Abstract
Previous studies have shown that human cytomegalovirus (CMV) is a potent elicitor of interferon-stimulated gene (ISG) expression. Induction of the interferon pathway does not require replication-competent virus, and envelope glycoprotein B (gB) from CMV is a viral structural component that can directly induce transcription of ISGs. Here we extend these earlier findings by defining the consequences of inducing the interferon pathway. We found that cells respond to CMV or soluble gB by establishing a functional antiviral state within cell types critical in CMV biology, such as fibroblasts and endothelial cells. We have also discovered new insights into the mechanism by which the pathway is initiated. Interferon regulatory factor 3 (IRF3), a key transcriptional regulator of cellular interferon responses, is activated by CMV virions and soluble gB. Thus, IRF3 becomes activated via “outside-in” signal transduction events. This is a novel mechanism of activation of this key transcription factor by viruses. In comparison to soluble gB (gB1-750), which comprises the entire ectodomain of gB, a truncation mutant encompassing only the amino-terminal region of gB (gB1-460) was markedly less effective at inducing antiviral responses. This indicates that the region of gB from residues 461 to 750 is important for initiation of the antiviral response. In addition, CMV and gB establish an antiviral state in alpha/beta interferon null cells, illustrating that primary induction of ISGs by CMV and gB is sufficient to establish the antiviral response and that interferon secretion is not necessary for the antiviral effect. Taken together, our findings reveal that CMV initiates a coordinated antiviral response through contact between gB and an as-yet-unidentified cell surface receptor(s).
Human cytomegalovirus (CMV), a member of the Herpesviridae family, is a ubiquitous pathogen that causes significant morbidity and mortality in humans. Immunocompromised patient groups, including chemically immunosuppressed transplant recipients and those afflicted with AIDS, are among the most severely affected by CMV (23, 28). Congenital CMV infection is a major cause of birth defects, often resulting in severe neurological and cognitive disorders (29, 32). In addition, persistent CMV infection has been associated with coronary artery disease (21).
CMV infection renders numerous and rapid changes upon cells. Several signal transduction pathway factors are affected, including the activation of mitogen-activated protein kinases, Rel family transcription factors, and phospholipid mediators (1-3, 49-52). Since many of the cellular factors that become activated in response to CMV are transcriptional regulators, it was predicted that cellular gene expression would be altered during infection. Transcriptional profiling studies revealed that the expression of hundreds of cellular genes is altered in cells that are exposed to CMV particles (10, 38, 53, 54). Notably, members of the interferon-stimulated gene (ISG) family are among the transcripts that are most dramatically up-regulated in response to CMV. ISGs are the subset of cellular genes that are strongly induced in response to alpha/beta interferon and are responsible for carrying out the antiviral activities attributed to interferons (41). Alpha/beta interferon is secreted by cells in response to viruses and restricts virus replication at the earliest stages of infection, thereby limiting virus spread while adaptive immune responses are mounted (41). Alpha/beta interferon is also an important component of the cytokine response induced by a vast array of pathogens, both viral and nonviral, that functions to recruit immune cells to the site of infection and promote cell-mediated and humoral immune responses.
Double-stranded RNA (dsRNA), a common replicative intermediate for RNA viruses, is typically the molecular trigger that initiates interferon responses (35). Infection by DNA viruses and retroviruses also elicits interferon responses; however, these viruses do not generate cytoplasmic dsRNA intermediates. Thus, DNA viruses and retroviruses likely trigger cellular antiviral responses by alternative means. Viral envelope glycoproteins are one factor that can contribute to interferon induction by viruses. For example, single amino acid changes in the glycoprotein M gene of transmissible gastroenteritis virus, a member of the coronavirus family, severely impairs the ability of the virus to induce interferon secretion (22). In addition, soluble forms of envelope glycoproteins from human immunodeficiency virus (HIV) and herpes simplex virus type 1 have been shown to elicit interferon secretion from peripheral blood mononuclear cells (4-6).
The observation that transcriptionally inactive CMV virions can induce ISG expression suggests that structural components of the virion are able to initiate these responses (10, 14, 53, 54). Members of our laboratory utilized a recombinant form of envelope glycoprotein B (gB) from CMV to show that gB is one structural factor that can directly induce ISG expression (8, 38). Microarray analysis revealed that cells treated with gB or CMV exhibit a high degree of coregulation, suggesting that much of the transcriptional modulation induced by CMV can be attributed to an interaction between gB and an unidentified cellular receptor(s) (38). Taken together, these studies strongly indicate that envelope glycoproteins represent at least one mechanism by which CMV can elicit interferon responses. However, the cellular factor(s) that initiates host antiviral responses to viral glycoproteins remains unidentified.
While the viral determinants responsible for initiating interferon responses are only beginning to be elucidated, great strides have been made with respect to the identification and definition of the cellular factors and mechanisms that mediate antiviral responses. The emerging model of interferon induction by viruses favors a biphasic response that allows for rapid and robust, yet tightly controlled, interferon production (44, 45). A key event early in the induction of these responses is the activation of interferon regulatory factor 3 (IRF3), a constitutively expressed protein that resides in the cytoplasm of cells. IRF3 becomes activated via the sequential phosphorylation of serine residues in its carboxy-terminal domain, and recent studies suggest that phosphorylation of IRF3 is carried out by IKKɛ and TBK1 (19, 36, 37). Once activated, IRF3 forms homodimers and translocates to the nucleus, where it associates with the general transcriptional coactivator p300/CBP (42, 48). Transcription of a subset of ISGs ensues, including one alpha interferon species and the single beta interferon gene (35, 47). This initial burst of interferon acts in an autocrine and paracrine manner through the alpha/beta interferon receptor and Jak-STAT pathway to induce a broader panel of ISGs, including another member of the IRF family, IRF7 (41). Newly synthesized IRF7 becomes activated via phosphorylation and forms heterodimers with IRF3 to promote increased expression of beta interferon and expression of additional alpha interferon genes that cannot be induced by IRF3 alone (33). The activity of ISGs induced by IRF- and interferon-dependent pathways causes cells to become refractory to viral replication.
The aim of this study was to further define the mechanism by which gB of CMV activates interferon responses in critical cell types for CMV pathogenesis, such as fibroblasts and endothelial cells. We report that cells exposed to CMV or gB respond by forming a fully functional antiviral state, further demonstrating that infection is not necessary for the induction of antiviral responses. We also show that gB activates IRF3 and ultimately induces secretion of beta interferon from human fibroblasts. Interferon production implies that the establishment of the antiviral state in response to CMV and gB is mediated, at least in part, by the autocrine and/or paracrine activity of interferon. However, the secondary activity of interferon is not required for the establishment of the antiviral state, as cells lacking alpha/beta interferon genes mount a functional antiviral response to both CMV and gB. This indicates that the initial, cell surface-generated induction of ISGs by both CMV and gB is sufficient for the establishment of the antiviral state within cells.
MATERIALS AND METHODS
Cells, reagents, and virus.
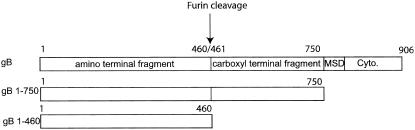
Normal human dermal fibroblasts (NHDFs) (Clonetics, San Diego, Calif.) and GRE cells (a generous gift from Ganes Sen, The Cleveland Clinic Foundation [7]) were grown at 37°C in 5% CO2 in Dulbecco's minimal medium (Life Technologies, Rockville, Md.) supplemented with 10% fetal bovine serum, 1% glutamine, and 1% penicillin-streptomycin-amphotericin B (Fungizone). Human umbilical vein endothelial cells (HUVEC), human aortic endothelial cells (HAEC), and human dermal microvascular endothelial cells (DMVEC) were all purchased from Clonetics. Endothelial cells were grown at 37°C in 5% CO2 in endothelial cell medium (Clonetics) supplemented with 10% fetal bovine serum, 0.3% glutamine, 3 mg of bovine brain extract per ml, 1 μg of hydrocortisone per ml, and 10 ng of human epidermal growth factor per ml. Interferon treatments were performed with a combination of alpha and beta recombinant human interferons (100 U/ml; BioSource International, Camarillo, Calif.). Soluble glycoprotein B (gB1-750) was constructed, produced, and purified as previously described (12). Soluble gB1-460 was constructed in our laboratory (M. Lopper and T. Compton, unpublished data) and purified as described previously (12). Figure 1 shows a schematic of gB1-750 and gB1-460. UV inactivation of AD169 virions was performed as described previously (15). VHL/E (kindly provided by W. James Waldman, The Ohio State University) was propagated in HUVEC as previously described (46).
FIG. 1.
Schematic diagram of gB constructs showing the region of wild-type gB represented by each truncation mutant. Constructs are shown with the amino terminus at the left, and prominent structural features are labeled with the corresponding amino acid residue number. The carboxy-terminal fragment comprises amino acid residues 461 to 906. gB1-750 comprises the entire ectodomain, while gB1-460 is truncated at the site of furin cleavage. Each truncation mutant has a six-histidine tag at its carboxy terminus. MSD, membrane-spanning domain; Cyto, cytoplasmic tail.
RNase protection analysis.
NHDFs or endothelial cells were washed twice with phosphate-buffered saline (PBS) and then mock infected, infected with CMV strain AD169 or VHL/E (multiplicity of infection [MOI] of 2 PFU/cell), or treated with soluble gB (13 μg/ml). All treatments were performed in serum-free media. At the indicated times posttreatment, total RNA was extracted from cells with RNA Stat-60 according to the manufacturer's instructions (Tel-Test Inc., Friendswood, Tex.). Purified RNA was quantified by spectrophotometric analysis, and 4 μg of each sample was used for RNase protection analysis. The cloning and generation of probes for 2′,5′-oligoadenylate synthetase (OAS), ISG of 54 kDa (ISG54), and actin have been previously described (38). RNase protection probes were generated from cloned genes by using the MAXIscript T7 kit (Ambion) and 32P-labeled UTP (Amersham Pharmacia). Gel-purified radioactively labeled probes and sample RNA were hybridized, digested with RNase, and separated by denaturing polyacrylamide gel electrophoresis (PAGE) according to the manufacturer's instructions (RPA III kit; Ambion). The protected samples were visualized by using a phosphorimager.
VSV plaque reduction assay.
Subconfluent NHDF, endothelial cell, or GRE monolayers were washed twice with PBS and then mock infected, treated with recombinant human alpha/beta interferons, infected with live or UV-inactivated CMV (strain AD169), or treated with soluble gB, as indicated. All treatments were performed in serum-free media. Following a 6-h incubation period at 37°C, the cells were washed once with PBS and then infected with 100 PFU of vesicular stomatitis virus (VSV) (New Jersey strain) per ml. VSV was adsorbed for 1 h at 37°C, the inoculum was removed, and the cells were overlaid with 2 ml of a 60:40 mixture of 2× Eagle's minimum essential medium (BioWhitaker, Walkersville, Md.) and 1% agarose. The cells were incubated at 37°C, and plaques were visualized by crystal violet staining at 48 h postinfection (0.5× PBS, 0.07% crystal violet, 5.5% formaldehyde).
Comparison of soluble and virion-associated gB.
A gB1-750 standard curve was prepared via twofold serial dilution. The standard curve and whole-virus preparations were transferred to a membrane (Immobilon-NC; Millipore) by use of a dot blot apparatus (MilliBlot system; Millipore) according to the manufacturer's instructions. gB was detected with gB-specific monoclonal antibody 27-78 (9), a horseradish peroxidase-conjugated secondary antibody, and chemiluminescent developing reagent. gB levels in virus preparations were quantitated by comparison to the gB1-750 standard curve.
Western blot analysis of IRF3 phosphorylation.
NHDFs seeded in 100-mm-diameter dishes were washed twice with PBS and then mock infected, infected with CMV (strain AD169; MOI of 0.1 PFU/cell), or treated with soluble gB (1 μg/ml) in the presence of cycloheximide (100 μg/ml). All treatments were performed in serum-free media. At 6 h posttreatment, cells were harvested in lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 5 mM EDTA, 1% Nonidet P-40, 30 mM NaF, 40 mM β-phosphoglycerate, 1 mM Na3VO4, 10% glycerol, protease inhibitors). After three freeze-thaw cycles, equivalent amounts of total cellular protein from each sample were separated via sodium dodecyl sulfate (SDS)-8% PAGE followed by immunoblotting. IRF3 was detected with an anti-IRF3 antibody (Santa Cruz Biotechnologies), a horseradish peroxidase-conjugated secondary antibody, and chemiluminescent developing reagent.
Cellular localization of IRF3.
NHDFs plated on coverslips were washed twice with PBS and then mock infected, infected with UV-inactivated CMV (strain AD169; MOI of 0.1 PFU/cell), or treated with gB (1 μg/ml). All treatments were performed in serum-free media. At 6 h posttreatment, the cells were fixed in 3% paraformaldehyde and IRF3 localization was visualized by indirect immunofluorescence, as previously described (30), with a primary antibody against IRF3 (Santa CruzBiotechnologies) and fluorescence-conjugated secondary antibody (Molecular Probes).
Beta interferon ELISA.
For enzyme-linked immunosorbent assays (ELISAs), subconfluent NHDF monolayers were washed twice with PBS and then mock infected, infected with live or UV-inactivated CMV (strain AD169; MOI of 0.1 PFU/cell), or treated with soluble gB (10 or 25 μg/ml). At 18 h posttreatment, the supernatants were harvested and cellular debris was removed by centrifugation at 12,000 × g for 5 min at 4°C. Beta interferon levels were measured by use of a human beta interferon ELISA kit (Fujirebio, Inc., Tokyo, Japan) according to the manufacturer's instructions.
RESULTS
CMV elicits the establishment of a functional antiviral state within cells.
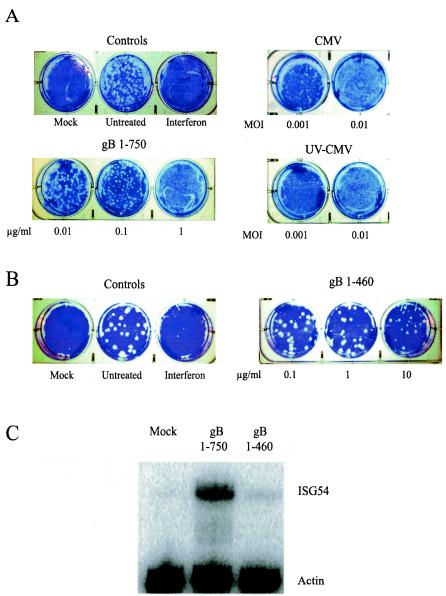
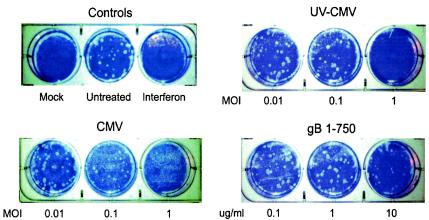
Upon infection with CMV (10, 53, 54) or exposure to soluble gB (8, 14, 38), transcription of ISGs is robustly induced. Based on these observations, we predicted that the physiological effects exerted on cells by CMV and soluble gB would be similar to those induced by alpha/beta interferon. Specifically, we hypothesized that induction of ISGs by CMV and soluble gB would result in the establishment of a functional antiviral state within cells. To test this hypothesis, we assessed the ability of CMV and soluble gB to inhibit plaque formation by VSV, which is sensitive to the effects of interferons. As shown in Fig. 2A, human fibroblasts treated with alpha/beta interferon or replication-competent or UV-inactivated CMV virions are resistant to VSV infection in a dose-dependent manner, indicating the establishment of a functional antiviral state. Breakthrough plaques are seen in cells treated with the lowest dose of infectious CMV, whereas no plaques are detected in the parallel experiment with UV-inactivated CMV. This result is consistent with the notion that CMV encodes gene products that are synthesized during viral replication that dampen host interferon responses (10).
FIG. 2.
Cell response to treatment with CMV, gB, and gB1-460. (A and B) CMV and gB trigger a functional antiviral state in cells. Human fibroblasts were stimulated as indicated (interferon, 100 U/ml). At 6 h poststimulation, the treatments were removed and the monolayers were challenged with approximately 100 PFU of VSV per well. The mock-treated cells were not infected with VSV. Plaque formation was visualized by crystal violet staining at 48 h postinfection. CMV replicates with much slower kinetics than VSV; thus, any visible plaque formation is the result of VSV, not CMV, growth. (C) gB1-460 minimally induces ISG activation. Human fibroblasts were mock treated or treated with gB1-750 or gB1-460 (1 μg/ml). At 8 h posttreatment, total RNA was harvested from cells and subjected to RNase protection analysis with ISG54- and actin-specific probes.
Based on prior observations that soluble gB elicits ISG induction in a manner similar to that by virions, we characterized cellular antiviral responses to two recombinant forms of gB, gB1-750 and gB1-460 (Fig. 1). gB1-750 comprises the entire ectodomain of gB and is a potent elicitor of ISGs (8, 14, 38). Consistent with its ability to transcriptionally up-regulate ISGs, gB1-750 elicits an antiviral response in a dose-dependent manner (Fig. 2A). We compared the gB content of whole-virus inocula to the input levels of soluble gB1-750 by serial dilution and Western blotting. This allowed for direct comparison between the amounts of soluble gB1-750 and virion-associated gB required to elicit the antiviral response. Our estimates indicate that gB levels are approximately equivalent between gB1-750 at 1 μg/ml and CMV at an MOI of 0.1 (data not shown). Based on these estimates, virion-associated gB is approximately 100-fold more effective at inducing the antiviral response than its soluble counterpart (Fig. 2A, compare gB1-750 at 1 μg/ml and UV-CMV at an MOI of 0.001). The difference in relative activity between soluble and virion-associated gB suggests that although contact between gB and cell surface receptors can elicit these responses, additional viral factors or possible valence differences between soluble and membrane-bound gB contribute to the overall induction of antiviral responses to CMV.
gB1-460 is a truncated form of gB1-750 that exhibits cell binding activity and is structurally similar to the corresponding region of gB in the context of gB1-750 (Lopper and Compton, unpublished data). In contrast to gB1-750, gB1-460 elicited a minimal antiviral response (Fig. 2B). For gB1-750, complete inhibition of VSV plaque formation was achieved at a concentration of 1 μg/ml; however, at 10-fold higher levels of gB1-460 (10 μg/ml), plaque formation is still apparent, although the plaque number and size are somewhat reduced. The decreased antiviral activity in response to gB1-460 correlates with its reduced ability to induce ISG expression compared to gB1-750 (Fig. 2C). The difference in activity between gB1-460 and gB1-750 implies that the region of gB between residues 461 and 750 is important for the initiation of cellular antiviral responses. Taken together, these observations demonstrate that the induction of ISGs in response to CMV via an interaction between gB and cell surface components results in the activation of a functional cellular antiviral response.
gB activates IRF3 and induces interferon secretion.
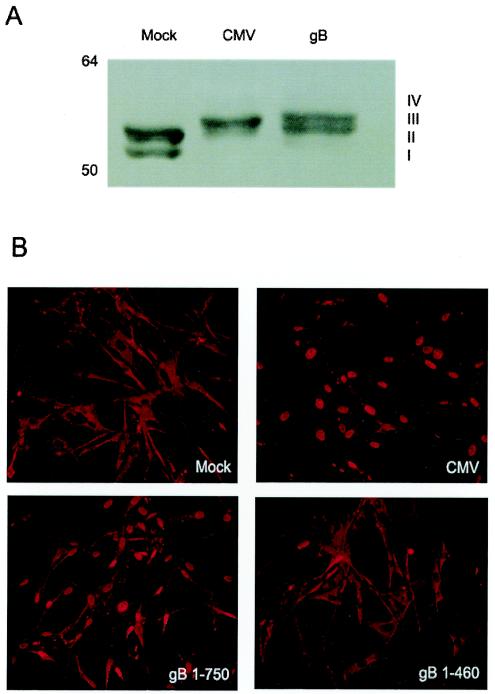
IRF3 activation is critical for the induction of cellular antiviral responses. Activated IRF3 becomes localized to the nucleus, wherein it promotes transcription of a number of ISGs, including selected alpha/beta interferon genes. Two laboratories have shown that CMV activates IRF3 (27, 31), and both CMV and soluble gB directly induce transcription of ISGs (8, 10, 53, 54). Based on these similarities, we hypothesized that IRF3 would be activated in response to soluble gB. Because activated IRF3 is phosphorylated within its carboxy-terminal domain, we assessed the phosphorylation status of IRF3 in response to CMV and gB by SDS-PAGE and Western blot analysis. In uninfected cells, two species of IRF3 are detectable (Fig. 3A). Form I corresponds to unphosphorylated IRF3 (∼55 kDa) and form II is amino-terminally phosphorylated IRF3, whose function has not yet been determined. Forms III and IV, the activated, carboxy-terminally phosphorylated forms of IRF3, are detectable in response to gB as well as CMV, indicating that IRF3 is activated by both stimuli (Fig. 3A).
FIG. 3.
Effects of CMV and gB on IRF3. (A) IRF3 becomes phosphorylated in response to CMV and gB. Human fibroblasts were mock or CMV infected (MOI = 0.1 PFU/cell) or treated with soluble gB (1 μg/ml) in the presence of cycloheximide for 6 h. Cell lysates were prepared and analyzed via SDS-8% PAGE followed by immunoblotting with an IRF3-specific antibody. The different phosphorylated forms of IRF3 are indicated. (B) CMV and gB induce IRF3 nuclear localization. Human fibroblasts were mock infected, infected with CMV or UV-inactivated CMV, or treated with soluble gB or the gB1-460 truncation in the presence of cycloheximide. At 6 h posttreatment, the cells were fixed and the cellular localization of IRF3 was determined by indirect immunofluorescence.
Another indicator of IRF3 activation is translocation to the nucleus. We performed indirect immunofluorescence experiments to determine if IRF3 traffics to the nucleus in response to CMV and soluble gB. As expected, IRF3 is translocated to the nucleus in response to both CMV and soluble gB (Fig. 3B). In contrast, the gB1-460 truncation mutant fails to induce IRF3 nuclear translocation, which correlates with the decreased ability of gB1-460 to trigger the antiviral state (Fig. 2A). This is the first demonstration of IRF3 activation from an external viral stimulus and further defines the cellular pathway utilized by viral glycoproteins to initiate interferon responses.
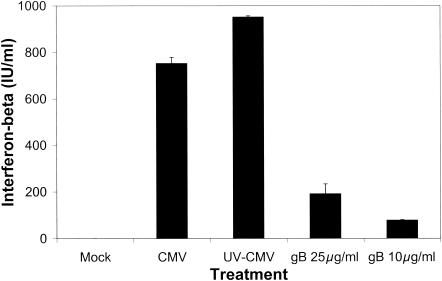
Since IRF3 activation results in the expression of a number of ISGs, including selected alpha/beta interferon genes, we predicted that beta interferon secretion would also be induced by soluble gB. Importantly, our virus and soluble gB stocks were found to be free of detectable interferon (data not shown). Figure 4 shows that beta interferon is secreted by human fibroblasts in response to soluble gB; however, the levels of beta interferon secreted in response to intact virus are much greater than those induced by soluble gB. Furthermore, the amount of soluble gB required to elicit measurable interferon secretion is much greater than that needed to trigger the establishment of the antiviral state. The differences between viral and soluble protein are consistent with the results from the antiviral assay, in which soluble gB induces a less potent antiviral response than gB in the context of whole virions.
FIG. 4.
CMV and gB induce beta interferon secretion. Human fibroblasts were mock infected, infected with CMV or UV-inactivated CMV, or treated with soluble gB. At 12 h posttreatment, cell supernatants were assayed for beta interferon by ELISA.
Primary ISG induction by CMV and gB contributes to the antiviral response.
Interferon secretion in response to CMV and soluble gB implies that the autocrine and paracrine activity of interferon contributes to the antiviral response induced by CMV and soluble gB (Fig. 2). However, in addition to alpha/beta interferons, activated IRF3 regulates the expression of a number of other ISGs, many of which have been shown to individually possess antiviral activity (14). To assess the contribution of primary gene induction by CMV and soluble gB to the antiviral response, we utilized GRE cells, a glioma cell line lacking the alpha/beta interferon loci (7). These cells cannot synthesize beta interferon or any species of alpha interferon, thus allowing direct evaluation of the contribution of primary ISG induction by CMV and soluble gB to the antiviral response. Figure 5 shows that the antiviral response is triggered in GRE cells treated with CMV virions but that higher doses of CMV are required to elicit the response in GRE cells than in fibroblasts. Although this disparity may be attributable to differences between the two cell types, it suggests that the autocrine and paracrine activity of interferons is not absolutely required to establish the antiviral state. The antiviral effect is also achieved with soluble gB, but as with intact virions, higher concentrations of the protein are required to elicit the effect in GRE cells than in fibroblasts. These data indicate that primary induction of ISGs by CMV and soluble gB contributes to the antiviral response, but a portion of the antiviral effect induced by CMV and gB can be attributed to the secondary phase of the response mediated by secreted interferons.
FIG. 5.
Primary induction of ISGs by CMV and gB contributes directly to the antiviral response. GRE cells were treated as indicated (interferon, 100 U/ml). At 6 h posttreatment, the inoculum was removed and the monolayers were challenged with approximately 100 PFU of VSV per well. Plaque formation was visualized by crystal violet staining at 48 h postinfection.
CMV induces ISGs and elicits the antiviral response in endothelial cells.
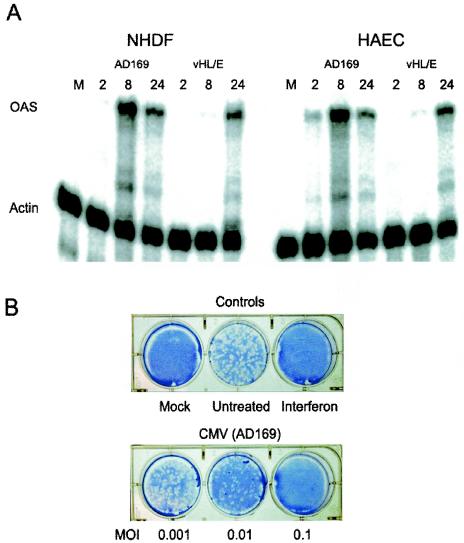
In vitro, the primary cell type used for the study of CMV is human fibroblasts. However, within infected individuals, CMV exhibits a broad cell tropism and is able to infect and replicate in a wide variety of cell types (16, 20, 26, 39, 40). Endothelial cells, which line all blood vessels within the body, are a critical conduit for virus dissemination during CMV infection in vivo and are predicted to act as a reservoir for viral persistence (18, 25). To measure the ability of CMV and soluble gB to elicit antiviral responses from endothelial cells, we utilized RNase protection assays to measure ISG expression in response to both stimuli. In addition to a laboratory-adapted strain of CMV (AD169) that has been passaged primarily in fibroblasts, we analyzed an endothelium-tropic CMV strain (VHL/E). HAEC responded to infection with both strains by up-regulating OAS expression (Fig. 6A). Interestingly, VHL/E induced OAS with much slower kinetics in both cell types than did the AD169 strain. In response to AD169, OAS expression is detectable as early as 2 h postinfection in endothelial cells, reaches a maximum by 8 h, and subsides by 24 h postinfection. In fibroblasts, the results are nearly identical, except that very little OAS is detectable at 2 h in response to AD169, suggesting that ISG induction occurs more rapidly in endothelial cells than in fibroblasts in response to AD169. Conversely, the endothelial strain VHL/E induced OAS expression with identical kinetics in both endothelial cells and fibroblasts. Similar to the case in fibroblasts, the induction of ISGs in endothelial cells also resulted in the establishment of a functional antiviral state (Fig. 6B).
FIG. 6.
Effects of CMV in endothelial cells. (A) CMV induces ISG expression in endothelial cells. Human fibroblasts or endothelial cells were mock infected (M) or infected with CMV (strain AD169 or VHL/E) at an MOI of 2 PFU/cell. At the indicated times postinfection, total cellular RNA was harvested and subjected to RNase protection analysis with OAS- and actin-specific probes. (B) CMV triggers an antiviral state in endothelial cells. HUVEC were stimulated as indicated (interferon, 100 U/ml). At 6 h poststimulation, the treatments were removed and the monolayers were challenged with approximately 100 PFU of VSV per well. Plaque formation was visualized by crystal violet staining at 48 h postinfection.
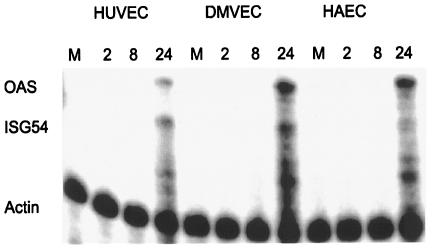
In addition to using the virus, we assessed the ability of soluble gB to induce ISG expression in endothelial cells. Soluble gB was able to elicit ISG expression in endothelial cells of three different origins, from umbilical veins (HUVEC), the dermal microvasculature (DMVEC), and the aorta (HAEC) (Fig. 7). Furthermore, soluble gB induces ISG expression with similar kinetics in all three endothelial cell types. Taken together, these data indicate that ISG expression in response to gB is a hallmark of CMV infection that is conserved across multiple biologically relevant cell types.
FIG. 7.
Soluble gB induces ISG expression in endothelial cells. Endothelial cells from three different sources were mock treated (M) or stimulated with soluble gB. At the indicated times posttreatment, total cellular RNA was harvested and subjected to RNase protection analysis with OAS-, ISG54-, and actin-specific probes.
DISCUSSION
Alpha/beta interferon secretion is vital for mounting a comprehensive immune response capable of effectively clearing viral pathogens. Although the events leading to the induction of interferon responses to viruses are beginning to be addressed, many questions remain. Unresolved issues include the identification of the viral determinants that elicit and the cellular factors that propagate interferon responses. RNA viruses trigger interferons via dsRNA replication intermediates, but the mechanisms by which retroviruses and DNA viruses initiate interferon responses are poorly understood. A number of reports, including several studies from our laboratory, indicate that viral glycoproteins contribute to the induction of interferon responses by viruses (4-6, 11, 22, 38). An emerging theme from these studies is that interferon induction by viruses can be activated in the absence of infection through an interaction between glycoproteins on the surface of the virus and receptors on the plasma membrane of the host cell.
For the present report, we further characterized the cellular antiviral response to envelope gB from CMV and defined components of the pathway that propagate the response to gB. We showed that a functional antiviral response can be induced in human fibroblasts and in endothelial cells by CMV virions as well as by a soluble form of gB (Fig. 2A and 6B). The ability of soluble gB to induce these responses is consistent with the hypothesis that cell contact, but not necessarily virus entry, is required to elicit a coordinated cellular antiviral response. We also showed that soluble gB activates IRF3 (Fig. 3), a key component of the cellular machinery that mediates antiviral responses. To our knowledge, this is the first report of a viral glycoprotein that activates IRF3. Both CMV virions and soluble gB elicit beta interferon secretion from fibroblasts (Fig. 4), implying that the functional antiviral state induced in response to CMV and soluble gB (Fig. 2A) is mediated, at least in part, by the autocrine and paracrine activity of secreted interferons. Since the activation of IRF3 can result in transcription of ISGs independent of interferon secretion, it is possible that direct ISG induction by CMV and soluble gB contributes to the antiviral response. To assess the involvement of primary gene induction by CMV and soluble gB in the absence of interferon feedback, we employed GRE cells, which lack the alpha/beta interferon loci (Fig. 5). The ability of CMV virions and soluble gB to establish an antiviral state within these cells revealed that direct ISG induction by CMV virions and soluble gB is sufficient to confer the antiviral state.
The VSV plaque reduction experiments (Fig. 2A; data not shown) and beta interferon ELISAs (Fig. 4) suggested that virion-associated gB is a more potent inducer of cellular antiviral responses than soluble gB. One possible explanation is that additional viral components contribute to the activation of interferon responses to CMV. Alpha/beta interferon is generated via an amplifiable loop requiring activation of IRF3, as well as IRF7 (34). The dramatic differences in beta interferon secretion between CMV virions and soluble gB (Fig. 4) may indicate that the entire activation loop is engaged upon infection with CMV, whereas only the initial events are triggered by soluble gB. Failure to comprehensively activate the loop could lead to a lesser degree of interferon secretion. Since transcriptionally inactivated virions elicit interferon secretion to levels comparable to those by replication-competent virions (Fig. 4), it follows that additional virion constituents are necessary to generate a comprehensive antiviral response to CMV; however, the identity of these factors remains to be identified.
Alternatively, soluble gB may not be presented to the cell in the same manner as gB within the context of the virion. During the course of virus binding and entry into cells, multiple gB molecules are likely to contact the cell surfaces. Consequently, virion-associated gB will assume a higher local valence than soluble gB will be able to achieve. gB clustered in this manner may be able to more effectively trigger the antiviral response. A detailed investigation of these possibilities is currently under way.
Within the context of the virion, gB is proteolytically processed by the host cell endoprotease furin immediately after amino acid residue 460 into two fragments that remain associated via disulfide linkages (24). The carboxy-terminal fragment is anchored within the viral envelope and possesses features shared by a number of viral fusion proteins (Lopper and Compton, unpublished data). Conversely, the amino-terminal fragment is predicted to possess the receptor binding activity of gB. gB1-750 comprises the entire ectodomain of gB and is processed by furin in a manner similar to membrane-anchored gB into amino- and carboxy-terminal fragments; the carboxy-terminal fragment is no longer embedded in a lipid bilayer. gB1-460 corresponds to the amino-terminal fragment resulting from furin cleavage and retains cell binding activity and structural features similar to that region in the context of gB1-750 (Lopper and Compton, unpublished data). Interestingly, gB1-460 is significantly less effective at triggering interferon responses than gB1-750 (Fig. 2). Furthermore, gB1-460 has a reduced ability to induce ISG expression (Fig. 2C), whereas gB1-750 is a potent inducer of ISGs (38). The fact that gB1-460 is significantly less efficient at inducing these responses is intriguing because this region of gB possesses cell binding activity and is predicted to interact with a cellular receptor(s) (Lopper and Compton, unpublished data). The reduced capacity of gB1-460 to elicit ISG induction indicates that the region of gB between residues 461 and 750 is important for the activation of cellular antiviral responses. In addition, previous studies from our laboratory have shown that a soluble gB construct comprising amino acids 1 to 692 is capable of inducing ISG expression, further reducing the region of gB responsible for triggering the antiviral response to lie between amino acids 461 and 692, a region thought to contribute to fusion (8). This suggests that components of the viral fusion machinery may be responsible for activating cellular antiviral responses to CMV and gB. Studies are under way to more precisely define the region of gB that induces interferon responses as well as the role that the viral fusion machinery plays in the response.
Reports indicate that CMV encodes one or more gene products that act to dampen cellular antiviral responses that are likely detrimental to viral replication. This hypothesis is rooted in the differential ISG expression levels between replication-competent CMV and UV-inactivated virus at later times postinfection. For replication-competent CMV, ISG expression begins to decline between 6 and 24 h postinfection; however, ISG levels are sustained in response to UV-inactivated virus (10). Figure 6A shows that the expression of OAS is high at 8 h postinfection and is notably diminished by 24 h postinfection. Consistent with these results, in VSV plaque reduction assays UV-inactivated CMV consistently confers a higher level of protection than replication-competent virus (Fig. 2A, compare the MOI of 0.001 PFU/ml for CMV and UV-CMV treatments). The down-regulation of ISG expression by live CMV likely allows replication of the indicator virus (VSV), whereas sustained levels of ISGs in response to UV-inactivated CMV limits VSV growth to a greater extent. Thus, our results are consistent with the notion that a CMV-encoded gene product attenuates the antiviral effects triggered during infection.
We also demonstrated that CMV and gB activate antiviral responses in endothelial cells. We assessed the antiviral response of human endothelial cells to two distinct CMV strains. The fibroblast-adapted AD169 strain is highly attenuated in humans and contains a large genomic deletion compared to clinical isolates (13, 17). In contrast, the endothelium-tropic VHL/E strain has been maintained exclusively in endothelial cells and is predicted to retain a higher degree of genetic similarity to CMV clinical isolates than to AD169 (46). Both AD169 and VHL/E induce ISG expression in endothelial cells as well as in fibroblasts; however, in each cell type VHL/E up-regulated ISG expression with slower kinetics and lower peak intensities than did AD169 (Fig. 6A). Many of the open reading frames that are missing from AD169, but present in clinical isolates, are hypothesized to play crucial roles in CMV clinical pathology. Thus, it is possible that the differential ISG induction by AD169 and VHL/E correlates with the ability of virulent CMV isolates to more adeptly avoid host immune responses than their attenuated counterparts.
An important area of interest is the identification of the cell membrane component(s) that detects viral glycoproteins and relays this information to the interior of the cell. One family of cell surface molecules that detect extracellular pathogens and activate host innate immune responses, such as interferon responses, is the Toll-like receptors (TLRs). TLRs are type I transmembrane proteins expressed predominantly on macrophages and dendritic cells, although many other cell and tissue types also express TLRs (43). TLRs function to detect bacteria, fungi, and viruses based on unique structural motifs present on these pathogens, and TLR activation results in a broad range of cellular responses, including secretion of inflammatory cytokines, expression of immune costimulatory molecules, and induction of antiviral responses. TLR2 was recently identified as a cell surface receptor that activates inflammatory cytokine secretion in response to CMV (15). Based on the connection between TLRs and the interferon pathway, an attractive hypothesis is that stimulation of TLR2 by CMV results in the activation of interferon responses. To date, only TLR3, TLR4, and TLR7 have been linked to interferon responses; however, studies to assess the contribution of TLR2 to antiviral responses have been performed with nonviral TLR ligands. We are actively pursuing whether TLR2 activation by CMV contributes to CMV-elicited antiviral responses.
The results described here further clarify the cellular events that are initiated upon interaction of CMV virions with the host cell membrane. These studies highlight the notion that interactions between the virus and cell surface components during virus entry can contribute to the induction of antiviral responses. The identification of viral determinants responsible for eliciting interferon responses to CMV, as well as of cellular factors that mediate these responses, is of paramount importance in efforts to understand CMV's immunomodulatory abilities.
Acknowledgments
We are grateful to W. James Waldman (The Ohio State University) for providing the VHL/E strain of CMV and to Matthew Lopper for generating purified gB1-460. The GRE cells were a kind gift from Ganes C. Sen (The Cleveland Clinic).
This work was supported by NIH grants R01 A134998 and R21 A154915 (to T.C.). K.W.B. was supported by NIH training grant T32 GM 072 15. S.T.P. is supported by NIH training grant T32 CA 09135-27.
REFERENCES
- 1.AbuBakar, S., I. Boldogh, and T. Albrecht. 1990. Human cytomegalovirus stimulates arachidonic acid metabolism through pathways that are affected by inhibitors of phospholipase A2 and protein kinase C. Biochem. Biophys. Res. Commun. 166:953-959. [DOI] [PubMed] [Google Scholar]
- 2.AbuBakar, S., I. Boldogh, and T. Albrecht. 1990. Human cytomegalovirus. Stimulation of [3H] release from [3H]-arachidonic acid prelabelled cells. Arch. Virol. 113:255-266. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht, T., I. Boldogh, M. Fons, S. AbuBakar, and C. Z. Deng. 1990. Cell activation signals and the pathogenesis of human cytomegalovirus. Intervirology 31:68-75. [DOI] [PubMed] [Google Scholar]
- 4.Ankel, H., M. R. Capobianchi, C. Castilletti, and F. Dianzani. 1994. Interferon induction by HIV glycoprotein 120: role of the V3 loop. Virology 205:34-43. [DOI] [PubMed] [Google Scholar]
- 5.Ankel, H., M. R. Capobianchi, F. Frezza, C. Castilletti, and F. Dianzani. 1996. Interferon induction by HIV-1-infected cells: a possible role of sulfatides or related glycolipids. Virology 221:113-119. [DOI] [PubMed] [Google Scholar]
- 6.Ankel, H., D. F. Westra, S. Welling-Wester, and P. Lebon. 1998. Induction of interferon-alpha by glycoprotein D of herpes simplex virus: a possible role of chemokine receptors. Virology 251:317-326. [DOI] [PubMed] [Google Scholar]
- 7.Bandyopadhyay, S. K., G. T. Leonard, Jr., T. Bandyopadhyay, G. R. Stark, and G. C. Sen. 1995. Transcriptional induction by double-stranded RNA is mediated by interferon-stimulated response elements without activation of interferon-stimulated gene factor 3. J. Biol. Chem. 270:19624-19629. [DOI] [PubMed] [Google Scholar]
- 8.Boyle, K. A., R. L. Pietropaolo, and T. Compton. 1999. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol. Cell. Biol. 19:3607-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Britt, W. J. 1984. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology 135:369-378. [DOI] [PubMed] [Google Scholar]
- 10.Browne, E. P., B. Wing, D. Coleman, and T. Shenk. 2001. Altered cellular mRNA levels in human cytomegalovirus-infected fibroblasts: viral block to the accumulation of antiviral mRNAs. J. Virol. 75:12319-12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capobianchi, M. R., H. Ankel, F. Ameglio, R. Paganelli, P. M. Pizzoli, and F. Dianzani. 1992. Recombinant glycoprotein 120 of human immunodeficiency virus is a potent interferon inducer. AIDS Res. Hum. Retrovir. 8:575-579. [DOI] [PubMed] [Google Scholar]
- 12.Carlson, C., W. J. Britt, and T. Compton. 1997. Expression, purification, and characterization of a soluble form of human cytomegalovirus glycoprotein B. Virology 239:198-205. [DOI] [PubMed] [Google Scholar]
- 13.Cha, T. A., E. Tom, G. W. Kemble, G. M. Duke, E. S. Mocarski, and R. R. Spaete. 1996. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J. Virol. 70:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chin, K. C., and P. Cresswell. 2001. Viperin (cig5), an IFN-inducible antiviral protein directly induced by human cytomegalovirus. Proc. Natl. Acad. Sci. USA 98:15125-15130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Compton, T., E. A. Kurt-Jones, K. W. Boehme, J. Belko, E. Latz, D. T. Golenbock, and R. W. Finberg. 2003. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J. Virol. 77:4588-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dankner, W. M., J. A. McCutchan, D. D. Richman, K. Hirata, and S. A. Spector. 1990. Localization of human cytomegalovirus in peripheral blood leukocytes by in situ hybridization. J. Infect. Dis. 161:31-36. [DOI] [PubMed] [Google Scholar]
- 17.Elek, S. D., and H. Stern. 1974. Development of a vaccine against mental retardation caused by cytomegalovirus infection in utero. Lancet i:1-5. [DOI] [PubMed] [Google Scholar]
- 18.Fish, K. N., C. Soderberg-Naucler, L. K. Mills, S. Stenglein, and J. A. Nelson. 1998. Human cytomegalovirus persistently infects aortic endothelial cells. J. Virol. 72:5661-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzgerald, K. A., S. M. McWhirter, K. L. Faia, D. C. Rowe, E. Latz, D. T. Golenbock, A. J. Coyle, S. M. Liao, and T. Maniatis. 2003. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4:491-496. [DOI] [PubMed] [Google Scholar]
- 20.Gnann, J. W., Jr., J. Ahlmen, C. Svalander, L. Olding, M. B. Oldstone, and J. A. Nelson. 1988. Inflammatory cells in transplanted kidneys are infected by human cytomegalovirus. Am. J. Pathol. 132:239-248. [PMC free article] [PubMed] [Google Scholar]
- 21.Hendrix, M. G., M. M. Salimans, C. P. van Boven, and C. A. Bruggeman. 1990. High prevalence of latently present cytomegalovirus in arterial walls of patients suffering from grade III atherosclerosis. Am. J. Pathol. 136:23-28. [PMC free article] [PubMed] [Google Scholar]
- 22.Laude, H., J. Gelfi, L. Lavenant, and B. Charley. 1992. Single amino acid changes in the viral glycoprotein M affect induction of alpha interferon by the coronavirus transmissible gastroenteritis virus. J. Virol. 66:743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ljungman, P. 1996. Cytomegalovirus infections in transplant patients. Scand. J. Infect. Dis. 100(Suppl.):59-63. [PubMed] [Google Scholar]
- 24.Lopper, M., and T. Compton. 2002. Disulfide bond configuration of human cytomegalovirus glycoprotein B. J. Virol. 76:6073-6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melnick, J. L., C. Hu, J. Burek, E. Adam, and M. E. DeBakey. 1994. Cytomegalovirus DNA in arterial walls of patients with atherosclerosis. J. Med. Virol. 42:170-174. [DOI] [PubMed] [Google Scholar]
- 26.Myerson, D., R. C. Hackman, J. A. Nelson, D. C. Ward, and J. K. McDougall. 1984. Widespread presence of histologically occult cytomegalovirus. Hum. Pathol. 15:430-439. [DOI] [PubMed] [Google Scholar]
- 27.Navarro, L., K. Mowen, S. Rodems, B. Weaver, N. Reich, D. Spector, and M. David. 1998. Cytomegalovirus activates interferon immediate-early response gene expression and an interferon regulatory factor 3-containing interferon-stimulated response element-binding complex. Mol. Cell. Biol. 18:3796-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pass, R. F. 2001. Cytomegalovirus, p. 2675-2706. In D. M. Knipe et al. (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 29.Pass, R. F., S. Stagno, G. J. Myers, and C. A. Alford. 1980. Outcome of symptomatic congenital cytomegalovirus infection: results of long-term longitudinal follow-up. Pediatrics 66:758-762. [PubMed] [Google Scholar]
- 30.Pietropaolo, R. L., and T. Compton. 1997. Direct interaction between human cytomegalovirus glycoprotein B and cellular annexin II. J. Virol. 71:9803-9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Preston, C. M., A. N. Harman, and M. J. Nicholl. 2001. Activation of interferon response factor 3 in human cells infected with herpes simplex virus type 1 or human cytomegalovirus. J. Virol. 75:8909-8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramsay, M. E., E. Miller, and C. S. Peckham. 1991. Outcome of confirmed symptomatic congenital cytomegalovirus infection. Arch. Dis. Child 66:1068-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539-548. [DOI] [PubMed] [Google Scholar]
- 34.Sato, M., N. Tanaka, N. Hata, E. Oda, and T. Taniguchi. 1998. Involvement of the IRF family transcription factor IRF-3 in virus-induced activation of the IFN-beta gene. FEBS Lett. 425:112-116. [DOI] [PubMed] [Google Scholar]
- 35.Sen, G. C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255-281. [DOI] [PubMed] [Google Scholar]
- 36.Servant, M. J., N. Grandvaux, B. R. Tenoever, D. Duguay, R. Lin, and J. Hiscott. 2003. Identification of the minimal phosphoacceptor site required for in vivo activation of interferon regulatory factor 3 in response to virus and double-stranded RNA. J. Biol. Chem. 278:9441-9447. [DOI] [PubMed] [Google Scholar]
- 37.Sharma, S., B. R. Tenoever, N. Grandvaux, G. P. Zhou, R. Lin, and J. Hiscott. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148-1151. [DOI] [PubMed] [Google Scholar]
- 38.Simmen, K. A., J. Singh, B. G. Luukkonen, M. Lopper, A. Bittner, N. E. Miller, M. R. Jackson, T. Compton, and K. Fruh. 2001. Global modulation of cellular transcription by human cytomegalovirus is initiated by viral glycoprotein B. Proc. Natl. Acad. Sci. USA 98:7140-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinzger, C., M. Kahl, K. Laib, K. Klingel, P. Rieger, B. Plachter, and G. Jahn. 2000. Tropism of human cytomegalovirus for endothelial cells is determined by a post-entry step dependent on efficient translocation to the nucleus. J. Gen. Virol. 81:3021-3035. [DOI] [PubMed] [Google Scholar]
- 40.Sinzger, C., H. Muntefering, T. Loning, H. Stoss, B. Plachter, and G. Jahn. 1993. Cell types infected in human cytomegalovirus placentitis identified by immunohistochemical double staining. Virchows Arch. A 423:249-256. [DOI] [PubMed] [Google Scholar]
- 41.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 42.Suhara, W., M. Yoneyama, I. Kitabayashi, and T. Fujita. 2002. Direct involvement of CREB-binding protein/p300 in sequence-specific DNA binding of virus-activated interferon regulatory factor-3 holocomplex. J. Biol. Chem. 277:22304-22313. [DOI] [PubMed] [Google Scholar]
- 43.Takeda, K., and S. Akira. 2003. Toll receptors and pathogen resistance. Cell. Microbiol. 5:143-153. [DOI] [PubMed] [Google Scholar]
- 44.Taniguchi, T., K. Ogasawara, A. Takaoka, and N. Tanaka. 2001. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 19:623-655. [DOI] [PubMed] [Google Scholar]
- 45.Taniguchi, T., and A. Takaoka. 2002. The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr. Opin. Immunol. 14:111-116. [DOI] [PubMed] [Google Scholar]
- 46.Waldman, W. J., W. H. Roberts, D. H. Davis, M. V. Williams, D. D. Sedmak, and R. E. Stephens. 1991. Preservation of natural endothelial cytopathogenicity of cytomegalovirus by propagation in endothelial cells. Arch. Virol. 117:143-164. [DOI] [PubMed] [Google Scholar]
- 47.Wathelet, M. G., C. H. Lin, B. S. Parekh, L. V. Ronco, P. M. Howley, and T. Maniatis. 1998. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Mol. Cell 1:507-518. [DOI] [PubMed] [Google Scholar]
- 48.Yoneyama, M., W. Suhara, Y. Fukuhara, M. Fukuda, E. Nishida, and T. Fujita. 1998. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 17:1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yurochko, A. D., and E. S. Huang. 1999. Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J. Immunol. 162:4806-4816. [PubMed] [Google Scholar]
- 50.Yurochko, A. D., E. S. Hwang, L. Rasmussen, S. Keay, L. Pereira, and E. S. Huang. 1997. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-kappaB during infection. J. Virol. 71:5051-5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yurochko, A. D., T. F. Kowalik, S. M. Huong, and E. S. Huang. 1995. Human cytomegalovirus upregulates NF-κB activity by transactivating the NF-κB p105/p50 and p65 promoters. J. Virol. 69:5391-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yurochko, A. D., M. W. Mayo, E. E. Poma, A. S. Baldwin, Jr., and E. S. Huang. 1997. Induction of the transcription factor Sp1 during human cytomegalovirus infection mediates upregulation of the p65 and p105/p50 NF-kappaB promoters. J. Virol. 71:4638-4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu, H., J. P. Cong, G. Mamtora, T. Gingeras, and T. Shenk. 1998. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:14470-14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu, H., J. P. Cong, and T. Shenk. 1997. Use of differential display analysis to assess the effect of human cytomegalovirus infection on the accumulation of cellular RNAs: induction of interferon-responsive RNAs. Proc. Natl. Acad. Sci. USA 94:13985-13990. [DOI] [PMC free article] [PubMed] [Google Scholar]