell number, and cell area of a leaf zone can appear complex, especially for cell area (Fig. 4F). However, this complex pattern can be simulated from two simple processes: (a) the three-phase model of leaf development presented in the introduction, and (b) the assumption that water deficit affects independently the processes of cell division and expansion. Therefore, it is logical that water deficit has virtually no effect on final cell area if it affects the RER and the RDR when both are maximum, as deficit affected them by similar proportions. A water deficit occurring later in leaf development still affects RER and RDR by the same proportion, but during a period when division rate has already decreased. This causes a major effect on expansion than on division processes, thereby causing maximum effect on individual cell area.
Spatial variability within the leaf of the effect of water deficit was accounted for by the same reasoning, taking into account the tip-to-base gradient of development in the leaf. Zones located near the leaf tip stopped exponential processes of expansion and cell division before those located near the leaf base. A water deficit experienced 200°Cd after leaf initiation had the same effect on the area, cell number, and cell area in the leaf tip as those observed in the leaf base for a water deficit experienced 280°Cd after leaf initiation. If origin of time was placed at the end of expansion of the considered zone, such as in Figure 7, common relationships were observed for all leaf zones between the timing of water deficits and their effects on final area, cell number, and cell area of all zones.
This analysis supports Green's theory (1976) that cell division and tissue expansion should be viewed as independent processes, because it gives a theoretical framework that accounts for experimental results. The alternative theory, which considers cell division and individual cell expansion as independent variables (Terry et al., 1971; Clough and Milthorpe, 1975; Lecoeur et al., 1995), is compatible with the existence of the negative after-effect of early water deficit, considered to be due to a reduction in cell number without effect on individual cell expansion rate (Lecoeur et al., 1995, 1996). However, it is not able to account in a simple way for the complex pattern of results shown in Figure 4.
CONCLUSION
Similar water deficits experienced at different phases of leaf development had contrasting effects on final area, cell number, and cell area in a given zone of the leaf, and these effects differed between zones. Considerable after-effect of early deficits on absolute increases in area and in cell number were observed after rewatering. This was in spite of the fact that the reductions in RER and RDR were similar for all timings of deficit and in all zones, and were observed during water deficits only. All observed behaviors could be simulated under the hypotheses that water deficit affects independently cell division and tissue expansion, and that leaf development follows in each zone a stable three-phase pattern in which duration of each phase is stable if expressed in thermal time (Granier and Tardieu, 1998b). We believe that the present analysis could facilitate further analysis of the effect of water deficit on expansion and cell division of dicot leaves, since the apparent variability of responses can be analyzed by using a simple model of leaf development.
ACKNOWLEDGMENTS
We thank Philippe Barrieu for ABA measurements and technical assistance during flow-cytometry experiments, Jean-Jacques Thioux for his help during the set up of the experiments, and Thierry Simonneau for critical comments on the manuscript.
Abbreviations:
- ASW
available soil water
- °Cd
degree days
- cdt
cell doubling time
- MB
middle-to-base zone
- MT
middle-to-tip zone
- RDR
relative cell division rate
- RER
relative expansion rate
- zone B
base zone
Footnotes
This work was supported by grants from the Institut National de la Recherche Agronomique and the Centre Technique Interprofessionnel des Oléagineux Métropolitains.
LITERATURE CITED
- Beemster GTS, Masle J, Williamson RE, Farquhar GD. Effects of soil resistance to root penetration on leaf expansion in wheat (Triticum aestivum L.): kinematic analysis of leaf elongation. J Exp Bot. 1996;304:1663–1678. [Google Scholar]
- Ben Haj Salah H, Tardieu F. Temperature affects expansion rate of maize leaves without change in spatial distribution of cell length. Analysis of the coordination between cell division and cell expansion. Plant Physiol. 1995;109:861–870. doi: 10.1104/pp.109.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Haj Salah H, Tardieu F. Control of leaf expansion rate of droughted maize plants under fluctuating evaporative demand. A superposition of hydraulic and chemical messages? Plant Physiol. 1997;114:893–900. doi: 10.1104/pp.114.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein N, Silk WK, Läuchli A. Growth and development of sorghum leaves under conditions of NaCl stress. Planta. 1993;191:433–439. [Google Scholar]
- Boyer JS. Leaf enlargement and metabolic rates in corn, bean and sunflower at various leaf water potential. Plant Physiol. 1970;46:233–235. doi: 10.1104/pp.46.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough BF, Milthorpe FL. Effect of water deficits on leaf development in tobacco. Aust J Plant Physiol. 1975;2:291–300. [Google Scholar]
- Dolezel J, Binarova P, Lucretti S. Analysis of nuclear DNA content in plant cells by flow cytometry. Biol Plant. 1989;31:113–120. [Google Scholar]
- Durand JL, Onillon B, Schnyder H, Rademacher I. Drought effects on cellular and spatial parameters of leaf growth in tall fescue. J Exp Bot. 1995;46:1147–1155. [Google Scholar]
- Gandar PW, Hall AJ. Estimating position-time relationships in steady-state one-dimensional growth zones. Planta. 1988;175:121–129. doi: 10.1007/BF00402889. [DOI] [PubMed] [Google Scholar]
- Granier C, Tardieu F. Spatial and temporal analyses of expansion and cell cycle in sunflower leaves. A common pattern of development for all zones of a leaf and different leaves of a plant. Plant Physiol. 1998a;116:991–1001. doi: 10.1104/pp.116.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier C, Tardieu F. Is thermal time adequate for expressing the effects of temperature on sunflower leaf development? Plant Cell Environ. 1998b;21:695–703. [Google Scholar]
- Green PB. Growth and cell pattern formation on an axis: critique of concepts, terminology, and mode of study. Bot Gaz. 1976;137:187–202. [Google Scholar]
- Green PB, Bauer K. Analyzing the changing cell cycle. J Theor Biol. 1977;68:299–315. doi: 10.1016/0022-5193(77)90167-9. [DOI] [PubMed] [Google Scholar]
- Heckenberger U, Roggatz U, Schurr U. J Exp Bot. 1998;49:181–191. [Google Scholar]
- Lecoeur J, Wery J, Sinclair TS. Model of leaf area expansion in field pea subjected to soil water deficits. Agronomy J. 1996;88:467–472. [Google Scholar]
- Lecoeur J, Wery J, Turc O, Tardieu F. Expansion of pea leaves subjected to short water deficit: cell number and cell size are sensitive to deficit at different periods of leaf development. J Exp Bot. 1995;46:1093–1101. [Google Scholar]
- Muller B, Stosser M, Tardieu F. Spatial distributions of tissue expansion and cell division rates are related to irradiance and to sugar content in the growing zone of maize roots. Plant Cell Environ. 1998;21:149–158. [Google Scholar]
- Nougarède A, Rondet P. Evénements structuraux et métaboliques dans les entre-noeuds des bourgeons axillaires du pois, en réponse à la levée de dominance. Can J Bot. 1978;56:1213–1228. [Google Scholar]
- Palmer SJ, Berridge DM, MacDonald AJS, Davies WJ. Control of leaf expansion in sunflower (Helianthus annuus L.) by nitrogen nutrition. J Exp Bot. 1996;47:359–368. [Google Scholar]
- Powell MJ, Davies MS, Francis D. The influence of zinc on the cell cycle in the root meristem of a zinc-tolerant and non-tolerant cultivar of Festuca rubra L. New Phytol. 1986;102:419–428. doi: 10.1111/j.1469-8137.1986.tb00668.x. [DOI] [PubMed] [Google Scholar]
- Quarrie SA, Whitford PN, Appleford NEJ, Wang TL, Cook SK, Henson IE. A monoclonal antibody to (S)-abscisic acid: its characterisation and use in a radioimmunoassay for measuring abscisic acid in crude extract of cereal and lupin leaves. Planta. 1988;173:330–339. doi: 10.1007/BF00401020. [DOI] [PubMed] [Google Scholar]
- Rawson HM, Turner NC. Recovery from water stress in five sunflower cultivars. II. The development of leaf area. Aust J Plant Physiol. 1982;9:449–460. [Google Scholar]
- Saab IN, Ho THD, Sharp RE. Translatable RNA populations associated with maintenance of primary root elongation and inhibition of mesocotyl elongation by abscisic acid in maize seedlings at low water potentials. Plant Physiol. 1995;109:593–601. doi: 10.1104/pp.109.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks MM, Silk WK, Burman P. Effect of water stress on cortical cell division rates within the apical meristem of primary roots of maize. Plant Physiol. 1997;114:519–527. doi: 10.1104/pp.114.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadras VO, Villalobos FJ, Ferreres E. Leaf expansion in field grown sunflower in response to soil and leaf water status. Agron J. 1993;8:564–570. [Google Scholar]
- Silk WK. Steady form from changing cells. Int J Plant Sci. 1992;153:49–58. [Google Scholar]
- Terry N, Waldron LJ, Ulrich A. Effects of soil moisture on the multiplication and expansion of cells in leaves of sugar beet. Planta. 1971;97:281–289. doi: 10.1007/BF00390207. [DOI] [PubMed] [Google Scholar]
- Van't Hof J. The regulation of cell division in higher plants. Brookhaven Symposia. 1973;25:152–165. [Google Scholar]
- Wolf O, Silk W, Plant R. Quantitative patterns of leaf expansion: comparison of normal and malformed leaf growth in Vitis vinifera. Am J Bot. 1986;73:832–846. [Google Scholar]