Abstract
The major human tRNALys isoacceptors, tRNA1,2Lys and tRNA3Lys, are selectively packaged into human immunodeficiency virus type 1 (HIV-1) during assembly, where tRNA3Lys acts as a primer for reverse transcription. Lysyl-tRNA synthetase (LysRS) is also incorporated into HIV-1, independently of tRNALys, via its interaction with Gag, and it is a strong candidate for being the signal that specifically targets tRNALys for viral incorporation. Expression of exogenous wild-type LysRS in cells results in an approximately twofold increase in the viral packaging of both LysRS and tRNALys. Herein, we show that this increase in tRNALys incorporation into virions is dependent upon the ability of LysRS to bind to tRNALys but not upon its ability to aminoacylate the tRNALys. COS7 cells were cotransfected with plasmids coding for both HIV-1 and either wild-type or mutant human LysRS, all of which are incorporated into virions with similar efficiency. However, N-terminally truncated LysRS, which binds poorly to tRNALys, does not increase tRNALys packaging into viruses, while C-terminally truncated LysRS, which binds to but does not aminoacylate tRNALys, still facilitates an increase in tRNALys packaging into virions.
For human immunodeficiency virus type 1 (HIV-1), tRNA3Lys serves as the primer tRNA for the reverse transcriptase-catalyzed synthesis of minus-strand strong-stop cDNA (22). During HIV-1 assembly, the major tRNALys isoacceptors, tRNA1,2Lys and tRNA3Lys, are selectively packaged into the virion (19). The viral precursor protein Gag-Pol interacts with tRNALys and is known to be involved in tRNALys incorporation into viruses or Gag viruslike particles (20, 23, 24). However, the identity of viral or host cell factors that specifically target the tRNALys isoacceptors for interaction with viral proteins is less clear. Since the tRNALys-binding protein, human lysyl-tRNA synthetase (LysRS), is also selectively packaged into the virion (6), it is a prime candidate for facilitating viral packaging of tRNALys. A viral population contains, on average, approximately 20 to 25 molecules of LysRS/virion (5), similar to the average number of tRNALys molecules/virion (16). The incorporation of LysRS into HIV-1 also appears to be specific. Previously published work (5, 6) indicates that HIV-1 does not contain isoleucyl tRNA synthetase, prolyl tRNA synthetase, or tryptophanyl tRNA synthetase, while unpublished work (R. Halwani, H. Javanbakht, S. Cen, S. Kim, K. Shiba, and L. Kleiman, submitted for publication) further indicates the additional absence of aminoacyl-tRNA synthetases for arginine, glutamine, methionine, and tyrosine in HIV-1.
LysRS has been shown to interact directly with Gag in vitro and is packaged efficiently into viruslike particles composed only of Gag (6), which do not selectively package tRNALys due to the absence of Gag-Pol (23). This finding indicates that LysRS incorporation into Gag particles occurs independently of tRNALys packaging, and this conclusion is further supported by the finding that mutant LysRS not containing tRNALys-binding domains is still incorporated into virions. Since Gag and Gag-Pol interact during viral assembly (25, 27), a likely model for tRNALys packaging into virions would involve a Gag/Gag-Pol complex interacting with a tRNALys/LysRS complex, with Gag interacting with Gag-Pol and LysRS and with Gag-Pol interacting with tRNALys.
Further support for a role of LysRS in tRNALys packaging into viruses comes from experiments in which COS7 cells were cotransfected with HIV-1 proviral DNA and a plasmid coding for wild-type LysRS. The expression of exogenous wild-type LysRS in the cell results in a maximum twofold increase in the incorporation of both total LysRS (endogenous and exogenous) and tRNALys into virions (14). In this work, we utilize this observation to study the effect of the expression of mutant LysRS species on both LysRS and tRNALys incorporation into viruses. We have previously shown that the ability of tRNA3Lys anticodon mutants to be incorporated into HIV-1 was directly correlated with their ability to be aminoacylated (18). However, in that report, aminoacylation was used to measure the ability of the mutant tRNALys to bind to LysRS, and it was unclear if aminoacylation of the tRNALys was in itself required for tRNALys packaging. In this report, we will show that binding of LysRS to tRNALys is required for the LysRS-facilitated increase in tRNALys incorporation into virions, but that the ability of LysRS to aminoacylate tRNALys is not required.
Incorporation of wild-type and mutant LysRS into HIV-1.
COS7 cells were cotransfected with a plasmid containing HIV-1 proviral DNA and a plasmid (pcDNA3.1 [Invitrogen]) containing cDNA coding for either full-length human LysRS (termed LysRS.F, to distinguish it from endogenous LysRS in COS7 cells) or a truncated LysRS variant in which the N-terminal 65 amino acids have been deleted (Δ1-65 LysRS). Transcription is driven by a cytomegalovirus promoter. This N-terminal region has been reported to contain a nonspecific tRNA binding domain in mammalian LysRS (11) and AspRS (13), both class IIb aminoacyl-tRNA synthetases. Figure 1 shows Western blot analyses of cell lysates probed with either anti-LysRS (A) or anti-β-actin (B). Lanes K in Fig. 1A and D contain purified human LysRS tagged at the N-terminal end with an MRGSHHHHHHSSGWVD sequence, which contains an His6 tag used for purifying the LysRS. As previously described (6), the primary cytoplasmic LysRS species migrates with an apparent molecular weight (Mr) of 68,000, while in virions produced from COS7 cells, both the 68K species and a smaller one with an Mr of 63K were found. The Δ1-65 LysRS migrates with an Mr of 61K (Fig. 1A, lane 3, and Fig. 1D, lane 3). The most rapidly migrating species seen in Fig. 1D have not been identified. The bands were quantitated, and the LysRS/β-actin ratios are shown in Fig. 1C and have been normalized to the LysRS/β-actin ratio in COS7 cells expressing only endogenous LysRS (Fig. 1A through C, lanes 1). Δ1-65 LysRS was expressed somewhat better than is LysRS.F, as shown by the higher LysRS/β-actin ratios (Fig. 1C).
FIG. 1.
Effect of expression of wild-type or N-terminal-truncated LysRS upon the cytoplasmic and viral concentrations of LysRS. HIV-1 was produced and isolated from HIV-1-transfected COS7 cells as previously described (6). Sucrose gradient-purified virions and cells were lysed by suspension in 1× radioimmunoprecipitation assay buffer (10 mM Tris [pH 7.4], 100 mM NaCl, 1% deoxycholate, 0.1% sodium dodecyl sulfate, 1% Nonidet P-40, protease inhibitor cocktail tablets [Boehringer Mannheim]). Western blot analysis was performed using either 300 μg of cellular protein or 10 μg of viral protein, as determined by the Bradford assay (1). The cellular and viral lysates were resolved by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis, which was followed by blotting onto nitrocellulose membranes (Gelman Sciences) and detection by antibody. Detection of protein on the Western blot utilized monoclonal antibodies or antisera that were specifically reactive with viral capsid (mouse antibody; Intracel), β-actin (mouse antibody; Sigma Aldrich), and human LysRS (rabbit antibody [26]). Western blots were analyzed by enhanced chemiluminescence (ECL kit; Amersham Life Sciences) using goat anti-mouse or donkey anti-rabbit (Amersham Life Sciences) as a secondary antibody, and the results were quantitated using the UN-SCAN-IT gel automated digitizing system. (A through C) Cytoplasmic concentration of LysRS. Western blot analysis of COS7 cell lysates, probed with either anti-LysRS (panel A) or anti-β-actin (panel B). Panel C shows the LysRS/β-actin ratio as determined by quantitative analysis of the bands in panels A and B. Lane K, purified His6-tagged human LysRS, containing the appended N-terminal MRGSHHHHHHSSGWVD sequence (6). The other lanes represent COS7 cells transfected with the following plasmids: lane 1, BH10.P-; lane 2, BH10.P- and LysRS.F; and lane 3, BH10.P- and Δ1-65 LysRS. BH10.P- is a simian virus 40-based vector that contains full-length wild-type HIV-1 proviral DNA containing an inactive viral protease (20, 23). LysRS.F contains cDNA encoding full-length (1 to 597 amino acids) human LysRS cloned into pcDNA3.1 (Invitrogen) and was constructed through PCR amplification of the cDNA as previously described (14). To produce an N-terminal-truncated Δ1-65 LysRS encoding amino acids 66 to 597, the sense primer was complementary to a sequence downstream of the sequence encoding the N-terminal amino acids. (D through F) Viral concentrations of LysRS. Western blot analysis of viral lysates probed with anti-LysRS (panel D) or anti-CA (panel E). Panel F shows the LysRS/Gag ratio determined from the data in panels D and E. Lanes 1 to 3 represent viruses produced from COS7 cells transfected with the following plasmids: lane 1, BH10.P-; lane 2, BH10.P- and LysRS.F; and lane 3, BH10.P- and Δ1-65 LysRS. Lane K, purified His6-tagged human LysRS. To purify this protein, the full-length LysRS PCR fragment was cloned into the bacterial expression vector pET-21b(+) (Clontech), which expresses the protein with a C-terminal His6 tag. The protein was overexpressed in E. coli and purified as previously described (26). The bar graphs in panels C and F represent the means of results of experiments performed at least three times, and the error bars represent standard deviations.
The packaging of LysRS into HIV-1 was examined next. Figure 1 shows Western blot analyses of viral lysates probed with either anti-LysRS (Fig. 1D) or anticapsid (CA) (Fig. 1E). Anti-CA detects the capsid sequences within the unprocessed Gag protein present in protease-negative viruses. The bands were quantitated, and the LysRS/Gag ratios are shown in Fig. 1F and have been normalized to the LysRS/Gag ratio for cells transfected with BH10.P- only (Fig. 1D through F, lanes 1). As previously reported (6), virions incorporating either endogenous LysRS (Fig. 1D, lane 1) or exogenous LysRS.F (Fig. 1D, lane 2) contain both full-length and smaller LysRS species. In addition to these species, the Δ1-65 LysRS species can also be seen in the viruses produced from cells expressing this LysRS variant (Fig. 1D, lane 3). The expression of exogenous LysRS.F or Δ1-65 LysRS results in increases in the total LysRS incorporated into virions (Fig. 1F), with more total LysRS incorporated into virions upon expression of Δ1-65 LysRS (Fig. 1D, lane 3) than upon expression of LysRS.F. This difference probably reflects the larger amount of Δ1-65 LysRS present in the cytoplasm (Fig. 1C). The N-terminal truncation itself is unlikely to have a direct effect upon LysRS incorporation, since LysRS is incorporated into particles via its interaction with Gag at LysRS sequences downstream of these N-terminal sequences (amino acids 208 to 259).
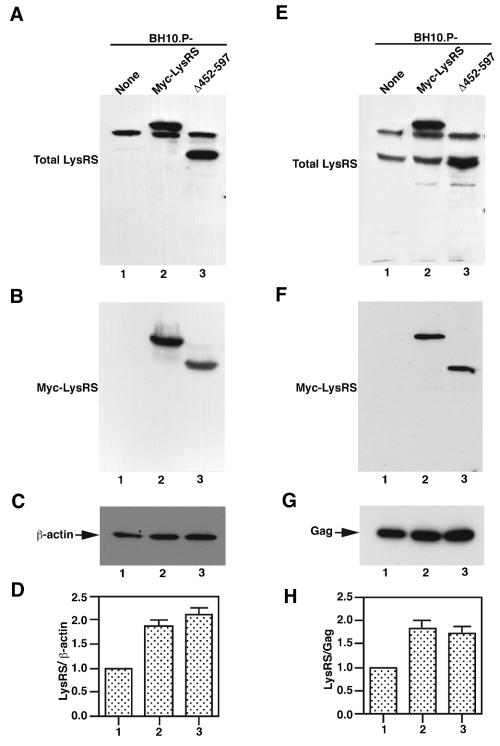
Factors regulating the ability of exogenous LysRS to be packaged into the virus were further examined through expression of two other mutant LysRS species: (i) a full-length LysRS variant containing extra amino acids of the Myc sequence at the N terminus (Myc-LysRS) and (ii) a LysRS mutant missing the C-terminal 145 amino acids (Δ452-597 LysRS), which also contains the N-terminal Myc. The deleted sequences in Δ452-597 LysRS include motif 3, a region believed to participate at the aminoacylation site of the enzyme (3, 9). The cytoplasmic expression of Myc-LysRS and Δ452-597 LysRS in COS7 cells cotransfected with BH10.P- is shown in Fig. 2 by Western blots of cell lysates probed with either anti-LysRS (A) anti-Myc (B), or anti-β-actin (C). In Fig. 2A, it can be seen that Myc-LysRS (lane 2) and Δ452-597 LysRS (lane 3) are distinguished from endogenous LysRS (lane 1) by their reduced and increased electrophoretic mobilities, respectively. Figure 2B shows Western blots probed with anti-Myc, which detects only the expression of exogenous LysRS variants. The LysRS (Fig. 2A)/β-actin ratios are plotted in Fig. 2D and have been normalized to the endogenous LysRS/β-actin ratio. Figure 2A indicates that the majority of cytoplasmic LysRS is either Myc-LysRS or Δ452-597 LysRS. The cytoplasmic expression of Myc-LysRS and Δ452-597 LysRS (Fig. 2D) appears to be somewhat greater than that found for Δ1-65 LysRS or LysRS.F (Fig. 1C). This result may be due to the different vectors used to express LysRS.F and Δ1-65 LysRS (pcDNA3.1) or Myc-LysRS and Δ452-597 LysRS (pcDNA1.0).
FIG. 2.
Effect of expression of Myc-LysRS and Δ452-597 LysRS upon the cytoplasmic and viral concentrations of LysRS. COS7 cells were cotransfected with BH10.P- and LysRS-encoding plasmids, viruses and cells were isolated and lysed, and the cell and viral lysates were analyzed by Western blotting, all as described in the legend of Fig. 1. (A through D) Cytoplasmic concentrations of LysRS. Western blot analysis of COS7 cell lysates, probed with either anti-LysRS (panel A), anti-Myc (panel B), or anti-β-actin (panel C). Panel D shows the LysRS/β-actin ratio as determined by quantitative analysis of the bands in panels A and C. The lanes in each panel represent COS7 cells transfected with the following plasmids: lane 1, BH10.P-; lane 2, BH10.P- and Myc-LysRS; and lane 3, BH10.P- and Δ452-597 LysRS. Myc-LysRS contains the Myc-containing sequence (MASMEQKLISEEDLNNG) appended to the N terminus of LysRS and was produced by cloning the PCR-amplified full-length LysRS cDNA into pcDNA1 (Invitrogen), into which sequences containing Myc had been inserted (7). Δ452-597 LysRS represents LysRS, whose C-terminal 146 amino acids were deleted and was also cloned into pcDNA1. (E through H) Western blots probing viral concentrations of LysRS. Viral lysates were probed with either anti-LysRS (panel E), anti-Myc (panel F), or anti-CA (panel G). Panel H shows the LysRS/Gag ratio determined from the data in panels E and G. Lanes in panels E through H represent viruses produced from cells transfected with: lane 1, BH10.P-; lane 2, BH10.P- and Myc-LysRS; and lane 3, BH10.P- and Δ452-597 LysRS. The bar graphs in panels D and H represent the means of experiments performed at least three times, and the error bars represent standard deviations.
Figure 2 also shows Western blots of viral lysates probed with either anti-LysRS (Fig. 2E), anti-Myc (Fig. 2F), or anti-CA (Fig. 2G). The bands were quantitated, and the total viral LysRS/Gag ratios are shown graphically in Fig. 2H and have been normalized to the LysRS/Gag ratio for cells transfected with BH10.P- only (lane 1). The results indicate that both exogenous Myc-LysRS and Δ452-597 LysRS are packaged into virions (Fig. 2E and F). We also note that unlike endogenous LysRS in viruses, which is processed to species with increased electrophoretic mobility (Fig. 1D, lanes 1 to 3, and Fig. 2E, lane 1), Myc-LysRS processing is not apparent, as indicated by the strong signal for full-length Myc-LysRS without any significant increase in the smaller band containing processed endogenous LysRS (Fig. 2E, lane 2).
Effect of the expression of exogenous wild-type and mutant LysRS upon cytoplasmic and viral concentrations of tRNALys isoacceptors.
Figure 3 shows the effect of expression of wild-type and mutant LysRS upon tRNALys concentrations in the cytoplasm of HIV-1-transfected COS7 cells and in the virions produced from these cells. Dot blot hybridization was used to determine the tRNALys/β-actin mRNA ratios in cellular RNA (Fig. 3A) or the tRNA3Lys/genomic RNA ratios in total viral RNA (Fig. 3B) (19). We observed, as shown in Fig. 3A, small increases (20 to 25%) in the cytoplasmic concentrations of the major tRNALys isoacceptors upon expression of LysRS.F, Δ1-65 LysRS, and Myc-LysRS, while expression of Δ452-597 LysRS induced no such increase. On the other hand, Fig. 3B indicates that incorporation of tRNALys isoacceptors is increased upon expression of LysRS.F, Myc-LysRS, and Δ452-597 LysRS but is actually decreased somewhat upon expression of Δ1-65 LysRS.
FIG. 3.
Effect of expression of wild-type or mutant LysRS upon the cellular and viral concentrations of tRNALys. COS7 cells were cotransfected with BH10.P- and LysRS-encoding plasmids, and viruses and cells were isolated as described in the legend of Fig. 1. Total cellular or viral RNA was extracted from cell or viral pellets by the guanidinium isothiocyanate procedure (8) and dissolved in 5 mM Tris buffer (pH 7.5). Human placental tRNA3Lys was purified as previously described (19). Hybridization to dot blots of cellular or viral RNA was carried out with DNA probes that were complementary to tRNA3Lys and tRNA1,2Lys (19), viral genomic RNA (4), and β-actin mRNA (DNA probe from Ambion). Dot blots of total cellular (A) or total viral (B) RNA were hybridized with DNA probes to either β-actin mRNA (panel A) or viral genomic RNA (panel B) and to either tRNA3Lys or tRNA1,2Lys (panels A and B). In the dot blots shown, the samples contained equal amounts of β-actin mRNA (panel A) or viral genomic RNA (panel B). Dot blots were analyzed by phosphorimaging, and the ratios of tRNALys/β-actin mRNA (panel A) and tRNALys/genomic RNA (panel B) were determined for cells cotransfected with BH10.P- and one of the following LysRS-encoding plasmids: none, LysRS.F, Δ1-65 LysRS, Myc-LysRS, or Δ452-597 LysRS. The bar graphs in panels A and B represent the means of results of experiments performed at least three times, and the error bars represent standard deviations.
While expression of wild-type LysRS results in an increase in cytoplasmic tRNALys as well, this is not responsible for the increased tRNALys packaging into the virion. Thus, an increase in cytoplasmic tRNALys resulting from expression of Δ1-65 LysRS does not result in an increase in tRNALys incorporation into virions, while the increased packaging of tRNALys into virions due to expression of Δ452-597 LysRS is not accompanied by any increase in tRNALys in the cytoplasm. The increases in the cytoplasmic production of tRNALys resulting from overexpression of LysRS.F, Myc-LysRS, and Δ1-65 LysRS, may reflect a control mechanism in which production of either tRNALys or LysRS is regulated to maintain a certain lysyl-tRNALys/LysRS ratio in the cytoplasm. However, the presence of unacylated tRNALys in the cell, such as might arise from tRNALys bound to the Δ452-597 LysRS mutant, might inhibit any further increase in tRNALys transcription. In yeast cells, uncharged tRNALys has been shown to act via a signal transduction pathway to activate the synthesis of LysRS through increased transcription of the LysRS gene (21). Presumably, this transcriptional regulation mechanism will maintain the optimum LysRS/tRNALys ratio to keep all tRNALys in a charged state, a state that could also be achieved by decreasing synthesis of new tRNALys.
Overexpression of either tRNA3Lys or tRNA2Lys in the cells containing a plasmid coding for either tRNALys isoacceptor will result in an increase in that specific tRNALys isoacceptor in the virion. However, the total tRNALys/virion ratio remains the same; i.e., an increase in one of the tRNALys isoacceptors results in a decrease in the other (14). The molecule that initially limits the tRNALys/virion ratio appears to be LysRS, since as shown previously (14) and herein, expression of LysRS results in an increase in all major tRNALys isoacceptors. Presumably, cellular LysRS and tRNALys are present in great excess relative to the amount packaged into virions, since infection of cells with HIV-1 does not noticeably reduce cell replication. It seems likely, therefore, that if LysRS represents an initially limiting factor for tRNALys incorporation into HIV-1, the tRNALys/LysRS complex that interacts with viral proteins may be a small pool separate from the bulk cytoplasmic pool. In fact, recent work indicates that the cellular source of viral LysRS may be newly synthesized LysRS (15)
The increase in viral tRNALys accompanying the increase in viral LysRS incorporation is not accompanied by an increase in Gag-Pol incorporation (14), and the limited increases in both viral LysRS and tRNALys levels that we observed may result from limited amounts of Gag-Pol incorporation. That viral LysRS and tRNALys levels can increase at all implies that there are a restricted number of unsaturated binding sites on the Gag/Gag-Pol complex for these molecules. This would also explain why Δ1-65 LysRS only creates a small decrease in tRNALys incorporation, since most of its binding might be to these unsaturated sites and is noncompetitive with wild-type endogenous LysRS/tRNALys. Further experimental increases in the cytoplasmic expression of LysRS has proven difficult, perhaps due to the possible feedback inhibition of LysRS expression by LysRS. This occurrence has been demonstrated in yeast cells for another class IIb aminoacyl-tRNA synthetase, AspRS, which specifically binds to its own mRNA and inhibits translation of this mRNA (12).
Ability of LysRS variants to bind or aminoacylate tRNA3Lys in vitro.
The ability of some of the LysRS variants to bind or aminoacylate tRNA3Lys in vitro was determined to see if this ability could explain the various abilities of LysRS species to facilitate tRNALys packaging. LysRS.F, Δ1-65 LysRS, and Δ452-597 LysRS, which were tagged at their C termini with His6, were partially purified from transformed bacteria by using Ni+ chromatography. Their relative binding affinities to human placental tRNA3Lys were compared using an electrophoretic band shift assay. Human tRNA3Lys was 3′-end labeled with [32P]pCp (2) and incubated with increasing amounts of each LysRS species. The resulting complexes were resolved on a native polyacrylamide gel. As shown in Fig. 4A, Δ1-65 LysRS shows significantly reduced binding to tRNA3Lys compared to LysRS.F, while binding of Δ452-597 LysRS is slightly less than that of LysRS.F. Thus, the relative binding affinities of the different LysRS variants are reflected in their ability to facilitate incorporation of tRNALys into virions. The reduced affinity of Δ1-65 LysRS for human placental tRNA3Lys in vitro is consistent with a report that an N-terminally truncated form of hamster LysRS displayed significantly reduced (about 100-fold) affinity for in vitro-synthesized tRNA3Lys transcripts relative to the full-length enzyme (11). The specificity of the binding reaction is shown in Fig. 4B, which demonstrates the inability of Escherichia coli total tRNA to bind to LysRS.F, Δ1-65 LysRS, and Δ452-597 LysRS.
FIG. 4.
Interaction and aminoacylation of tRNA3Lys with wild-type and mutant LysRS in vitro. tRNA3Lys was purified from human placenta as previously described (19). Wild-type and mutant LysRS variants were expressed in E. coli and purified as described for wild-type LysRS in the legend of Fig. 1. (A) The interaction between tRNA3Lys and wild-type LysRS, Δ1-65 LysRS, or Δ452-597 LysRS was measured using an electrophoretic band shift assay. tRNA3Lys was labeled with the [32P]pCp 3′-end labeling technique as previously described (2). In 20 μl of binding buffer (20 mM Tris-HCl [pH 7.4], 75 mM KCl, 10 mM MgCl2, and 5% glycerol), 5 nM labeled tRNALys was incubated with different concentrations of either wild-type or mutant human LysRS (0.06, 0.3, or 1.5 μM) for 15 min on ice and was then analyzed by native 6% polyacrylamide gel electrophoresis that was carried out at 4°C. Mock, no LysRS. (B) The interaction between 3′-end labeled [32P]pCp E. coli total tRNA (Roche) and wild-type LysRS.F, Δ1-65 LysRS, or Δ452-597 LysRS was measured using an electrophoretic band shift assay. (C) In vitro aminoacylation of tRNA3Lys by LysRS. The reactions were conducted at 30°C in a final volume of 100 μl containing a 10 mM concentration of enzyme (wild-type LysRS, Δ1-65 LysRS, or Δ452-597 LysRS), 50 mM HEPES (pH 7.5), 0.1 mg of bovine serum albumin per ml, 20 mM KCl, 10 mM MgCl2, 20 mM β-mercaptoethanol, 4 mM ATP, 20 μM lysine, 0.3 μC of [3H]lysine (Amersham) per ml, and 0.4 mg of calf liver tRNA (Sigma) per ml. At 2-min intervals, 10-μl aliquots were spotted onto trichloroacetic acid (TCA)-soaked Whatman 3MM filters. The filters were washed three times with 10% TCA and counted by scintillation counting.
A correlation between the ability of tRNALys to be packaged into viruses and its ability to be aminoacylated in vivo has been reported (18). In that work, COS7 cells were cotransfected with HIV-1 proviral DNA and with plasmids coding for anticodon mutants of tRNA3Lys. The ability of these mutant tRNAs to be aminoacylated in vivo was correlated with their ability to be packaged into HIV-1. Since the tRNALys anticodon is an important site for interaction with LysRS (10), aminoacylation was used as an indication of tRNA3Lys binding to endogenous LysRS, and the correlation showed the importance of LysRS binding to tRNA3Lys for its incorporation into the virus. Whether aminoacylation itself is required for tRNA3Lys packaging, however, could not be determined from those experiments.
The Δ452-597 LysRS lacks motif 3, whose sequences contribute to the catalytic aminoacylation site (3, 9). We therefore tested the ability of this and other mutant LysRS species to aminoacylate tRNA3Lys in vitro by using these enzymes to aminoacylate calf liver tRNA (Sigma) with [3H]lysine in a standard aminoacylation reaction. The aminoacylation time course for three LysRS variants is shown in Fig. 4C. As expected, Δ452-597 LysRS is severely inhibited in its aminoacylation ability. Thus, the ability of LysRS to facilitate tRNALys packaging into virions is not correlated with the enzyme's ability to aminoacylate tRNA3Lys. Δ1-65 LysRS, although having a weaker binding affinity for tRNA3Lys, still showed an intermediate ability to aminoacylate tRNA3Lys. This finding is supported by a report that indicated that the N-terminal domain of hamster LysRS, while not essential for aminoacylation, improved the docking of the acceptor arm of tRNA3Lys into the active site of the enzyme (11).
For HIV-1, the tRNA3Lys is not acylated (17), a condition which is probably required to allow the terminal 3′ adenosine of tRNA3Lys to be extended by reverse transcriptase. It is not known if only uncharged cytoplasmic tRNALys is targeted by viral protein for incorporation into viruses or if charged tRNALys is deacylated after binding to viral proteins. Thus, it is possible that a Gag/LysRS complex might bind to uncharged tRNALys without acylating it or Gag might instead induce deacylation of the charged tRNALys within a tRNALys/LysRS complex.
REFERENCES
- 1.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 2.Bruce, A. G., and O. C. Uhlenbeck. 1978. Reactions at the termini of tRNA with T4 RNA ligase. Nucleic Acids Res. 5:3665-3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavarelli, J., G. Eriani, B. Rees, M. Ruff, M. Boeglin, A. Mitschler, F. Martin, J. Gangloff, J. C. Thierry, and D. Moras. 1994. The active site of yeast aspartyl-tRNA synthetase: structural and functional aspects of the aminoacylation reaction. EMBO J. 13:327-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cen, S., Y. Huang, A. Khorchid, J. L. Darlix, M. A. Wainberg, and L. Kleiman. 1999. The role of Pr55gag in the annealing of tRNA3Lys to human immunodeficiency virus type 1 genomic RNA. J. Virol. 73:4485-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cen, S., H. Javanbakht, S. Kim, K. Shiba, R. Craven, A. Rein, K. Ewalt, P. Schimmel, K. Musier-Forsyth, and L. Kleiman. 2002. Retrovirus-specific packaging of aminoacyl-tRNA synthetases with cognate primer tRNAs. J. Virol. 76:13111-13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cen, S., A. Khorchid, H. Javanbakht, J. Gabor, T. Stello, K. Shiba, K. Musier-Forsyth, and L. Kleiman. 2001. Incorporation of lysyl-tRNA synthetase into human immunodeficiency virus type 1. J. Virol. 75:5043-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, T., and S. Richard. 1998. Structure-function analysis of Qk1: a lethal point mutation in mouse quaking prevents homodimerization. Mol. Cell. Biol. 18:4863-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chomczynski, P., and N. Sacchi. 1987. RNA isolation from cultured cells. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 9.Cusack, S., M. Hartlein, and R. Leberman. 1991. Sequence, structural and evolutionary relationships between class 2 aminoacyl-tRNA synthetases. Nucleic Acids Res. 19:3489-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cusack, S., A. Yaremchuk, and M. Tukalo. 1996. The crystal structures of T. thermophilus lysyl-tRNA synthetase complexed with E. coli tRNALys and a T. thermophilus tRNALys transcript: anticodon recognition and conformational changes upon binding of a lysyl-adenylate analogue. EMBO J. 15:6321-6334. [PMC free article] [PubMed] [Google Scholar]
- 11.Francin, M., M. Kaminska, P. Kerjan, and M. Mirande. 2002. The N-domain of mammalian lysyl-tRNA synthetase is a functional tRNA binding domain. J. Biol. Chem. 277:1762-1769. [DOI] [PubMed] [Google Scholar]
- 12.Frugier, M., and R. Giege. 2003. Yeast aspartyl-tRNA synthetase binds specifically its own mRNA. J. Mol. Biol. 331:375-383. [DOI] [PubMed] [Google Scholar]
- 13.Frugier, M., L. Moulinier, and R. Giege. 2000. A domain in the N-terminal extension of class IIB eukaryotic aminoacyl-tRNA synthetases is important for tRNA binding. EMBO J. 19:2371-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabor, J., S. Cen, H. Javanbakht, M. Niu, and L. Kleiman. 2002. Effect of altering the tRNA3Lys concentration in human immunodeficiency virus type 1 upon its annealing to viral RNA, GagPol incorporation, and viral infectivity. J. Virol. 76:9096-9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo, F., S. Cen, M. Niu, H. Javanbakht, and L. Kleiman. 2003. Specific inhibition of the synthesis of human lysyl-tRNA synthetase results in decreases in tRNALys incorporation, tRNA3Lys annealing to viral RNA, and viral infectivity in human immunodeficiency virus type 1. J. Virol. 77:9817-9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang, Y., J. Mak, Q. Cao, Z. Li, M. A. Wainberg, and L. Kleiman. 1994. Incorporation of excess wild type and mutant tRNA3Lys into human immunodeficiency virus type 1. J. Virol. 68:7676-7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, Y., A. Shalom, Z. Li, J. Wang, J. Mak, M. A. Wainberg, and L. Kleiman. 1996. Effects of modifying the tRNA3Lys anticodon on the initiation of human immunodeficiency virus type 1 reverse transcription. J. Virol. 70:4700-4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Javanbakht, H., S. Cen, K. Musier-Forsyth, and L. Kleiman. 2002. Correlation between tRNA3Lys aminoacylation and incorporation into HIV-1. J. Biol. Chem. 277:17389-17396. [DOI] [PubMed] [Google Scholar]
- 19.Jiang, M., J. Mak, A. Ladha, E. Cohen, M. Klein, B. Rovinski, and L. Kleiman. 1993. Identification of tRNAs incorporated into wild-type and mutant human immunodeficiency virus type 1. J. Virol. 67:3246-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khorchid, A., H. Javanbakht, M. A. Parniak, M. A. Wainberg, and L. Kleiman. 2000. Sequences within Pr160gag-pol affecting the selective packaging of tRNALys into HIV-1. J. Mol. Biol. 299:17-26. [DOI] [PubMed] [Google Scholar]
- 21.Lanker, L., J. L. Bushman, A. G. Hinnebusch, H. Trachsel, and P. P. Mueller. 1992. Autoregulation of the yeast lysyl-tRNA synthetase gene GCD5/KRS1 by translational and transcriptional control mechanisms. Cell 70:647-657. [DOI] [PubMed] [Google Scholar]
- 22.Leis, J., A. Aiyar, and D. Cobrinik. 1993. Regulation of initiation of reverse transcription of retroviruses, p. 33-47. In A. M. Skalka and S. P. Goff (ed.), Reverse transcriptase, vol. 1. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 23.Mak, J., M. Jiang, M. A. Wainberg, M.-L. Hammarskjold, D. Rekosh, and L. Kleiman. 1994. Role of Pr160gag-pol in mediating the selective incorporation of tRNALys into human immunodeficiency virus type 1 particles. J. Virol. 68:2065-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mak, J., and L. Kleiman. 1997. Primer tRNAs for reverse transcription. J. Virol. 71:8087-8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park, J., and C. D. Morrow. 1992. The nonmyristylated Pr160gag-pol polyprotein of human immunodeficiency virus type 1 interacts with Pr55gag and is incorporated into viruslike particles. J. Virol. 66:6304-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiba, K., T. Stello, H. Motegi, T. Noda, K. Musier-Forsyth, and P. Schimmel. 1997. Human Lysyl-tRNA synthetase accepts nucleotide 73 variants and rescues E. coli double-defective mutant. J. Biol. Chem. 272:22809-22816. [DOI] [PubMed] [Google Scholar]
- 27.Smith, A. J., N. Srinivasakumar, M.-L. Hammarskjöld, and D. Rekosh. 1993. Requirements for incorporation of Pr160gag-pol from human immunodeficiency virus type 1 into viruslike particles. J. Virol. 67:2266-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]