Abstract
The influenza virus genome replicates and forms a viral ribonucleoprotein complex (vRNP) with nucleoprotein (NP) and RNA polymerases in the nuclei of host cells. vRNP is then exported into the cytoplasm for viral morphogenesis at the cell membrane. Matrix protein 1 (M1) and nonstructural protein 2/nuclear export protein (NS2/NEP) work in the nuclear export of vRNP by associating with it. It was previously reported that influenza virus production was inhibited in Madin-Darby canine kidney (MDCK) cells cultured at 41°C because nuclear export of vRNP was blocked by the dissociation of M1 from vRNP (A. Sakaguchi, E. Hirayama, A. Hiraki, Y. Ishida, and J. Kim, Virology 306:244-253, 2003). Previous data also suggested that a certain protein(s) synthesized only at 41°C inhibited the association of M1 with vRNP. The potential of heat shock protein 70 (HSP70) as a candidate obstructive protein was investigated. Induction of HSP70 by prostaglandin A1 (PGA1) at 37°C caused the suppression of virus production. The nuclear export of viral proteins was inhibited by PGA1, and M1 was not associated with vRNP, indicating that HSP70 prevents M1 from binding to vRNP. An immunoprecipitation assay showed that HSP70 was bound to vRNP, suggesting that the interaction of HSP70 with vRNP is the reason for the dissociation of M1. Moreover, NS2 accumulated in the nucleoli of host cells cultured at 41°C, showing that the export of NS2 was also disturbed at 41°C. However, NS2 was exported normally from the nucleus, irrespective of PGA1 treatment at 37°C, suggesting that HSP70 does not influence NS2.
Influenza virus is an enveloped RNA virus belonging to the orthomyxovirus family. The viral genome consists of eight segments of negative-sense RNA which are bound to viral RNA polymerases and nucleoprotein (NP) to form a viral ribonucleoprotein complex (vRNP). When a host cell is invaded, influenza virus delivers its vRNP into the nucleus and replicates its genome. vRNP also acts as a template for mRNAs encoding virus-specific proteins. Although the viral proteins are synthesized in the cytoplasm, NP and RNA polymerases are imported into the nucleus to form new vRNP with a replicated genome. The assembly of influenza viral components, however, occurs at the plasma membrane. Therefore, new vRNP must be exported from the nucleus into the cytoplasm for viral offspring production (16). Matrix protein 1 (M1) and nonstructural protein 2/nuclear export protein (NS2/NEP) are known to be necessary for the nuclear export of vRNP (4, 19, 20, 21, 24, 30). Both proteins also migrate into the nucleus and associate with vRNP (10, 19, 28, 32, 33) for transportation via the cellular machinery for nuclear export, dependent on chromosome region maintenance 1 (CRM1) protein (9, 18, 21).
It was previously reported that influenza virus production is suppressed at 41°C in Madin-Darby canine kidney (MDCK) cells but is normal at 37°C (24). Virus-specific proteins are synthesized and vRNP is formed even at 41°C; however, vRNP cannot be exported from the nucleus at 41°C. This failure in vRNP export is due to M1 not interacting with vRNP, demonstrating that the association of M1 with vRNP is essential for the nuclear export of vRNP. To investigate why M1 is not bound to vRNP at 41°C, viral proteins were labeled with [35S]methionine at 37°C, and the M1-vRNP complex formed at 37°C was chased after the culture temperature was raised to 41°C. The temperature rise caused the release of M1 from vRNP; the M1-vRNP complex formed at 37°C was dissociated at 41°C. However, in this experiment, it was found incidentally that the dissociation of the M1-vRNP complex was inhibited if the infected cells were treated with cycloheximide, a protein synthesis inhibitor (24). M1 remained associated with vRNP even at 41°C in the presence of cycloheximide. This result suggested that some protein or proteins synthesized only at 41°C were related to the dissociation of the M1-vRNP complex. We believed that cycloheximide inhibited the synthesis of such an obstructive protein(s) and then the M1-vRNP complex remained intact, even at 41°C. To identify the proteins that inhibited the association of M1 with vRNP, we focused on the heat shock protein (HSP) family for candidates.
The HSP family consists of proteins that are typically induced by high culture temperatures. HSPs mainly work as molecular chaperons and were originally known to prevent proteins from degenerating from heat shock or some other stress (11, 22). For canine cells, including MDCK cells, seven kinds of HSP, namely H11 kinase (L. Wang and C. Depre, unpublished data; the protein number for the database is AAM90297.1), HSP27 (17), α-crystallin A chain (7), HSP60 (8), HSP70 (27), glucose-regulated protein 75 (GRP75) (8), and GRP94 (5), are registered in the database. Since HSPs that are localized in the nucleus and induced only at 41°C are highly likely to be concerned with the dissociation of the M1-vRNP complex, HSP27 and HSP70, which are reported to be localized in the nucleus (2, 3, 11, 29), were investigated in particular. Our preliminary experiments showed that HSP27, however, was expressed both at 37 and 41°C and was also distributed in the nucleus at 37°C. In contrast, expression of HSP70 was significantly induced and localized in the nucleus only at 41°C. Moreover, expression of HSP70 can be induced by prostaglandins (1, 26). Thus, for this report, the relationship between HSP70 and the suppression of influenza virus production was examined. We found that nuclear export of vRNP was also inhibited via the dissociation of the M1-vRNP complex when HSP70 was induced by prostaglandin A1 (PGA1) at 37°C.
MATERIALS AND METHODS
Cell culture.
MDCK cells were cultured in minimum essential medium (MEM) supplemented with 10% calf serum and antibiotics as described previously (15, 24).
Viral infection.
Infection with influenza A virus (A/Aichi/2/68/H3N2) was described elsewhere (24, 31). Briefly, 106 MDCK cells were cultured for 48 h at 37°C in a 35-mm-diameter dish, and the virus was inoculated at a multiplicity of infection of 2 × 103 at 4°C for 60 min. The cells were washed with phosphate-buffered saline (PBS) and then cultured in Dulbecco's modified Eagle's medium with 10% calf serum. When the cells were treated with PGA1, 20 μg of PGA1 (Wako Pure Chemical Industries, Osaka, Japan) per ml was added to the culture medium 2 h before the infection. The cells were infected as described above, and then the culture was continued in medium containing 20 μg of PGA1 per ml. For the control cells without PGA1, a volume of ethanol equal to that of PGA1 in the other experiments was added to the medium.
Virus production in the culture medium was measured by hemagglutinating activity (HA) on chick red blood cells, as described elsewhere (25).
Antibodies.
Anti-influenza virus polyclonal antibody and anti-NP and anti-M1 monoclonal antibodies were prepared in our laboratory (24). Anti-NS2 rabbit polyclonal antibody was a kind gift from Peter M. Palese (Mount Sinai School of Medicine, New York, N.Y.) and was used for immunoblotting. For fluorescent staining, anti-NS2 mouse polyclonal antibody was prepared. Briefly, cDNA for NS2 (a gift from P. Palese) (21) was inserted into the EcoRI and XhoI sites of a pGEX-5X-1 vector, and NS2 protein fused to glutathione S-transferase (GST) was induced in Escherichia coli DH5α cells by use of isopropyl-β-d(−)-thiogalactopyranoside. GST-NS2 was extracted and purified with glutathione-Sepharose 4B (Amersham Pharmacia Biotech AB, Uppsala, Sweden) as described elsewhere (14), and NS2 was removed from GST by treatment with factor Xa (Itoham Foods, Inc., Hyogo, Japan). Free GST, uncut NS2-GST, and factor Xa were removed by the addition of a mixture of glutathione-Sepharose and benzamidine-Sepharose 6B (Amersham Pharmacia Biotech AB) (9:1 [vol/vol]). Four-week-old BALB/c female mice were immunized with purified NS2 with adjuvant, and antisera were obtained. Anti-HSP70 monoclonal antibody was purchased from StressGen Biotechnologies (Victoria, British Columbia, Canada). This antibody reacts only to the HSP70 with a molecular mass of 72 kDa, induced by heat shock, among the members of the HSP70 family.
Immunoblotting.
Immunoblotting was performed as described previously (24). The proteins that reacted with the specific antibodies were detected with Western Lightning Chemiluminescence Reagent Plus (Perkin-Elmer Life Sciences, Inc., Norwalk, Conn.) and visualized by optimum exposure to Fuji medical X-ray film RX-U (Fuji Photo Film Co., Tokyo, Japan) (12). The intensity of the bands was measured with NIH Image analysis software (National Institutes of Health, Bethesda, Md.).
Indirect immunofluorescent staining.
NP, M1, and NS2 were fixed with a mixture of acetone and methanol (1:1 [vol/vol]) and stained as described previously (24). For staining of HSP70, the cells were fixed with 3.7% formaldehyde in PBS for 20 min at room temperature and treated with 0.2% Triton X-100 in PBS for 5 min on ice. The cells were treated with anti-HSP70 monoclonal antibody for 60 min on ice, followed by fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin goat antibody (Cappel/ICN Pharmaceuticals, Inc., Aurora, Ohio) for 30 min on ice.
Immunoprecipitation.
Immunoprecipitation was also performed as described previously (24). Infected cells that were untreated or treated with PGA1 were harvested in TNE buffer (10 mM Tris-HCl [pH 7.8], 1% NP-40, 150 mM NaCl, 1 mM EDTA, and 25 μg of aprotinin per ml) and lysed. The lysate was incubated with anti-NP monoclonal antibody for 4 h at 4°C and then with protein A-Sepharose CL-4B beads (Amersham Pharmacia Biosciences) for 2 h at 4°C. The beads were harvested by centrifugation and washed with TNE buffer.
RESULTS
Induction of HSP70 in infected MDCK cells at 41°C.
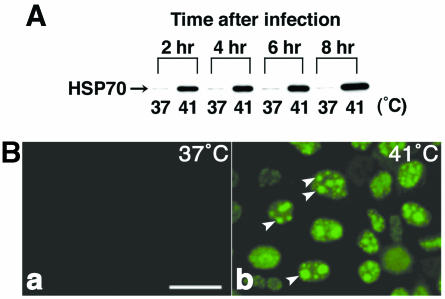
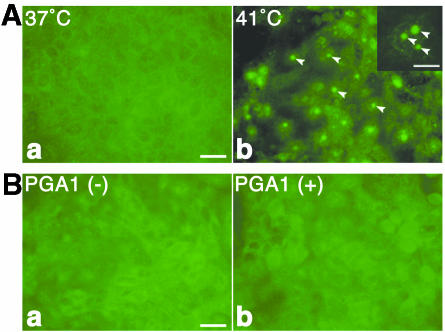
When MDCK cells are infected with influenza A virus (A/Aichi/2/68/H3N2) and cultured at 37°C, some of the synthesized viral proteins, such as NP and M1, are imported into the nucleus up to 2 h after infection. These proteins start to migrate from the nucleus into the cytoplasm from about 4 h after infection, forming the M1-vRNP complex. Newly formed virions are then detected in the culture medium from 6 h after infection (24). However, in infected cells cultured at 41°C, the viral proteins are not exported into the cytoplasm and remain in the nucleus because of the dissociation of M1 from vRNP. To examine the possibility that HSP27 or HSP70 is related to the inhibition of nuclear export at 41°C, we first monitored the expression patterns of both HSPs by immunoblotting during the infectious period in MDCK cells cultured at 37 or 41°C. Their distributions were also compared at both temperatures by immunofluorescent staining. HSP27 was already expressed at 37°C and was not increased much by raising the temperature to 41°C (data not shown). HSP27 was also distributed in the nucleus at both temperatures. HSP27 did not seem to participate in the inhibition of nuclear export at 41°C. On the other hand, the induction of HSP70 was significant at 41°C. When the infected cells were cultured at 37°C, HSP70 was slightly detected by immunoblotting (Fig. 1A) and was not observed at all by immunostaining (Fig. 1B). However, in the culture at 41°C, its expression dramatically increased within 2 h of incubation and remained expressed thereafter (Fig. 1A). In noninfected cells, the expression pattern of HSP70 was similar to that in infected ones; the induction was seen after a 2-h incubation at 41°C. Immunostaining showed that HSP70 synthesized at 41°C was specifically localized in the nucleus 2 h after infection (data not shown) and was also distributed in the nucleolus after 4 h of incubation (Fig. 1B, arrowheads). HSP70 was more likely to be related to the inhibition of nuclear export at 41°C than was HSP27.
FIG. 1.
Expression of HSP70 in MDCK cells infected with influenza virus at 41°C. (A) MDCK cells were infected with influenza virus and cultured at 37 or 41°C for the indicated times. The cells were lysed, and immunoblotting was performed with anti-HSP70 monoclonal antibody. The expression of HSP70 increased after 2-h of incubation at 41°C, but not at 37°C. (B) Infected MDCK cells were cultured at 37°C (a) or 41°C (b) for 6 h and stained immunofluorescently with anti-HSP70 antibody. HSP70 was observed only at 41°C and was localized specifically in the nuclei and nucleolus (arrowheads). Bar, 25 μm.
Induction of HSP70 at 37°C by PGA1.
It is known that PGA1 induces the expression of HSP70 (1, 26). For confirmation of whether HSP70 is the cause of the disturbance in nuclear export of viral proteins, the expression of HSP70 was induced at 37°C by PGA1 and its influence on virus production was investigated.
To determine the concentration of PGA1 required to induce HSP70 in our experimental system, we cultured noninfected MDCK cells in the medium with various concentrations of PGA1, between 5 and 40 μg/ml, for 24 h at 37°C. The cells degenerated in the presence of 30 μg or more of PGA1 per ml. The concentration was decreased to 20 μg/ml or less, and the amount of HSP70 was estimated by immunoblotting after 6 h at 37°C. The result was that 20 μg of PGA1 per ml induced the highest expression level (data not shown). We then examined when HSP70 starts to be induced by 20-μg/ml PGA1 in noninfected MDCK cells. HSP70 began to increase after 4 h of treatment with PGA1 at 37°C (Fig. 2A). As shown in Fig. 1A, when cultured at 41°C, HSP70 was induced after 2 h of incubation. To modify the timing of the start of HSP70 induction by PGA1 to equal that at 41°C, MDCK cells were pretreated with PGA1 for 2 h before the infection. Namely, MDCK cells were precultured with PGA1 for 2 h at 37°C, infected with the virus, and then cultured further with PGA1 at 37°C. By this modulation of the treatment with PGA1, the induction of HSP70 began 2 h after infection, similar to the culture at 41°C (Fig. 2B). However, the expression of HSP70 by PGA1 gradually decreased from 8 h after infection, unlike expression at 41°C (Fig. 2B, compare the expression level at 6 h with PGA1 to that at 12 h), implying that the effect of PGA1 is temporary.
FIG. 2.
Induction of HSP70 by PGA1 at 37°C. (A) MDCK cells were cultured in medium including PGA1 for the indicated intervals at 37°C. HSP70 started to be induced after 4 h of treatment with PGA1. (B) Expression of HSP70 in infected MDCK cells by treatment with PGA1. MDCK cells were pretreated with PGA1 or an equal volume of ethanol as a control for 2 h at 37°C and then infected with influenza virus. The cells were further cultured in the presence of PGA1 (+) or ethanol (−) at 37°C for the indicated times, and extracts were prepared for immunoblotting with anti-HSP70 monoclonal antibody. By pretreatment with PGA1, HSP70 could be induced 2 h after infection. (C) MDCK cells 6 h after infection with (b) or without (a) PGA1 were stained immunofluorescently with anti-HSP70 antibody. HSP70 was induced by PGA1 (b) and was localized more strongly in the nuclei (arrows) than in the cytoplasm and was not in the nucleolus (arrowheads). Bar, 25 μm.
The distribution of HSP70 induced by PGA1 at 37°C was observed in infected cells. HSP70 induced by PGA1 was distributed both in the nucleus and the cytoplasm, but nuclear staining was stronger than cytoplasmic staining (Fig. 2C, arrows). Localization in the nucleolus was not seen (Fig. 2C, arrowheads).
These results show that PGA1 is basically capable of inducing HSP70 in our experimental system, although the expression is not sustainable and its localization is somewhat different from that at 41°C.
Suppression of virus production by PGA1.
For examination of whether the induction of HSP70 influences virus production, the cells treated with PGA1 were infected with influenza virus in the same way as for Fig. 2B, and virus production was assayed. HA in the culture medium was measured at various time points after infection as an indicator of virus production (Fig. 3A). In control cells without PGA1, HA increased 6 h after infection and reached a plateau within 24 h. Corresponding to the increase in HA, a cytopathic effect (CPE) by viral infection was observed (Fig. 3B, panels a, c, and e). Cells detaching from the dish were seen from 12 h on (Fig. 3B, panel a [arrows]) and almost all of the cells had degenerated 24 h after infection (Fig. 3B, panels c and e), whereas in the presence of PGA1, virus production was hardly detected until 12 h after infection (Fig. 3A). Even at 24 h, HA remained at one-fourth that of the control. CPE was also not observed until 12 h after infection (Fig. 3B, panel b). However, after 48 h, HA increased to a similar level as that in the control, despite PGA1 treatment (Fig. 3A). Concomitant with the increase in HA, CPE was also seen from 24 h on (Fig. 3B, panel d [arrows]) and almost all of the cells had detached from the dish after 48 h (Fig. 3B, panel f). As shown in Fig. 2B, expression of HSP70 induced by PGA1 decreased after 8 h. The increase in HA and the appearance of CPE in the cells treated with PGA1 seemed to correspond to the decrease in HSP70. To clarify this point, expression of HSP70 was monitored by immunoblotting, focusing especially on expression after 12 h, which is when HA began to rise even with PGA1. As expected, the induction of HSP70 decreased from 12 h after infection (Fig. 3C and D) and its expression level at 24 h was reduced to less than half of that at 8 h (Fig. 3D). These results indicate that virus production increases with a decrease in HSP70. They also show that virus production is suppressed by PGA1 when a certain amount of HSP70 remains, suggesting that HSP70 is related to the suppression of virus production.
FIG. 3.
Influenza virus production in cells treated with PGA1. MDCK cells were treated with PGA1 and infected with the virus in the same way as for Fig. 2. (A) Virus production in the culture medium was assayed by measuring HA. In control cells without PGA1 (○), HA increased after 6 h and reached a plateau 24 h after infection. In cells treated with PGA1 (•), HA began to be detected 24 h after infection. (B) Phase-contrast images of cells infected with PGA1. MDCK cells treated with (b, d, and f) or without (a, c, and e) PGA1 were infected with the virus and cultured for 12 h (a and b), 24 h (c and d), or 48 h (e and f). CPE by infection was observed after 12 h in the cells without PGA1 treatment (arrows in panel a) and almost all of the cells had degenerated after 24 h (c and e). In the cells treated with PGA1, CPE was not seen at 12 h (b) but began to be observed from 24 h (arrows in panels d and f). Bar, 50 μm. (C) Expression of HSP70 in cells infected with PGA1. Total protein extracts were prepared from the infected cells in the presence (+) or absence (−) of PGA1 as shown in panels A and B and were blotted with anti-HSP70 antibody. HSP70 gradually decreased 12 h later in the presence of PGA1. (D) The intensities of the bands shown in panel C were measured by NIH Image software. The intensities were compared as relative percentages to that in the cells with PGA1 8 h after infection.
Inhibition of nuclear export of viral proteins by PGA1.
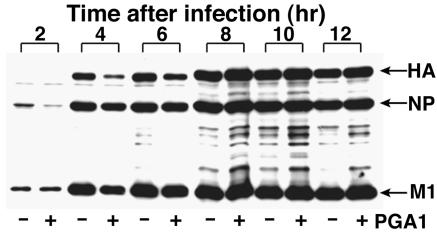
For examination of which steps of virus production were inhibited by PGA1, the synthesis of viral proteins was investigated by immunoblotting. The cells treated with PGA1 were infected with the virus in the same way as for Fig. 2B, and the extracts were blotted with anti-influenza virus polyclonal antibody at various time points after infection (Fig. 4). Even in the presence of PGA1, major viral proteins HA, NP, and M1 were detected at almost normal levels 2 to 4 h after infection, although the intensity of the bands seemed to be slightly less than those of control cells without PGA1 until 6 h and although some nonspecific bands were seen. This result indicated that the synthesis of viral proteins was not inhibited by PGA1 and that the suppression of virus production by PGA1 was due to the disturbance of some steps after viral protein synthesis.
FIG. 4.
Synthesis of virus-specific proteins in the presence of PGA1. The extracts from the infected cells that were untreated (−) or treated with PGA1 (+) in a similar way to that described for Fig. 2B were immunoblotted with anti-influenza virus polyclonal antibody. The viral proteins were synthesized irrespective of PGA1 treatment.
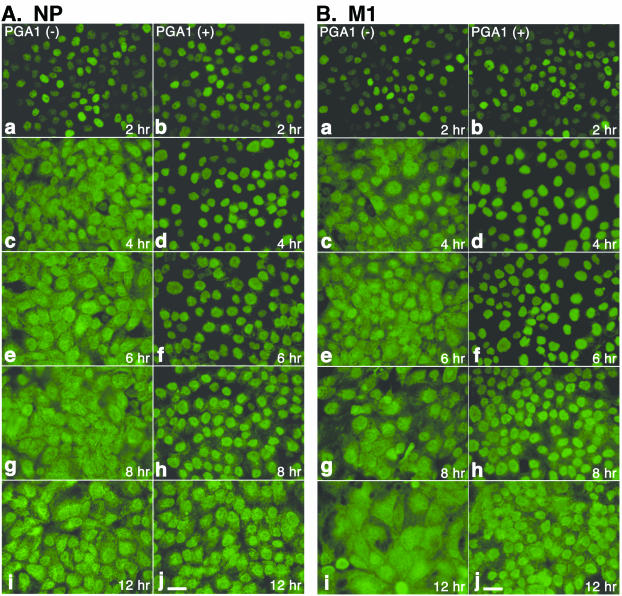
For further investigation of the inhibitory mechanism of PGA1, the distribution of viral proteins was observed in detail by immunofluorescence staining. It was recognized in a previous report that nuclear export of NP and M1 was prevented in cells cultured at 41°C (24). We observed whether NP and M1 were also not transported from the nucleus into the cytoplasm by treatment with PGA1 (Fig. 5). Both NP (Fig. 5A) and M1 (Fig. 5B) were already localized in the nuclei 2 h after infection, irrespective of PGA1 treatment (panels a and b), showing that their import into the nucleus occurred after synthesis. In the absence of PGA1, they began to be exported into the cytoplasm from 4 h on (Fig. 5, panels c), and the cells were almost evenly stained after 6 h (Fig. 5, panels e, g, and i). However, in the cells treated with PGA1, both proteins remained in the nuclei until 6 h after infection (Fig. 5, panels d and f). Although cytoplasmic staining was slightly detected after 8 h, nuclear retention of both proteins was significant even after 8 h (Fig. 5, panels h). These observations suggest that nuclear export of viral proteins is also blocked by treatment with PGA1. After 12 h, when the expression of HSP70 decreased (Fig. 3C and D), both NP and M1 were exported to the cytoplasm and almost no differences were observed between the cells that were untreated and those that were treated with PGA1, corresponding to the increase in virus production shown in Fig. 3 (Fig. 5, panels i and j). The inhibitory effect of PGA1 on the nuclear export of viral proteins was also seen when expression of HSP70 remained, suggesting that HSP70 is related to the inhibition of nuclear export.
FIG. 5.
Distribution of NP and M1 in MDCK cells infected with PGA1. The cells treated with PGA1 (b, d, f, h, and j) or untreated cells (a, c, e, g, and i) were infected with the virus and fixed after culturing for 2 h (a and b), 4 h (c and d), 6 h (e and f), 8 h (g and h), or 12 h (i and j). The cells were stained with anti-NP (A) or -M1 (B) monoclonal antibody. NP and M1 remained in the nuclei in the cells treated with PGA1 until 8 h after infection. Bar, 25 μm.
Dissociation of M1 from vRNP by PGA1.
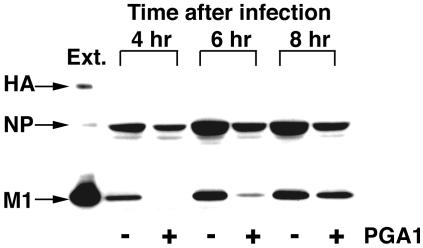
As reported previously (24), M1 does not associate with vRNP in infected cells at 41°C and this is one of the reasons that nuclear export of vRNP is inhibited. Therefore, we examined whether M1 was also not associated with vRNP when nuclear export was inhibited by PGA1. MDCK cells treated with PGA1 were infected with the virus and cultured for the intervals shown in Fig. 6. vRNP was immunoprecipitated from the extract by anti-NP antibody, and the interaction of M1 with vRNP was detected by immunoblotting with anti-influenza virus polyclonal antibody (Fig. 6). In the cells treated with PGA1, M1 was not coprecipitated with NP after 4 h. M1 was slightly detected 6 h after infection, but the amount was extremely reduced compared with that of the control. It was therefore shown that the association of M1 with vRNP was inhibited by PGA1 as it was with culturing at 41°C. However, an almost similar level of M1 to that in the control was coprecipitated, even with PGA1 treatment, after 8 h. The association of M1 with vRNP seemed to be due to the reduction of HSP70.
FIG. 6.
Dissociation of M1 from vRNP in cells treated with PGA1. Infected MDCK cells were cultured with (+) or without (−) PGA1 as for the other figures, and total proteins were extracted after the indicated times. vRNP was immunoprecipitated with anti-NP monoclonal antibody, and the precipitates were blotted with anti-influenza virus polyclonal rabbit antibody. Ext., total protein extract from the infected cells cultured for 6 h at 37°C without PGA1, indicating the position of each viral protein. M1 was hardly coprecipitated with vRNP until 6 h, but an association was detected after 8 h of infection.
Association of HSP70 with vRNP.
As described above, the M1-vRNP complex was not formed by PGA1 treatment, even at 37°C. The complex formed when the induction of HSP70 decreased 8 h after the infection, suggesting that HSP70 is closely related to the dissociation of M1 from vRNP. Since HSP70 is a molecular chaperone, one of its characteristics is binding with other proteins. To understand the mechanism by which HSP70 inhibited the association between M1 and vRNP, we considered the possibility that HSP70 is directly bound to some viral proteins included in the M1-vRNP complex.
For investigation of this possibility, the interaction of HSP70 with vRNP was first assayed by an immunoprecipitation method, as for Fig. 6. Infected MDCK cells were cultured at 37 or 41°C for 6 h, and vRNP was immunoprecipitated with anti-NP antibody. The association of HSP70 was detected by immunoblotting with specific antibody. As shown in Fig. 7A, HSP70 clearly coprecipitated with vRNP in the infected cells cultured only at 41°C. In addition, when HSP70 was induced by PGA1 at 37°C, HSP70 coprecipitated with vRNP 4 and 6 h after infection (Fig. 7B). However, 8 h after infection, HSP70 did not coprecipitate with vRNP, even in the presence of PGA1. As shown in Fig. 6, M1 failed to associate with vRNP until 6 h after infection in the cells treated with PGA1, but after 8 h, M1 was bound to vRNP even in the presence of PGA1. Therefore, M1 cannot associate with vRNP when HSP70 is associated with vRNP. These results suggest that the association of HSP70 with vRNP is one of the reasons for the inhibition of M1-vRNP complex formation.
FIG. 7.
Interaction of HSP70 with vRNP. (A) MDCK cells were infected with the virus and cultured at 37 or 41°C for 6 h. Immunoprecipitation was performed with anti-NP antibody as for Fig. 6, and HSP70 in the precipitates was detected by immunoblotting. HSP70 coprecipitated with vRNP in the cells cultured at 41°C. (B) MDCK cells were treated with PGA1 and infected with the virus. After culturing for the indicated times after infection with (+) or without (−) the addition of PGA1, the protein extracts were prepared and vRNP was immunoprecipitated with anti-NP antibody. The association of HSP70 was detected by immunoblotting. HSP70 interacted with vRNP in the infected cells with PGA1 until 6 h, but not after 8 h of infection. Ext., total protein extract from the infected cells cultured at 41°C for 6 h, indicating the position of HSP70. The band under HSP70 is the band of the heavy (H) chain of the immunoglobulin G used for the immunoprecipitation assay.
The behavior of NS2.
NS2 is also reported to be necessary for the nuclear export of vRNP (20, 21). We examined whether NS2 was influenced by culturing at 41°C or by PGA1. Immunoblotting showed that NS2 was synthesized normally at 41°C or under PGA1 treatment (data not shown). The distribution of NS2 was observed for the cells cultured at 41°C or treated with PGA1 at 37°C by immunofluorescence staining (Fig. 8). In control cells cultured at 37°C after infection, NS2 was almost evenly distributed in almost all of the cells after 4 h (Fig. 8A). However, in the cells cultured at 41°C, a peculiar distribution of NS2 was seen; NS2 was concentrated in the nucleolus 4 h after infection (Fig. 8A, arrowheads). This observation indicated that the nuclear export of NS2 was also disturbed at 41°C.
FIG. 8.
Distribution of NS2 in infected MDCK cells. (A) MDCK cells infected with the virus were cultured at 37°C (a) or 41°C (b) for 6 h. The cells were immunostained fluorescently with anti-NS2 mouse polyclonal antibody. At 41°C, NS2 accumulated in the nucleolus (arrowheads). The inset in panel b shows a magnification of a nucleus. (B) Infected MDCK cells were cultured with (b) or without (a) PGA1 for 6 h at 37°C as for the other figures, and NS2 was stained. NS2 was detected all over the cells, regardless of whether the cells were treated with PGA1 or not. Bar, 25 μm. Bar in inset, 10 μm.
However, when the infected cells were cultured with PGA1 at 37°C, the distribution of NS2 was similar to that for the control without PGA1; almost all of the cells were evenly stained irrespective of the presence of the drug (Fig. 8B). This result suggested that HSP70 did not affect the nuclear export of NS2.
DISCUSSION
As reported previously, influenza virus production was suppressed at 41°C, although virus-specific proteins were expressed. The suppression at 41°C was due to the dissociation of M1 from vRNP, which disturbed the nuclear export of vRNP (24). During our investigations, a certain protein or proteins synthesized only at 41°C were suggested to interfere with the association of M1 with vRNP. This report shows that HSP70 is a likely candidate for such obstruction against the formation of the M1-vRNP complex. There have been no reports investigating the relationship of HSPs to the nuclear export of influenza virus vRNP, although a few studies have reported that HSPs have a suppressive effect on influenza virus infection (6, 23).
The induction of HSP70 by PGA1 at 37°C inhibited virus production. The effect by PGA1 appears to be identical to that by culturing at 41°C. In fact, M1 did not interact with vRNP and the nuclear export of viral proteins was disturbed by treatment with PGA1. These results strongly suggest that the induction of HSP70 is associated with the dissociation of M1 from vRNP. Moreover, it was clarified that HSP70 was bound to vRNP (Fig. 7). This result indicates that HSP70 is directly related to interference of the association of M1 with vRNP. The induction of HSP70 by PGA1 was temporary. The expression of HSP70 gradually decreased after 8 h despite the presence of the drug. Corresponding to the reduction of HSP70, HSP70 separated from vRNP (Fig. 7) and all events involved in viral infection were recuperated; the association of M1 with vRNP was restored (Fig. 6), the viral proteins began to be exported into the cytoplasm, and then virus production was detected in the medium. The recovery of virus production with the reduction of HSP70 supports the close relationship of HSP70 with the inhibition of virus production. These results indicate that the binding of HSP70 to vRNP is the reason for the dissociation of M1 from vRNP.
To obtain more direct proof that HSP70 inhibits nuclear export of vRNP, we carried out small interfering RNA (siRNA) targeting of HSP70. MDCK cells were transfected beforehand with siRNA for HSP70 at 41°C and infected with the virus, and then we observed whether nuclear export was recovered. The result was that nuclear export of NP occurred even at 41°C by siRNA targeting of HSP70 (data not shown). However, in this experiment, the expression of HSP70 also faded in the control cells without siRNA during the advance treatment with siRNA and the culturing after infection at 41°C; the expression of HSP70 was temporary in the culture at 41°C. We were not able to determine whether siRNA targeting caused the restoration of nuclear export. We also tried microinjection of a specific antibody against HSP70 and the transfection of a plasmid encoding HSP70. However, both experiments did not show clear results. At present, it is impossible to set up suitable experimental systems to directly confirm the involvement of HSP70 in the inhibition of nuclear export. The possibility still remains that other proteins, besides HSP70, are involved in blocking nuclear export of vRNP.
The induction of HSP70 by prostaglandins was reported to inhibit the production of influenza virus (6, 23). However, these studies reported that the synthesis of viral proteins was mainly suppressed. Conti et al. (6) showed that the induction of HSP70 by PGA1 blocks the synthesis of virus-specific proteins and the transport of hemagglutinin into the cell membrane. Their data seem to differ from ours. Although the exact reasons are not known at present, different experimental systems, especially host cells, may be responsible. As reported previously (12, 13), virus production obviously relies on factors in host cells. We also observed that virus production did not occur when LLC-MK2 cells, which were used as host cells by Conti et al., were infected with A/Aichi/2/68/H3N2, which was used in our experimental system (data not shown).
NS2 is another factor required for nuclear export of vRNP (20, 21). In a recent model (21), NS2 was described to interact with M1 and work as an adaptor between the M1-vRNP complex and CRM1, the cellular machinery for the nuclear export system. As shown in Fig. 8B, NS2 was normally exported from the nucleus into the cytoplasm despite the induction of HSP70 by PGA1. On the other hand, M1 was not exported from the nucleus in the presence of PGA1, by which M1 did not interact with vRNP (Fig. 5B and 6). If M1 were simply bound to NS2, M1 could be transported together with NS2 into the cytoplasm irrespective of whether M1 forms a complex with vRNP or not. These results suggest that NS2 does not associate with M1 unless the M1-vRNP complex is formed and that the association of M1 with vRNP is an essential event for nuclear export of vRNP. In contrast, NS2 was uniquely localized in the nucleolus at 41°C (Fig. 8A). Concerning this, M1 was also distributed in the nucleolus at 41°C, but not at 37°C (24). As with M1 and NS2, HSP70 induced by PGA at 37°C did not enter the nucleolus (Fig. 2C), whereas at 41°C, it was found in the nucleolus (Fig. 1B). The distribution of these proteins in the nucleolus seems to be due to some influence of the high culture temperature, but not to the expression of HSP70 itself. Further investigations are in progress.
Acknowledgments
We thank Peter M. Palese (Mount Sinai School of Medicine, New York, N.Y.) for the antibody and cDNA for NS2/NEP.
This work was supported in a part by grants from the Japanese Private School Promotion Foundation.
REFERENCES
- 1.Amici, C., L. Sistonen, M. G. Santoro, and R. I. Morimoto. 1992. Antiproliferative prostaglandins activate heat shock transcription factor. Proc. Natl. Acad. Sci. USA 89:6227-6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrigo, A. P., J. P. Suhan, and W. J. Welch. 1988. Dynamic changes in the structure and intracellular locale of the mammalian low-molecular-weight heat shock protein. Mol. Cell. Biol. 8:5059-5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arrigo, A. P., and W. J. Welch. 1987. Characterization and purification of the small 28,000-dalton mammalian heat shock protein. J. Biol. Chem. 262:15359-15369. [PubMed] [Google Scholar]
- 4.Bui, M., E. G. Wills, A. Helenius, and G. R. Whittaker. 2000. Role of the influenza virus M1 protein in nuclear export of viral ribonucleoproteins. J. Virol. 74:1781-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cala, S. E., and L. R. Jones. 1994. GRP94 resides within cardiac sarcoplasmic reticulum vesicles and is phosphorylated by casein kinase II. J. Biol. Chem. 269:5926-5931. [PubMed] [Google Scholar]
- 6.Conti, G., P. Portincasa, S. Visalli, and C. Chezzi. 2001. Inhibition by prostaglandin PGA1 on the multiplication of influenza virus is a dose-dependent effect. Virus Res. 75:43-57. [DOI] [PubMed] [Google Scholar]
- 7.De Jong, W. W., F. J. van der Ouderaa, M. Versteeg, G. Groenewoud, J. M. van Amelsvoort, and H. Bloemendal. 1975. Primary structures of the alpha-crystallin A chains of seven mammalian species. Eur. J. Biochem. 53:237-242. [Google Scholar]
- 8.Dunn, M. J., J. M. Corbett, and C. H. Wheeler. 1997. HSC-2DPAGE and the two-dimensional gel electrophoresis database of dog heart proteins. Electrophoresis 18:2795-2802. [DOI] [PubMed] [Google Scholar]
- 9.Elton, D., M. Simpson-Holley, K. Archer, L. Medcalf, R. Hallam, J. McCauley, and P. Digard. 2001. Interaction of the influenza virus nucleoprotein with the cellular CRM1-mediated nuclear export pathway. J. Virol. 75:408-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenspan, D., M. Krysta, S. Nakada, H. Arnheiter, D. S. Lyles, and P. Palese. 1985. Expression of influenza virus NS2 nonstructural protein in bacteria and localization of NS2 in infected eucaryotic cells. J. Virol. 54:833-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendrick, J. P., and F. U. Hartl. 1993. Molecular chaperone functions of heat-shock proteins. Annu. Rev. Biochem. 62:349-384. [DOI] [PubMed] [Google Scholar]
- 12.Hirayama, E., Y. Ishida, M. Sugimoto, A. Hiraki, and J. Kim. 2003. Characterization of temperature-sensitive HVJ (Sendai virus) infection in Vero cells: inhibitory mechanism of viral production at 41°C. Intervirology 46:86-95. [DOI] [PubMed] [Google Scholar]
- 13.Ishida, Y., A. Hiraki, E. Hirayama, Y. Koga, and J. Kim. 2002. Temperature-sensitive viral infection: inhibition of hemagglutinating virus of Japan (Sendai virus) infection at 41°C. Intervirology 45:125-135. [DOI] [PubMed] [Google Scholar]
- 14.Isobe, A., E. Hirayama, and J. Kim. 1998. Characterization of myogenin expression in myotubes derived from quail myoblasts transformed with a temperature sensitive mutant of Rous sarcoma virus. Cell Struct. Funct. 23:57-67. [DOI] [PubMed] [Google Scholar]
- 15.Kim, J., T. Adachi, Y. Yoneda, and Y. Okada. 1990. Fusion of Madin-Darby canine kidney cells by HVJ (Sendai virus): absence of direct association of virus particles with the site of membrane fusion. Eur. J. Cell Biol. 51:128-134. [PubMed] [Google Scholar]
- 16.Lamb, R. A., and R. M. Krug. 2001. Orthomyxoviridae: the viruses and their replication, p. 1487-1532. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 17.Larsen, J. K., W. T. Gerthoffer, E. Hickey, and L. A. Weber. 1995. Cloning and sequencing of a cDNA encoding the canine HSP27 protein. Gene 161:305-306. [DOI] [PubMed] [Google Scholar]
- 18.Ma, K., A. M. Roy, and G. R. Whittaker. 2001. Nuclear export of influenza virus ribonucleoproteins: identification of an export intermediate at the nuclear periphery. Virology 282:215-220. [DOI] [PubMed] [Google Scholar]
- 19.Martin, K., and A. Helenius. 1991. Nuclear transport of influenza virus ribonucleoproteins: the viral matrix protein (M1) promotes export and inhibits import. Cell 67:117-130. [DOI] [PubMed] [Google Scholar]
- 20.Neumann, G., M. T. Hughes, and Y. Kawaoka. 2000. Influenza A virus NS2 protein mediates vRNP nuclear export through NES-independent interaction with hCRM1. EMBO J. 19:6751-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Neill, R. E., J. Talon, and P. Palese. 1998. The influenza virus NEP (NS2 protein) mediates the nuclear export of viral ribonucleoproteins. EMBO J. 17:288-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsell, D. A., and S. Lindquist. 1993. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu. Rev. Genet. 27:437-496. [DOI] [PubMed] [Google Scholar]
- 23.Pica, F., A. T. Palamara, A. Rossi, A. de Marco, C. Amici, and M. G. Santoro. 2000. Δ12-pronstaglandin J2 is a potent inhibitor of influenza A virus replication. Antimicrob. Agents Chemother. 44:200-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakaguchi, A., E. Hirayama, A. Hiraki, Y. Ishida, and J. Kim. 2003. Nuclear export of influenza viral ribonucleoprotein is temperature-dependently inhibited by dissociation of viral matrix protein. Virology 306:244-253. [DOI] [PubMed] [Google Scholar]
- 25.Salk, J. E. 1944. A simplified procedure for titrating hemagglutinating capacity of influenza-virus and the corresponding antibody. J. Immunol. 49:87-98. [Google Scholar]
- 26.Santoro, M. G., E. Garaci, and C. Amici. 1989. Prostaglandins with antiproliferative activity induce the synthesis of a heat shock protein in human cells. Proc. Natl. Acad. Sci. USA 86:8407-8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu, K., A. Iguchi, R. Gomyou, and Y. Ono. 1999. Influenza virus inhibits cleavage of the HSP70 pre-mRNAs at the polyadenylation site. Virology 254:213-219. [DOI] [PubMed] [Google Scholar]
- 28.Ward, A. C., L. A. Castelli, A. C. Lucantoni, J. F. White, A. A. Azad, and I. G. Macreadie. 1995. Expression and analysis of the NS2 protein of influenza A virus. Arch. Virol. 140:2067-2073. [DOI] [PubMed] [Google Scholar]
- 29.Welch, W. J., and J. R. Feramisco. 1984. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J. Biol. Chem. 259:4501-4513. [PubMed] [Google Scholar]
- 30.Whittaker, G., M. Bui, and A. Helenius. 1996. Nuclear trafficking of influenza virus ribonucleoproteins in heterokaryons. J. Virol. 70:2743-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamaoka, M., M. Homma, and H. Hotta. 1995. MDCK cell cultures supplemented with high concentrations of trypsin exhibit remarkable susceptibility to influenza C virus. Arch. Virol. 140:937-944. [DOI] [PubMed] [Google Scholar]
- 32.Yasuda, J., S. Nakada, A. Kato, T. Yoyoda, and A. Ishihama. 1993. Molecular assembly of influenza virus: association of the NS2 protein with virion matrix. Virology 196:249-255. [DOI] [PubMed] [Google Scholar]
- 33.Ye, Z., T. Liu, D. P. Offringa, J. Mclnnis, and R. A. Levandowski. 1999. Association of influenza virus matrix protein with ribonucleoproteins. J. Virol. 73:7467-7473. [DOI] [PMC free article] [PubMed] [Google Scholar]